Abstract
Occlusive wound dressings are utilized clinically to accelerate wound healing and improve the final appearance of scars. In vivo and in vitro evidence suggests that one mechanism for this effect is maintenance of normal hydration in the epidermis, although the molecular signals remain uncharacterized. We sought to elucidate histological changes and some of the molecular signals involved in this effect in a rat model of wound semi-occlusion. We utilized a rat linear incision model with surgical tape occlusion. Histological stains and quantitative real-time PCR analysis were used to characterize the cellular and molecular effects of semi-occlusion on the wound healing response. Semi-occluded wounds demonstrated decreased epidermal thickness and cellularity and less mitotic epidermal activity when compared with non-occluded control wounds. Associated dermal cellularity was similarly attenuated by semi-occlusion. Finally, levels of proinflammatory cytokines IL-1-alpha and TNF-alpha were significantly decreased on postoperative day 3 at the transcriptional level when compared with non-occluded wounds. Semi-occlusive wound treatments significantly decrease epidermal thickness, cellularity, mitotic activity and dermal cellularity as well as transcriptional levels of important epidermal mediators of inflammation in a rat incisional wound model.
Keywords: occlusion, rat, proliferation, epithelium, scar, wound healing, IL-1, TNF-alpha
Introduction
Cutaneous wound healing is a highly concerted process that can be divided into three partially overlapping stages: i) an initial inflammatory phase, ii) a proliferative phase characterized by an increase in cell number and matrix deposition, and iii) a remodeling phase involving inflammatory and mesenchymal cell apoptosis and matrix reorganization (1, 2). Optimal wound healing occurs when the cascade is able to transition efficiently through all three phases. Prompt restoration of homeostasis after the initial injury allows for expeditious entry into the final phases of wound healing and minimizes scarring (3–7).
A moist wound environment has been demonstrated to accelerate re-epithelialization (8). Winter showed more than forty years ago that partial thickness wounds heal more rapidly when covered with dressings that prevented eschar formation (9). At least eight randomized and 27 non-randomized trials have proven the efficacy of silicone gel occlusion in improving the appearance of scars (10). Although the mechanism of action has been the source of speculations, substantial evidence indicates that its occlusive properties with epidermal hydration are responsible for the antiscarring effect (11–19).
While the role of wound hydration has been appreciated clinically, it has not been clearly defined at the molecular level. Facilitated keratinocyte migration over a moist wound surface and a consistent increase of growth factors and proteinases in wound fluids have all been suggested as theories to explain scar reduction in occluded wounds (13, 15, 18). An increasing body of evidence, including evidence from our laboratory’s rabbit ear model, implicates the semi-occlusive characteristics of the dressing, with hydration of the epidermis and secondary paracrine signaling to the dermis (13, 15, 19). For instance, in a coculture setting, hydrated keratinocytes reduced collagen production in the underlying fibroblasts (20). Furthermore, semi-occlusion with silicone gel sheeting of full-thickness dermal ulcer wounds in the rabbit ear exerted a significant reduction in epithelial thickness indicating a faster re-epithelialization (Mustoe et al., unpublished observation). A recent clinical study demonstrated that paper tape semi-occlusion of cesarean section incisional wounds decreased both the incidence and severity of hypertrophic scars (16, 17).
We hypothesize that early restoration of an epidermal water barrier by a semi-occlusive dressing would result in observable changes in epidermal/dermal proliferation in the setting of an identical initial injury. We also postulated that semi-occlusion would lead to a significant reduction of key proinflammatory epidermal cytokines in early stages of wound healing. We utilized a paper taping method for semi-occlusion which is widely used clinically and found to be beneficial in improving the early and late outcomes after surgical wound closure.
Material and Methods
Animals & Wound Model
A rat linear incisional model was utilized as previously described (22). A total of eighty-nine young (3–6 months) white female Fisher rats (260–320 g) were used for all experiments. Animals were housed according to the National Institutes of Health guidelines for the care and use of laboratory animals. All procedures were approved by the Northwestern University Animal Care and Use Committee. After animals were anesthetized via intraperitoneal injection, two parallel 5 cm long incisions were made 1.5 cm on each side of the spine down to the panniculus carnosus. Wounds were closed with intradermal 5–0 polypropylene sutures in a running fashion. One wound was randomly picked for semi-occlusive treatment by rolling the dice (1–3 meant left side, 4–6 right side of the spine) and covered with two layers of Steri-Strips® (3M Health Care, St. Paul, MN, USA) after applying a liquid adhesive (Mastisol®: Ferndale Inc., Ferndale, MI, USA). The second wound was solely covered with Mastisol®, thus every animal served as it own control. Wounds were harvested at post-operative day (POD) 3, 7, 10, 14 and 21.
Transepidermal water loss (TEWL)
In order to demonstrate that two layers of Steri-Strip do have semi-occlusive properties we assessed the transepidermal water loss (TEWL) in three different treatment groups: 1) mastisol + control 2) mastisol + two layers of steristrip + control and 3) mastisol + one layer of Tegaderm + control. We chose Tegaderm® since this polyurethane dressing doubtlessly does have semi-occlusive properties.
Dressings were removed after one hour and then the TEWL was assessed in standardized environmental conditions (21° Celsius room temperature, 35% Air Humidity).
BrdU-Staining and Histological Analysis
Animals at POD3 (n=8) received an intraperitoneal injection (6mg/100g body weight) of bromodeoxyuridine solution (BrdU, Sigma, St. Louis, MO, USA, Cat. #B5002) two hours prior to harvest. Immediately after euthanizing the rats, a random section of both wounds was fixed in a 10%-Zinc-Formalin solution for further histological processing. Tissue sections were paraffin embedded, processed, and either stained with hematoxylin and eosin (H&E), (POD3, n= 28; POD7, n=30; POD10, n=31) or examined via immunohistochemistry for BrdU uptake. We used a cell proliferation kit (Zymed Lab. Inc., San Francisco, CA) to detect BrdU-positive nuclei. The percentage of BrdU-positive cells was determined by dividing the number of BrdU-positive nuclei by the total number of nuclei in the epidermal basal cell layer and multiplied by 100.
