Summary
Transforming growth factor-β (TGF-β) and Forkhead box p3-expressing T-regulatory (Treg) cells are critical in maintaining self-tolerance and immune homeostasis. The immune suppressive functions of TGF-β and Treg cells are widely acknowledged and extensively studied. Nonetheless, recent studies revealed the positive roles for TGF-β and Treg cells in shaping the immune system and the inflammatory responses. This review discusses our and other’s efforts in understanding the negative (Yin) as well as the positive (Yang) roles for TGF-β and Treg cells in immune regulation.
Keywords: TGF-β, Treg, Foxp3, Th2, IL-17
Introduction
Complex immune strategies have evolved in mammals to defend against foreign pathogens to maintain health. The two fundamental arms of the immune response, adaptive and innate immunity, together form a defensive front against these pathogens. Innate immunity, mediated by cells such as macrophages, is less specific and works by recognizing classes of micro-organisms. T and B cells are the major cells carrying out the adaptive immune responses through their highly antigen-specific surface receptors. By using quasi-random recombinational mechanisms, thymic derived T cells can potentially generate infinite numbers of specificities, being reactive to foreign as well as self-antigens. Self-reactive T cells can cause autoimmune diseases in the host if they are not tightly controlled. Multiple processes therefore are in place to suppress the generation or the function of self-reactive T cells. Self-reactive T cells can be deleted in the thymus as well as in the periphery. Such elimination processes are incomplete, however, resulting in small populations of mostly low-affinity self-reactive T cells in the periphery that can potentially initiate an autoimmune response. Fortunately, active immune suppressive mechanisms exist to suppress the function of these auto-reactive T cells. Dysregulated immune suppression often results in immune disorders. Autoimmunity and inflammatory diseases can be caused by decreased immune-suppression, while cancers are often associated with increased immune-suppression. Great progress has been made in understanding the cellular and molecular components of immune suppression. Active immune suppression is mediated mostly through cytokines or through specialized cells. The pleiotropic cytokine, transforming growth factor-β (TGF-β), and the immunosuppressive cell, previously called suppressor cells (1) and now usually termed T regulatory (Treg) cells, play critical roles in suppressing the immune response. For years, we have investigated the immune suppressive functions of TGF-β and Tregs under normal and immune-pathological conditions. Recently, our and other’s studies suggest that, in a cell type and environment-dependent fashion, TGF-β and Tregs might also positively regulate immune responses. Thus, while TGF-β and Tregs are dominantly viewed as critical mediators for immune suppression, they in fact exert both ‘Yin’ (negative) and ‘Yang’ (positive) effects on the immune system. Here, we will review the evolution of our views on the functions of TGF-β and Tregs in the immune regulation.
TGF-β and its signaling
Consisting of a family of pleiotropic cytokines, TGF-β regulates multifaceted cellular functions including proliferation, differentiation, migration, and survival (2). Bone morphogenetic proteins (BMPs), activins, and growth differentiation factors (GDFs) also belong to the TGF-β family (3).
TGF-β1, -β2, and -β3 are the three isoforms that have been identified in mammals. While sharing similar functions, these isoforms are differentially expressed in a spatially and temporally dependent manner (4). In the immune system, TGF-β1 is the isoform predominantly expressed. TGF-β is synthesized in an inactive form, pre-pro-TGF-β precursor. Additional stimuli are required to liberate active TGF-β, enabling it to exert its function by binding to its receptor (6–10). Through proteolytic processing, active TGF-β is produced through its dissociation from latency associated protein (LAP) or latent-TGF-β-binding protein (LTBP), whose association keeps TGF-β inactive (5). The active form of TGF-β can function in either a cell surface-bound form or a soluble form (11, 12).