The sections for measuring the epidermal thickness were taken from a random area of the mid-third of a wound. All tissue samples were measured in a blinded fashion by two independent examiners using light microscopy and a calibrated lens reticule. The results were averaged. Epithelial thickness was measured from the basement membrane of the epidermis to the stratum corneum. The epidermal, dermal and peripanniculous wound area was evaluated for cellularity employing a semi-quantitative scale (0–4), in which higher numbers indicate increased cellularity.
RNA extraction and quantitative real-time PCR
RNA extraction procedures and quantitative real-time PCR were carried out as previously described (23). Briefly, the remaining wound tissue was excised with surgical blades from the surrounding unwounded skin in a 1-mm range to both sides of the incision. These wound strips were digested enzymatically overnight with dispase (Invitrogen, Carlsbad, CA, USA, Cat. 17105-041). The next day, the epithelium was pulled off from the dermis and homogenized using a rotor-stator homogenizer (PowerGen 125, Fisher Scientific, Hanover Park, IL, USA). RNA was extracted from epidermal cells employing a mini-column based lysis and extraction kit (Qiagen Inc., Valencia, CA, USA: Qia-Shredder and RNeasy Minikit, Cat. 79656 and 74106) following the manufacturer’s instructions and using intermediary proteinase K and RNAse-free DNAse steps to prevent genomic DNA carryover. The integrity and quantity for each RNA sample was evaluated on an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Palo Alto, CA, USA).
Both the reverse transcriptionm and the polymerase chain reaction were carried out in the same wells of 96-well Micro Amp optical plates. Each QRT-PCR reactions typically contained 1× RT-PCR reaction mix, 900nM of forward and reverse primers, 250 nM of Taqman probe and 100ng of RNA. The reactions for 18S included 300nM of forward and reverse primers and 10ng of RNA together with 1× RT-PCR mix. The reverse transcriptase reaction was set at 48° C for 30 minutes followed by 10-minute heating at 95° C. Subsequently, PCR cycling (45 cycles) started at a denaturation temperature of 95° C for 15 seconds followed by annealing/extension temperature of 60° C for 1 minute with all reactions in triplicates. The threshold cycle or CT values were averaged from each reaction and the gene expression level was calculated as relative level to the calibrator gene 18S. Every primer and probe was validated prior to its use. Primers and probes are designed by ‘Primer Express’ software (Applied Biosystems, Foster City, CA, USA). Rat-specific taqman primer and probe sequences for tumor necrosis factor alpha and interleukin-1-alpha were validated by and purchased from Applied Biosystems.
Statistical analysis
All wounds were harvested and evaluated in a paired fashion. Data is shown as mean ± SEM. Statistical analysis was carried out employing a paired, two-tailed Student t-test to detect statistical significance (Excel, Microsoft Inc., CA, USA). Results with a p-value < 0.05 were considered significant.
Results
Transepidermal Water Loss Measurements (TEWL)
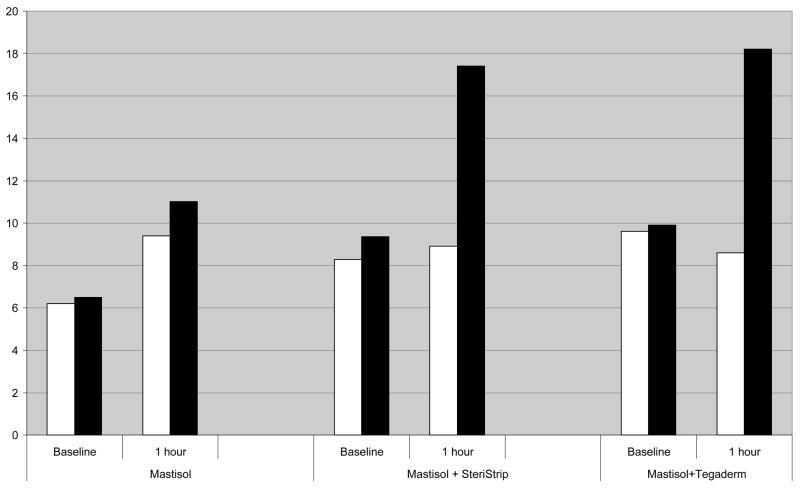
First, in order to demonstrate that a two-layered sheeting with paper tape (Steri-Strip) has semi-occlusive properties, we measured the TEWL after one hour of treatment in three different groups (Mastisol alone, two layers of Steri-Strip with Mastisol or one layer of Tegaderm with Mastisol). Our findings that are representatively summarized in Figure 1 clearly demonstrate that i) Mastisol alone as a liquid adhesive has no relevant semi-occlusive properties and therefore serves as an adequate control and ii) two layers of steri-strip are equally semi-occlusive as one layer of the established semi-occusive polyurethane dressing Tegaderm®.
Figure 1.
Figure 1 shows representative results of one hour treatment and subsequent measurement of transepidermal water loss (TEWL) in three different treatment groups (mastisol alone, mastisol + two layers of steri-strip and mastisol + one layer of polyurethane sheeting (Tegaderm®)). TEWL was assessed in standardized environmental conditions (21° Celsius room temperature, 35% Air Humidity). Baseline values express TEWL at t=0 (white column is control, full column value is prior to semi-occlusion at t=0). White columns at one-hour sections represent TEWL of non-occluded skin, full columns represent TEWL values after one hour of semi-occlusive treatment. Two layers of paper tape semi-occlusion have comparable occlusive properties as one layer of polyurethane dressing.