Activated TGF-β binds to a heterodimeric receptor complex consisting of a type I and II trans-membrane serine/threonine kinase subunits. Although more than 35 TGF family members have been identified, only five type I [activin-like receptor kinase (ALK) family] and seven type II receptors have been reported (3). Binding of the TGF-β dimer with the tetrameric ALK5 and TGFβRII receptor complex initiates signaling cascades (13). Much of our knowledge of TGF-β downstream signaling is from the studies performed in non-lymphoid cells. TGF-β binding tethers the type II receptor with ALK5 and triggers the phosphorylation and activation of ALK5 by the type II receptor. Intracellular signal transduction of TGF-β is mediated to a great degree via Smad proteins (13, 14). The eight vertebrate Smads identified thus far are grouped into three categories: five receptor-associated Smads (R-Smad1, 2, 3, 5, 8), one common Smad (Co-Smad4), and two inhibitory Smads (I-Smad6, 7). Upon TGF-β stimulation, activated ALK5 phosphorylates R-Smad-2 and -3. Phosphorylated R-Smads associate with Co-Smad4 and translocate into the nucleus to bind to DNA containing a Smad binding element (SBE) (15–18). As an inhibitory Smad protein, I-Smad7 suppresses TGF-β signaling (19). At least two mechanisms are used by I-Smad7 to inhibit TGF-β signaling: one is by competing with R-Smads for the binding to ALK5, and the other is by recruiting ubiquitin ligase complexes to degrade ALK5 via the proteasome (20, 21). In addition, Smad-independent TGF-β signaling pathways have been reported (22, 23). Through mechanisms yet to be determined, rapid activation of Ras-Erk (extracellular signal-regulated kinase), TAK-MKK4-JNK (TGF-β-activated kinase-mitogen-activated protein kinase kinase 4-c-Jun N-terminal kinase), TAK-MKK3/6-p38, Rho-Rac-cdc42 MAPK, and PI3K (phosphatidylinositol 3-kinase)-Akt pathways occurs when cells are treated with TGF-β (24). MAPKs also coordinate with Smads to modulate TGF-β responses (25–27). Moreover, TGF-β receptors activate TRIP-1 (TGF-β receptor-interacting protein-1) and PP2A (phosphatase 2A) through direct protein binding to regulate translation initiation (28–30). Therefore, TGF-β exerts its regulation of target cell function via many different mechanisms. T-cell-specific target genes of TGF-β are largely unknown, however, the expression of genes important for T-cell differentiation and function, such as GATA3, T-bet, STAT4 (signal transducer and activator of transcription 4), IFN-γ (interferon-γ), and granzyme-B, are suppressed by TGF-β (31–35).
Tregs
The concept of immune suppression was proposed in the early 1970s by Gershon et al. (1, 36, 37). Being unable to identify the cell types performing immune suppression impeded the validation of this concept and hampered development in this research area. It was not until a study performed by Sakaguchi et al. (38) in 1995 that a subset of T cells with markedly increased expression of CD25 was discovered as suppressor T cells and later referred to as Tregs (38). In recent years, substantial progress has been made in identifying different types of Treg cells as well as in understanding how these cells are generated and function. Based on cell surface markers or cytokine secretion profiles, Tregs can be generally grouped into two categories: naturally occurring Tregs (nTregs) and acquired Treg (aTregs).
nTregs
A subset of CD4+ T cells that develops in the thymus and constitutively expresses cell surface IL-2 receptor α chain (CD25) is termed nTregs. CD4+CD25+ nTregs comprise approximately 10% of peripheral CD4+ T cells in mice and humans. nTregs are critical for maintaining self-tolerance, as disruption of thymic development or peripheral maintenance of these cells invariably results in the development of autoimmunity. A cluster of cell surface molecules besides CD25, such as cytotoxic T-lymphocyte antigen-4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor family related gene (GITR), and lymphocyte activation antigen-3 (LAG-3), have also been used to differentiate nTregs from conventional T cells (39). TGF-β is expressed in nTregs at high levels in a cell surface-bound form (12, 40). nTreg cells suppress immune responses in an antigen-independent fashion in vitro and in vivo (39, 41–43). Forkhead box protein 3 (Foxp3), an X-linked transcription factor belonging to forkhead family, is specifically and highly expressed in nTreg. Thus, Foxp3 is currently used as the most reliable molecular marker for nTregs.