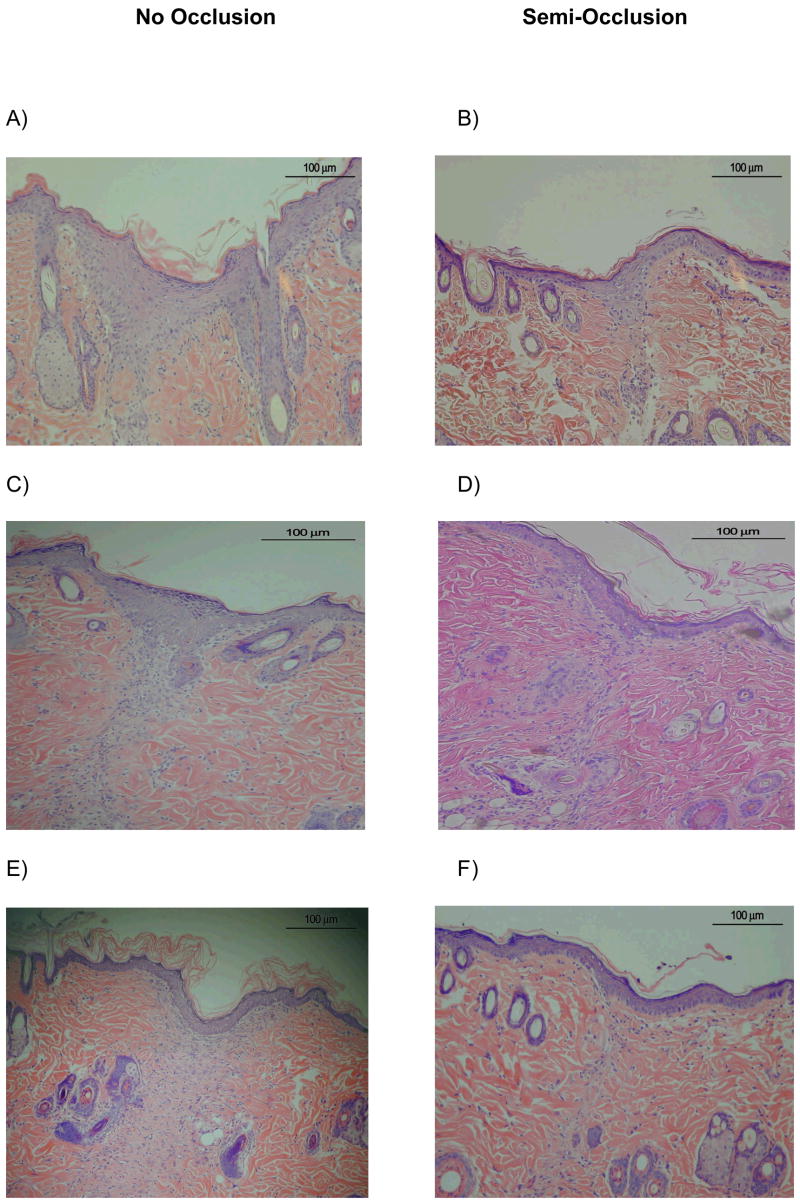
We compared histological changes in semi-occluded and non-occluded incisional wounds on POD 3, 7, 10, 14 and 21. At POD 3, 7 and 10, semi-occluded wounds demonstrated enhanced wound healing histologically when compared to non-occluded controls. On POD 3, semi-occluded wounds displayed a flatter epithelium with a clearly developed stratum corneum and stratum disjunctum (outermost sloughing layer of stratum corneum), indicative of a mature epithelium that has undergone multiple cell passages (Fig. 2). At POD 7, wound healing with semi-occlusion appeared to be nearly completed. In contrast, non-occluded wounds at this time point still showed a hyperproliferative epithelium implying ongoing repair with significantly higher dermal cellular activity compared to semi-occluded wounds (Fig. 2). Epidermal thickness at POD 7 was significantly reduced in occluded wounds (n= 11; 83,63 μm±11,35 vs. 45,45 μm±7,13, p<0,05, Fig. 3). Epidermal and dermal cellularity were also significantly reduced in occluded wounds at POD 7 (n=12; 2,125±0,15 vs. 1,25±0,12, p<0,05) and (n=12; 2,7±0,13 vs. 1,5±0,1, p<0,001), respectively (Fig 4). At POD 10, wound healing was fully accomplished in semi-occluded wounds and the epithelial thickness equaled the thickness of the surrounding non-wounded epithelium. In non-occluded wounds dermal cellularity was still increased, suggesting a prolongation of the inflammatory phase of wound healing. Wounds examined on POD 14 (Fig. 5 A+B) and POD 21 (Fig. 5 C+D) did not exert any significant histological alterations whether treated (Fig. 5 A+C) or untreated (Fig. 5 B+D).
Figure 2.
H&E-stained histological sections on POD 3, 7 and 10 (Magnification: 100x). From POD 3 (A+B), via POD7 (C +D) to POD 10 (E+F), semi-occluded wounds showed a more matured and flattened epithelium with less epithelial and especially dermal cellularity count as opposed to non-occluded wounds. This indicates accelerating properties of semi-occlusive dressings on the wound healing process and specifically on re-epithelialization along with scar ameliorating effects via reduced dermal proliferation.
Figure 3.
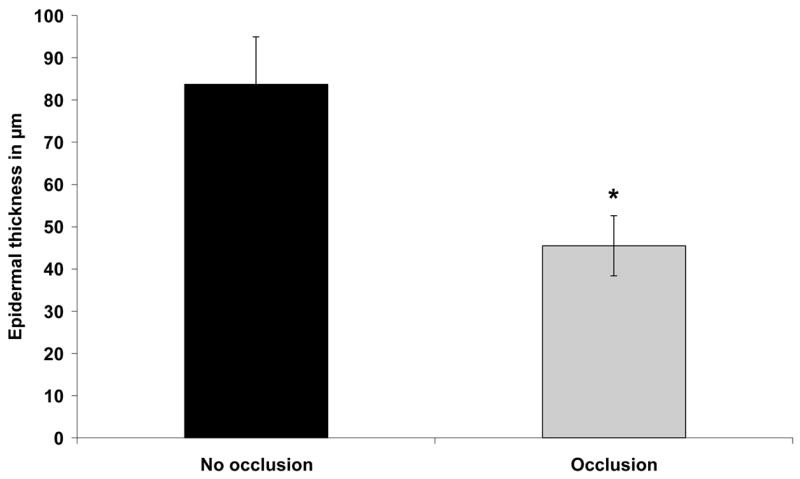
Epidermal thickness in semi-occluded versus non-occluded wounds was reduced by almost 50% on POD 7 (n= 11; 83,63 μm±11,35 vs. 45,45 μm±7,13, p<0,05).
Figure 4.
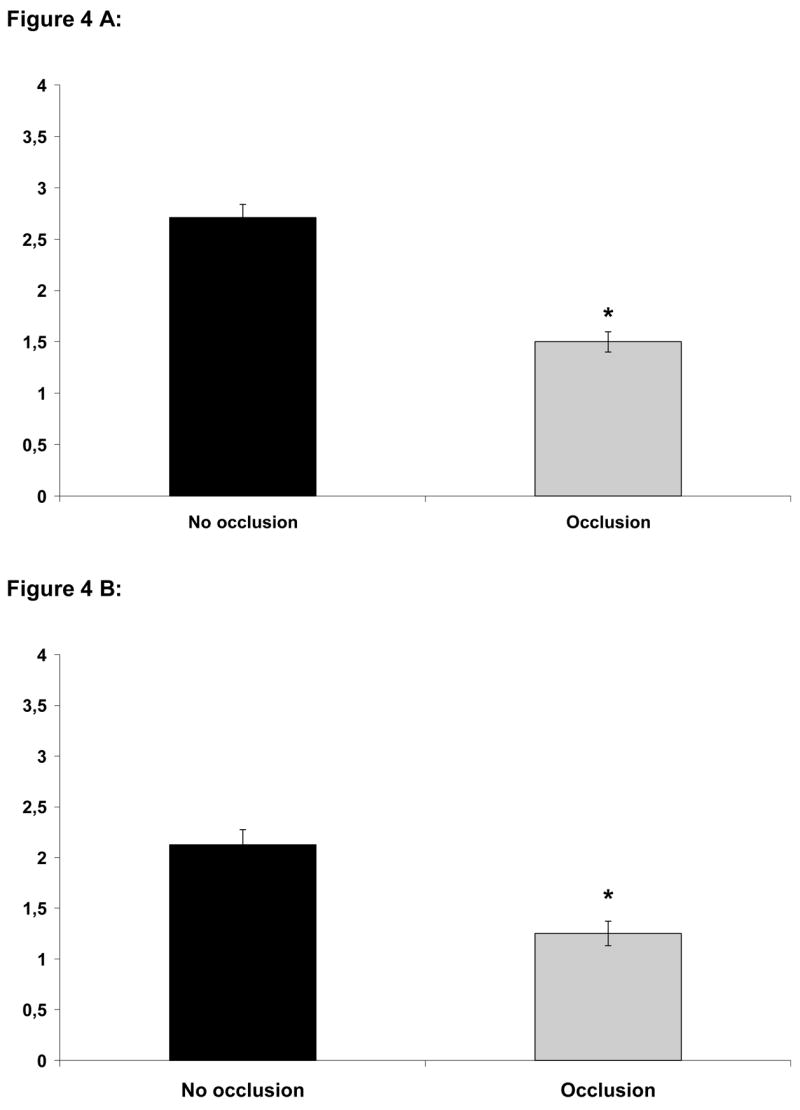
A) Dermal cellularity corresponding predominantly to newly formed granulation tissue was significantly elevated on POD 7 in non-occluded wounds (n=12; 2,7±0,13 vs. 1,5±0,1, p<0,001). B) Epidermal cellularity on POD 7 was significantly increased by approximately 70% in non-occluded wounds (n=12; 2,125±0,15 vs. 1,25±0,12, p<0,05)
Figure 5.
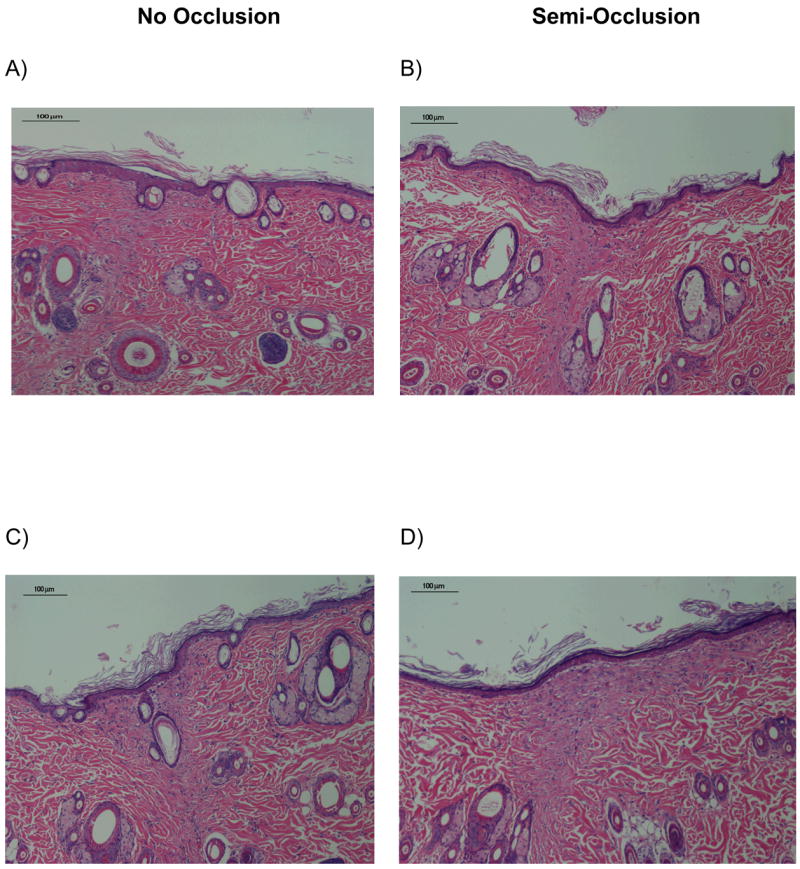
H&E stained sections of control (A+C) and treated (B+D) wounds on POD 14 (A+B) and 21 (C+D), (100×). No significant histological differences in terms of epidermal thickness and epidermal/dermal cellularity were found.