aTregs
Conventional T cells are able to gain immune suppressive activities and become aTregs. Tr1 (T regulatory1) and Th3 (T-helper 3) cells are two reported types of aTregs. Tr1 cells are often found within the intestinal mucosa and suppress immune reactions towards a variety of cognate antigens (44). There is no particular surface marker associated with Tr1 cells. However, these cells produce increased levels of IL-10 and TGF-β (45). Tr1 cells do not express Foxp3 (46), suggesting that it is a subset of Tregs distinct from nTregs.
Th3 is another aTreg subset induced primarily from naive CD4+ T cells after ingestion of a foreign antigen via the oral route, thereby eliciting oral tolerance (47, 48). While no particular surface marker is associated with these cells, Foxp3 is expressed in Th3 cells (49). In addition, TGF-β is produced at elevated levels by Th3 cells (48). Whether Th3 cells form a distinct aTreg subset or are activated nTregs remains unknown.
TGF-β inhibits immune responses
Identified as a growth factor for transformed tumor cells, TGF-β in fact inhibits the proliferation of non-transformed cells, such as epithelial cells and fibroblasts. The inhibitory effect of TGF-β on the immune system was first described in 1986, where TGF-β was found to inhibit the proliferation of human B and T cells (50, 51). However, the definitive genetic evidence to support the critical roles of TGF-β in suppressing immune responses in vivo were not presented until the analysis of TGF-β-deficient mice (52, 53). Since then, using genetic modified mouse models, our and others’ laboratories have carried out extensive studies to evaluate the immune functions of the components of TGF-β signaling networks, including their receptors and intracellular signaling molecules, and furthered the understanding of the suppressive role for TGF-β in the immune system. TGF-β regulates the adaptive immunity components, such as T cells, as well as the innate immunity components, such as natural killer (NK) cells (54–60). TGF-β suppresses immune responses through two means: inhibiting the function of inflammatory cells and promoting the function of Treg cells (Fig. 1).
Fig. 1. TGF-β inhibits and promotes immune responses by modulating the functions of immune cells.
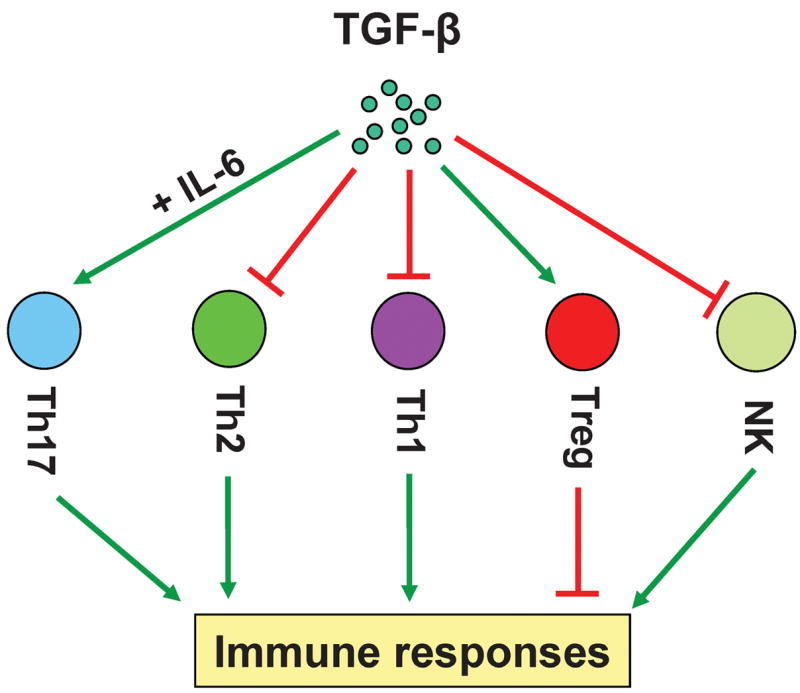
To inhibit immune responses, TGF-β suppresses the functions of Th1 and Th2 CD4+ effector cells and NK cells, and promotes the generation of Treg cells. To promote immune responses, TGF-β induces the generation of Th17 cells in combination with IL-6.
TGF-β inhibits the function of inflammatory cells
Multiple types of immune cells can be regulated by TGF-β. The role of TGF-β in controlling T-cell functions and immune responses has been studied extensively (61). The anti-proliferative function of TGF-β on T cells was first documented by studies performed in vitro using activated human T cells (51). TGF-β can suppress T -cell proliferation by inhibiting the production of interleukin-2 (IL-2), a lymphokine known to potently activate T cells, NK cells, and other of the immune system cells. Addition of exogenous IL-2 partially relieved TGF-β-mediated suppression (51). TGF-β suppresses IL-2 production in T cells potentially through direct inhibition of IL-2 promoter activity. A cis-acting enhancer DNA element was identified to be critical in suppressing IL-2 production by TGF-β (62). In addition, R-Smad3 is critical in mediating TGF-β-inhibited IL-2 production, as TGF-β failed to suppress IL-2 production in murine T cells that lack this gene (63). Moreover, the Smad-binding element has been located upstream of the human IL-2 promoter, which is important for Smad-mediated transcriptional suppression of IL-2 (64). Because addition of exogenous IL-2 did not fully reconstitute T-cell proliferation (51), TGF-β inhibits T-cell proliferation also through IL-2-independent mechanisms that warrant further investigation.
TGF-β regulates cell proliferation through controlling the expression of cell cycle regulators. Upon TGF-β treatment, cyclin-dependent kinase inhibitors (CKIs), such as p15, p21, and p27, are upregulated, while cell cycle promoting factors, such as c-myc, cyclin D2, CDK2, and cyclin E, are downregulated (65–70). The functional significance of these expression changes in TGF-β-induced inhibition and how TGF-β modulates these genes in T cells remain to be elucidated. Although TGF-β inhibits naive T-cell proliferation, it has minimal effects on activated T cells, which may be due to reduced TGF-β receptor II expression on these cells (71). It nevertheless suggests that TGF-β functions in a cell type-dependent fashion.
To perform immune function, naive CD4+ T cells differentiate into three major types of effector T cells following activation (72). Based on their cytokine production, CD4+ effector T cells, also named Th cells, can be profiled as Th1, Th2, and Th17 cells (73, 74). Th1 cells produce IFN-γ and lymphotoxin (LT), Th2 cells secrete IL-4, IL-13, and IL-5, and Th17 cells express IL-17 and IL-22 (75). While TGF-β partially suppresses T-cell proliferation, we have found that TGF-β potently inhibits effector functions of activated T cells and thus their differentiation into Th1 or Th2 effector cells under tissue culture conditions (76). Further studies revealed that TGF-β regulates effector T-cell function through multiple mechanisms. Distinct sets of transcription factors are preferentially expressed in and are important for Th cell differentiation. These include T-bet and Stat4 for Th1 differentiation and Gata-3 and Stat6 for Th2 differentiation (72). While the detailed mechanism remains unknown, our and others’ studies showed that T-bet and Gata-3 expression is inhibited by TGF-β (32, 33, 77, 78), possibly, in the latter case, through a mechanism via blocking Itk kinase activity and calcium influx (31). Interestingly, effector cytokine production by fully differentiated Th2 cells is unaffected by TGF-β, while Th1 cells remain susceptible to TGF-βksuppression (79). Therefore, TGF-β exerts most of its inhibitory effects on the establishment of effector cell functions. Our recent study demonstrated that the Th1 polarizing condition promotes CD122 (IL-2 receptor β chain) expression through T-bet, thereby enhancing the clonal expansion and survival of Th1 cells (80). Addition of TGF-β suppressed CD122 upregulation under Th1-skewing conditions. Therefore, TGF-β also limits Th1 effector cell numbers through inhibiting the upregulation of CD122. It was also noted that TGF-β inhibited T-cell differentiation independent of T-cell proliferation (81). Thus, TGF-β potentially regulates T-cell proliferation and effector functions through discrete mechanisms, with the greatest effects on suppressing their differentiation. While TGF-β inhibits the production of pro-inflammatory cytokines, it promotes T-cell production of IL-10, an anti-inflammatory cytokine, likely through direct activation of the IL-10 promoter via Co-Smad4 (82).