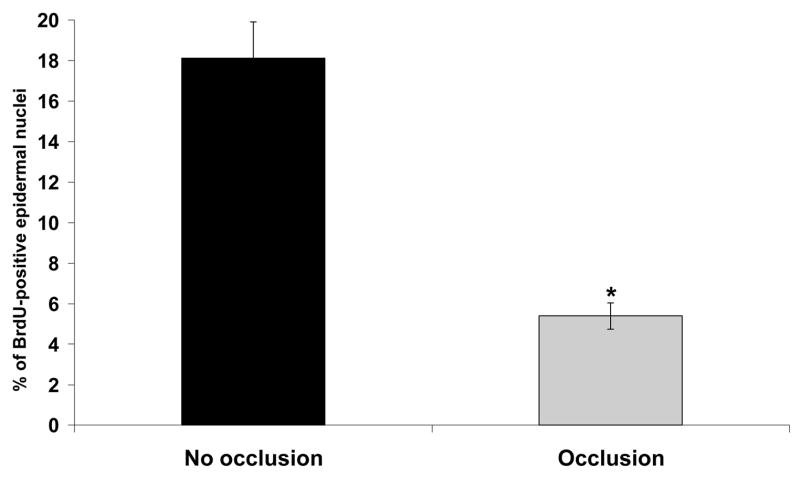
To further elucidate the observed histological changes in the epithelium of semi-occluded wounds, we employed BrdU staining in order to determine mitotic activity in the epidermis of treated and control wounds on POD 3 (Fig. 6 A+B). Only epithelial cells of the basal layer showed a detectable uptake of BrdU indicating the location of the highest proliferative activity. Semi-occluded wounds showed a significant reduction in BrdU-uptake at POD 3 (p< 0.001; Fig. 6 and 7) compared to non-occluded wounds closely paralleling our histological findings in a sense such that the semi-occluded epidermis is relatively more mature and therefore more quiescent.
Figure 6.
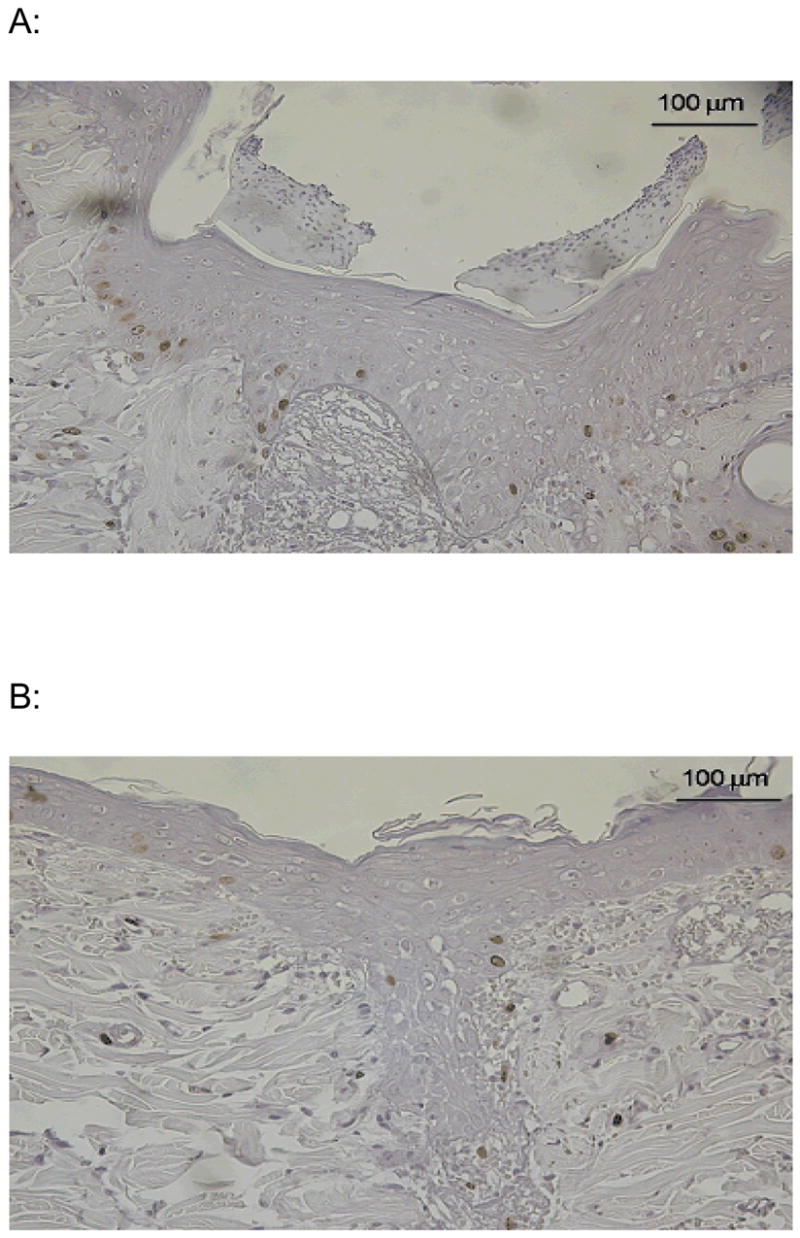
Representative BrdU-stained histological sections on POD 3. Only nuclei of the basal epidermal layer stored BrdU indicating proliferative activity. Epidermal basal nuclei of non-occluded wounds (A) showed significantly increased proliferation compared to semi-occluded wounds (B).
Figure 7.
Percentage of BrdU-positive epidermal nuclei compared to all epidermal nuclei. Non-occluded wounds showed a dramatically increased proliferative activity in the epidermal basal cell layer compared to semi-occluded wounds (n=8; 18,11%±1,8 vs. 5,39±0,65, p<0,001).