Besides regulating CD4+ T cells, TGF-β controls CD8+ T-cell proliferation and effector functions. The expression of effector molecules by CD8+ T cells, such as IFN-γ and perforin, is inhibited by TGF-β (83–86). Recent studies showed that TGF-β is important for Treg-induced inhibition of the exocytosis of granules and cytolytic function of CD8+ T cells (87).
The critical role for TGF-β in regulating immune suppression under physiological conditions has been demonstrated by studying mouse models where TGF-β signaling was altered. TGF-β1−/− mice develop a multifocal inflammatory disease associated with increased inflammatory cytokine production (52, 53, 88). This phenotype is predominantly mediated through T cells, as depletion of CD4+ T cells or crossing TGF-β1−/− mice onto an major histocompatibility complex (MHC) class II null background prevented this inflammation (89). However, from these studies, it was not clear whether T cells are direct targets of TGF-β, since TGF-β1 acts on multiple cell types. Indeed, TGF-β plays important role in suppressing the function of innate immunity. By expressing a transgene encoding a dominant negative form of TGFβRII under the control of CD11c promoter (CD11cTGFDNR), we blocked TGF-β signaling in NK cells and dendritic cells (DCs) (57). Blockade of TGF-β signaling in NK cells caused the accumulation of a large pool of NK cells secreting copious amounts of IFN-γ. Such increased IFN-γ induced Th1 differentiation of CD4+ T cells in these mice and resulted in their resistance to Leishmania infection. However, blockade of TGF-β signaling in DCs from these mice did not affect DC homeostasis or IL-12 production, suggesting TGF-β differentially affects NK cells and DCs. In addition, TGFβRII deletion facilitated generation of a highly pathogenic T-cell subset exhibiting hallmarks of NK cells. These TGFβRII-deficient NK-like T cells highly elevated IFN-γ expression (90). Further studies are warranted to elucidate TGF-β function in the generation and function of innate components and the underlying mechanisms.
To further investigate the intrinsic function of TGF-β in T cells, several groups including ours have used transgenic approaches to block TGF-β signaling in T cells by expressing dominant negative TGF-β receptors (56, 58). In this effort, we generated mice expressing a dominant-negative form of TGFβRII from the CD4 promoter (CD4-dnTβRII), whose CD4+ and CD8+ T cells are refractory to TGF-β signaling. These mice developed an autoimmune inflammatory phenotype associated with uncontrolled CD4+ T-cell differentiation into Th1 effector cells (56). Without TGF-β signaling, both CD4+ and CD8+ T cells from CD4-dnTβRII mice displayed increased effector functions, which led to drastically increased immune rejection of B16 melanoma and EL4 lymphoma in vivo (91). Nonetheless, CD4-dnTβRII mice displayed much less immune pathology than TGF-β1−/− mice. This is possibly due to insufficient expression of the transgenes or incomplete inhibition of TGF-β signaling. Subsequent studies demonstrated that deletion of TGF-βRII in the bone marrow cells results in an immune pathology similar to that found in TGF-β1−/− mice (92). However, the contribution of T cells to such a phenotype remained undetermined. Recently, more definitive studies from our laboratory have uncovered the essential role of TGF-β signaling in controlling the development, homeostasis, and tolerance of T cells through both Treg-dependent and independent mechanisms (80). Mice with T cell specific TGF-βRII deletion (4cre-RII/RII) developed a progressive wasting disease and succumbed to death by five weeks of age. In these mice, great numbers of leukocytes infiltrated into multiple non-lymphoid organs, auto-antibody levels were elevated, and peripheral T cells displayed activated phenotypes. In addition, deficiency of TGFβRII caused mice to develop fatal autoimmune diseases similar to the TGF-β1−/− mice. This phenotype can be attributed to hyper-activation and exaggerated Th1 effector functions of immune cells, especially T cells (80, 92). These findings are in accordance with the results from Rudensky’s laboratory (90), where a different strain of T cell specific TGFβRII knockout mice were used.