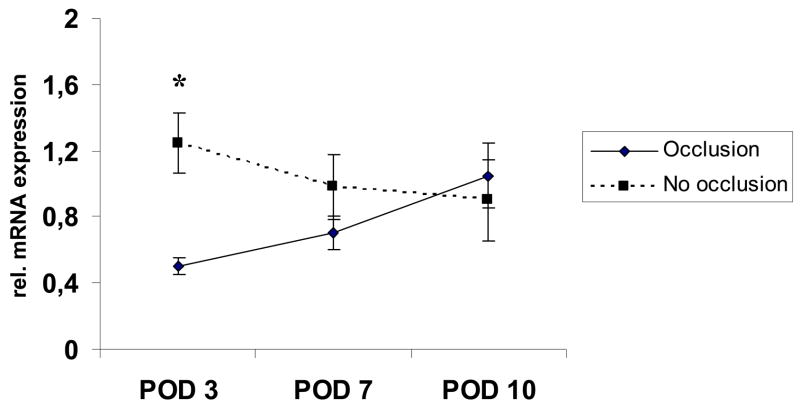
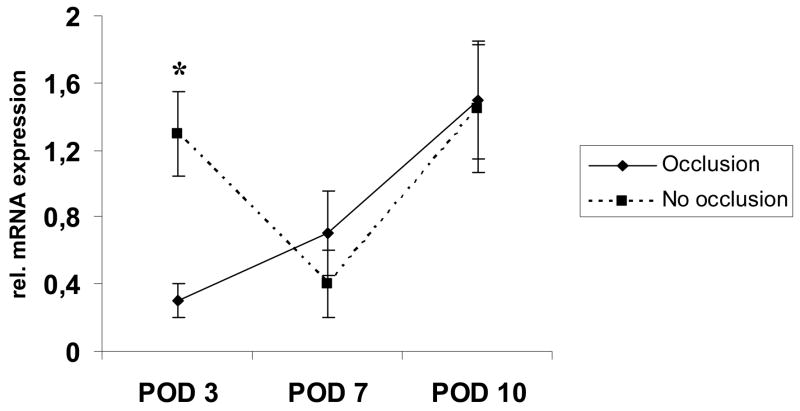
We then investigated expression patterns of IL-1-alpha and TNF-alpha in the extracted epidermal RNA to detect alterations in these proinflammatory cytokines. IL-1-alpha was significantly decreased on POD 3 in semi-occluded wounds and showed a nearly linear positive regression towards POD 10 equaling expression levels of non-occluded wounds. In contrast, IL-1-alpha levels in non-occluded wounds continuously decreased with wound healing approximating completion (Fig. 8).
Figure 8.
Selective epidermal relative IL-1-alpha mRNA-expression levels on POD 3, 7 and 10. IL-1-alpha mRNA expression was increased on POD 3 and 7 in non-occluded wounds, but only POD 3 reached significance (n=6; 1,18±0,26 vs. 0,59 ±0,06, p<0,05). On POD 10, IL-1-alpha mRNA expression was almost equal in both groups.
TNF-alpha was also significantly reduced in early stages of semi-occluded wound healing (n=6, p< 0.05, POD 3) and showed a nearly linear increase until POD 10 (Fig. 9).
Figure 9.
Epidermal expression of TNF-alpha-mRNA on POD 3, 7 and 10 in semi-occluded and non-occluded wounds. TNF-alpha expression was significantly downregulated in semi-occluded wounds on POD 3 but not at later timepoints. Comparable to IL-1-alpha, mRNA-TNF-alpha expression patterns were almost even in semi-occluded and non-occluded wounds on POD 10 paralleling the histological findings.
Discussion
Perhaps the most critical barrier function of the epidermis is to prevent water loss from the liquid environment that all cells exist in (24). The epidermis consists of several layers. There is a basal cell layer from which cell proliferation occurs, and which is in contact via the basement membrane with the underlying dermis. As such, the basal cell layer is the source of any epidermal paracrine signaling. Above that layer, the stratum granulosum contains keratinocytes connected by tight junctions which form a water barrier that is absolutely necessary for survival. The outer surface is the stratum corneum, which consists of multiple layers of terminally differentiated cells that have lost their nuclei, with layers of extracellular lipids between each cell layer which form the outer water barrier. The epidermal water content can be measured as well as the transepidermal water loss (TEWL) as markers of the water barrier homeostasis (Figure 1). Alterations in epidermal water content homeostasis due to low humidity for instance result in compensatory changes in the epidermis, such as increased epidermal thickness. The generation of cornified keratinocytes that insures the integrity of the physical water barrier involves a complex, multi-step differentiation process, influenced by a large number of signaling pathways including the protein C (PKC) family (25).
After wounding, there is a period of time when water barrier has not yet been restored, and homeostatic mechanisms result in epidermal proliferation along with altered paracrine signaling. It has been suggested that restoration of the normal water barrier through the use of semi-occlusive wound dressings helps to ameliorate the inflammatory and proliferative phases of the wound healing cascade, resulting in an improvement in the resultant scar. During the early stages of normal wound healing, keratinocytes migrate and make a single-layered epidermis to cover the wound bed. Then keratinocytes proliferate and differentiate to become multiple-layered stratified epidermis, which leads to the increase in the thickness of newly formed epidermis. At the later stages of re-epithelialization, as keratinocytes continue maturation, they tend to become more flattened and thinner in shape. Therefore, our histological findings of a flattened and more quiescent epithelium in semi-occluded wounds imply an advancing effect of such dressings on epidermal maturation in later stages of wound healing (POD 3, 7 and 10).
Wound semi-occlusion of ear punch wounds in a rabbit hypertrophic scar model clearly demonstrates an improvement in scar elevation and appearance (12, 14, 19). In vivo experiments using human keratinocyte-fibroblast coculture systems suggests that this effect is mediated through actions on the level of hydration of keratinocytes (20, 26).
Although the rat does not form hypertrophic scars per se, the results we have presented clearly demonstrate that the rat incisional model is capable of recapitulating the decreased inflammation and proliferation associated with wound semi-occlusion.
Here, we found a reduction of mRNA IL-1-alpha and TNF-alpha in vivo in the epidermis of semi-occluded versus non-occluded full thickness wounds at POD 3. TNF-alpha and IL-1 are cytokines that originate from the epithelium of the skin and their proinflammatory character has been demonstrated in many studies for multiple organs and tissues (27). Upon production, IL-1-alpha and TNF-alpha can trigger cytokine cascades with a subsequent upregulation of IL-6 (28), IL-8 (29, 30), granulocyte/macrophage-colony-stimulating factor (GM-CSF) (31) and proinflammatory cytokines such as intracellular adhesion molecule (ICAM) (29). We specifically investigated these two cytokines based on previous in vitro findings from our laboratory demonstrating that TNF-alpha and IL-1 are possible paracrine keyplayers in the setting of hydrated keratinocytes/fibroblast cocultures (26). Different murine models of water barrier disruption (e.g. chemical, mechanical and dietary) have demonstrated an increase in epidermal cytokines, such as IL-1 and TNF-alpha, in a time-dependent fashion and a subsequent return to normal as soon as barrier restoration occurred (32–35). In another study, IL-1-alpha was significantly increased in the epidermis of mice that were kept under low-humidity conditions 48 hours prior to barrier disruption by paper stripping (36). IL-1-alpha release directly after water barrier pertubation was significantly higher in mice that were kept in a low-humidity environment as opposed to high-humidity conditions (36). The results of this research strongly support our hypothesis of an ameliorating effect of epidermal hydration on the secretion of proinflammatory cytokines like IL-1-alpha and TNF-alpha.
In our study, transcriptional TNF-alpha levels exterted a significant reduction at POD 3 and gradually increased until POD 10 in an almost linear fashion paralleling histological barrier restoration. Other studies revealed that TNF-alpha is several fold elevated after chronic (dietary) or acute (mechanical and chemical) water barrier disruption (33, 35).
Previously, the impact of occlusion on water barrier disruption has been investigated with variable results regarding epidermal cytokine expression. For instance, latex occlusion after acute water barrier disruption by acteone or paper stripping treatment failed to reduce the initial elevation of TNF-alpha, IL-1 alpha and IL-1-beta 2.5 hours after epidermal injury (37). In contrast, occlusive treatment for 24 to 48 hours of chronic water barrier perturbation of skin of essential-fatty-acid-deficient mice, that naturally contain elevated levels of mRNAs encoding TNF-alpha, IL-1-alpha and IL-1-beta, caused a reduction of such cytokines. Finally, occlusion of uninjured normal mouse skin selectively reduced IL-1-alpha expression but not TNF-alpha or IL-1-beta (37).
In our study, we have covered full thickness inicisonal wounds immidiately after wounding and semi-occlusive dressings remained in place upon harvest. Meticulous care was taken, that wounds were fully covered at all times. The ameliorating effect of semi-occlusive dressings on epidermal proinflammatory cytokine expression, as shown in this study, is clearly dependent on the duration of semi-occlusion and the time of onset since hydration of the epidermis along with the proposed subsequent alteration in epidermal/dermal paracrine signaling is unlikely to be achieved in the very early stages after water barrier disruption. Moreover, the different murine models employed for water barrier disruption are only partially comparable to the complex phases of wound healing in our model.
We specifically chose an incisional as opposed to an excisional wound model since we wished to focus on the time after epithelialization is complete which in a superficial incision model is within 24 hours. Furthermore, the incisional model best represents surgical incisions and iatrogenic injuries seen everyday in the surgical theatre. Furthermore, dermal injury was well defined and controlled so that the variable we were testing, treatment of the epidermis with a semi-occlusive dressing, would not be overwhelmed by healing changes in the dermis. In an excisional model with a larger area to re-epithelialize, cytokine changes might have differed to a larger extent from wound edge to center as opposed to an incisional model. Furthermore, we have demonstrated that the employed semi-occlusive dressing method results in less epidermal cellularity, less epidermal proliferation, and less proinflammatory epidermal signaling compared to non-occluded control wounds. Due to the very few amounts of epithelial cells that can be obtained from rat wound tissue, we could not perform quantitative protein analysis in this study. The results presented here, support our hypothesis that a transient restoration of a perturbed water barrier by semi-occlusive dressings in the setting of full thickness wounds mitigates the inflammatory stimulus along with a reduction in epidermal and dermal proliferation.
Acknowledgments
This research was supported by the National Institutes of Health (NIH grant#: R01-GM063825)
References
- 1.Singer AJ, Clark RA. Cutaneous Wound Healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 3.Garner WL. Epidermal regulation of dermal fibroblast activity. Plast Reconstr Surg. 1998;102:135–9. doi: 10.1097/00006534-199807000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Mustoe TA, Cooter RD, Gold MH, Hobbs FD, Ramelet AA, Shakespeare PG, Stella M, Teot L, Wood FM, Ziegler UE. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–71. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Mustoe TA. Scars and keloids. BMJ. 2004;328:1329–30. doi: 10.1136/bmj.328.7452.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su CW, Alizadeh K, Boddie A, Lee RC. The problem scar. Clin Plast Surg. 1998;25:451–65. [PubMed] [Google Scholar]
- 7.Atiyeh BS, El-Musa KA, Dham R. Scar quality and physiologic barrier function restoration after moist and moist-exposed dressings of partial-thickness wounds. Dermatol Surg. 2003;29:14–20. doi: 10.1046/j.1524-4725.2003.29002.x. [DOI] [PubMed] [Google Scholar]
- 8.Svensjo T, Pomahac B, Yao F, Slama J, Eriksson E. Accelerated healing of full-thickness skin wounds in a wet environment. Plast Reconstr Surg. 2000;106:602–12. [PubMed] [Google Scholar]
- 9.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–4. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 10.Poston J. The use of silicone gel sheeting in the management of hypertrophic and keloid scars. J Wound Care. 2000;9:10–6. doi: 10.12968/jowc.2000.9.1.26342. [DOI] [PubMed] [Google Scholar]
- 11.Sawada Y, Sone K. Hydration and occlusion treatment for hypertrophic scars and keloids. Br J Plast Surg. 1992;45:599–603. doi: 10.1016/0007-1226(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 12.Saulis AS, Chao J, Telser A, Mogford JE, Mustoe TA. Silicone occlusive products in the treatment of hypertrophic scar in the rabbit ear hypertrophic scar model. Aesthetic Surg J. 2002;22:147–153. doi: 10.1067/maj.2002.123023. [DOI] [PubMed] [Google Scholar]
- 13.Kloeters O, Kim JYS, Mustoe TA. Occlusive Dressing Reduces Inflammatory Response and Alters Connexin Expression in a Rat Incisional Model. Wound Repair Regen. 2005;13:A26. [Google Scholar]
- 14.Kloeters O, Tandara A, Mustoe TA. The Hypertrophic Scar Model in the Rabbit Ear –A reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen. 2007;15:S40–S45. doi: 10.1111/j.1524-475X.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 15.Tandara AA, Mustoe TA, Mogford JE. Impact of Hydration on MMP Activity. Wound Repair Regen. 2004;12:A6. [Google Scholar]
- 16.Atkinson JA, McKenna KT, Barnett AG, McGrath DJ, Rudd M. A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer's skin tension lines. Plast Reconstr Surg. 2005;116:1648–56. doi: 10.1097/01.prs.0000187147.73963.a5. [DOI] [PubMed] [Google Scholar]
- 17.Mustoe TA, Kloeters O. Discussion [Google Scholar]; Atkinson JA, McKenna KT, Barnett AG, McGrath DJ, Rudd M. A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer's skin tension lines. Plast Reconstr Surg. 2005;116:1648–56. doi: 10.1097/01.prs.0000187147.73963.a5. discussion 1657–58. [DOI] [PubMed] [Google Scholar]
- 18.Suetake T, Sasai S, Zhen Yx, Tagami H. Effects of silicone gel sheet on the stratum corneum hydration. Br J Plast Surg. 2000;53:503–507. doi: 10.1054/bjps.2000.3388. [DOI] [PubMed] [Google Scholar]
- 19.Tandara AA, Kloeters O, Mustoe TA. Reduction of Scarring by Silicone in the Rabbit Ear. Model Wound Repair Regen. 2005;13:A4. doi: 10.1111/j.1524-475X.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 20.Chang CC, Kuo YF, Chiu MS, et al. Hydration, not silicone, modulates the effects of keratinocytes on fibroblasts. J Surg Research. 1995;59:705–711. doi: 10.1006/jsre.1995.1227. [DOI] [PubMed] [Google Scholar]
- 21.Suetake T, Sasai S, Zhen YX, Ohi T, Tagami H. Functional analyses of the stratum corneum in scars. Sequential studies after injury and comparison among keloids, hypertrophic scars, and atrophic scars. Arch Dermatol. 1996;132:1453–8. [PubMed] [Google Scholar]
- 22.Mustoe TA, Weber DA, Krukowski M. Enhanced healing of cutaneous wounds in rats using beads with positively charged surfaces. Plast Reconstr Surg. 1992;89:891–7. [PubMed] [Google Scholar]
- 23.Kloeters O, Jia SX, Roy N, Schultz GS, Leinfellner G, Mustoe TA. Alteration of Smad3 signaling in ischemic rabbit dermal ulcer wounds. Wound Repair Regen. 2007;15:341–9. doi: 10.1111/j.1524-475X.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 24.Madison KC. Barrier function of the skin: “la raison d’être” of the epidermis. J Invest Dermatol. 2003;121:231–41. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 25.Denning MF. Epidermal keratinocytes: regulation of multiple cell phenotypes by multiple protein kinase C isoforms. Int J Biochem Cell Biol. 2004;36:1141–6. doi: 10.1016/j.biocel.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Tandara AA, Kloeters O, Mogford JE, Mustoe TA. Hydrated keratinocytes reduce collagen-synthesis by fibroblasts via paracrine mechanisms. Wound Repair Regen. 2007;15:497–504. doi: 10.1111/j.1524-475X.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 27.Uchi H, Terao H, Koga T, Furue M. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(Suppl 1):S29–38. doi: 10.1016/s0923-1811(00)00138-9. [DOI] [PubMed] [Google Scholar]
- 28.Kirnbauer R, Kock A, Schwarz T, Urbanski A, Krutmann J, Borth W, Damm D, Shipley G, Ansel JC, Luger TA. IFN-beta 2, B cell differentiation factor 2, or hybridoma growth factor (IL-6) is expressed and released by human epidermal cells and epidermoid carcinoma cell lines. J Immunol. 1989;142:1922–8. [PubMed] [Google Scholar]
- 29.Griffiths CE, Barker JN, Kunkel S, Nickoloff BJ. Modulation of leucocyte adhesion molecules, a T-cell chemotaxin (IL-8) and a regulatory cytokine (TNF-alpha) in allergic contact dermatitis (rhus dermatitis) Br J Dermatol. 1991;124:519–26. doi: 10.1111/j.1365-2133.1991.tb04943.x. [DOI] [PubMed] [Google Scholar]
- 30.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung HF, Leonard EJ, Oppenheim JJ. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883–93. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kupper TS, Lee F, Birchall N, Clark S, Dower S. Interleukin 1 binds to specific receptors on human keratinocytes and induces granulocyte macrophage colony-stimulating factor mRNA and protein. A potential autocrine role for interleukin 1 in epidermis. J Clin Invest. 1988;82:1787–92. doi: 10.1172/JCI113792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–7. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood LC, Feingold KR, Sequeira-Martin SM, Elias PM, Grunfeld C. Barrier function coordinately regulates epidermal IL-1 and IL-1 receptor antagonist mRNA levels. Exp Dermatol. 1994;3:56–60. doi: 10.1111/j.1600-0625.1994.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 34.Tsai JC, Feingold KR, Crumrine D, Wood LC, Grunfeld C, Elias PM. Permeability barrier disruption alters the localization and expression of TNF alpha/protein in the epidermis. Arch Dermatol Res. 1994;286:242–8. doi: 10.1007/BF00387595. [DOI] [PubMed] [Google Scholar]
- 35.Ashida Y, Ogo M, Denda M. Epidermal interleukin-1 alpha generation is amplified at low humidity: implications for the pathogenesis of inflammatory dermatoses. Br J Dermatol. 2001;144:238–43. doi: 10.1046/j.1365-2133.2001.04007.x. [DOI] [PubMed] [Google Scholar]
- 36.Wood LC, Elias PM, Sequeira-Martin SM, Grunfeld C, Feingold KR. Occlusion lowers cytokine mRNA levels in essential fatty acid-deficient and normal mouse epidermis, but not after acute barrier disruption. J Invest Dermatol. 1994;103:834–8. doi: 10.1111/1523-1747.ep12413597. [DOI] [PubMed] [Google Scholar]