T-bet, encoded by the Tbx21, gene is a transcription factor that is critical for IFN-γ production and Th1 differentiation of CD4+ T cells (93). We attempted to alleviate/rescue the Th1-type immune disorder observed in 4cre-RII/RII mice by creating 4cre-RII/RII mice deficient in the Tbx21 gene. Much to our surprise, CD4+ T cells from 4cre-RII/RII-Tbx21−/− mice remained activated but with much less IFN-γ production. Therefore, TGF-β suppresses T-cell activation through a T-bet-independent mechanism, while T-bet remains essential for IFN-γ expression. In addition, CD4+ T-cell numbers were found to be decreased in these mice, likely due to decreased expression of CD122, a receptor that is important for both IL-2 and IL-15 signaling. Further analysis revealed that Th1-skewing conditions preferentially upregulated CD122 on CD4+ T cells in vitro in a T-bet dependent manner (80). More interestingly, addition of TGF-β inhibited the upregulation of CD122 on CD4+ T cells, suggesting that physiologically TGF-β limits CD4+ effector T-cell numbers through controlling IL-2- and IL-15-driven T-cell expansion. As TGF-β potently inhibits T-bet expression in Th1 cells (32), it remains to be addressed whether TGF-β inhibits CD122 expression through T-bet-dependent and/or -independent mechanisms.
The T-cell activation phenotype observed in 4cre-RII/RII mice might be attributed to decreased Treg numbers in the periphery (80). However, using a transfer model, we and others found that the existence of substantial numbers of wildtype Treg cells, which apparently cannot prevent the spontaneous activation of T cells lacking TGF-βRII (80, 94). It thus suggests that wildtype Tregs are not able to suppress T cells that cannot respond to TGF-β. This conclusion is consistent with previous reports (12, 95, 96). It does not however establish a Treg-independent role of TGF-β in controlling T-cell activation, since Treg-mediated suppression might be through a TGF-β dependent mechanism. Compelling evidence to support that TGF-β controls T-cell activation through a Treg-independent mechanism came from the analysis of 4cre-RII/RII mice with the OTII TCR transgene on a Rag1−/− background, where endogenous Foxp3+ Treg cells failed to develop. Substantial portions of T cells from these mice displayed an activated phenotype, while only a small percentage of these cells produced effector cytokines, which could be due to lack of stimulation from cognate antigens. Treg-independent TGF-β-dependent regulation of immune functions is an area that is poorly understood, and future studies are needed to pursue the mechanisms involved.
Leukocytes and stromal cells are both able to generate TGF-β; which source of TGF-β is important and whether TGF-β regulates T-cell function as an autocrine, a paracrine, or an endocrine cytokine are interesting questions. We generated T-cell-specific TGF-β1 knockout mice and addressed contribution of T-cell-produced TGF-β1. These mice developed lethal imunopathology in multiple organs, associated with enhanced T-cell proliferation, activation, and differentiation. In a transfer model, TGF-β1 produced by Treg cells was shown to be required for Th1 cell differentiation and the onset of inflammatory bowel disease, while TGF-β1 generated by conventional T cells also contributed to the inhibitory effects (97). These findings demonstrated that to regulate T cells, TGF-β1 functions through an autocrine or a paracrine but not an endocrine mechanism. Whether such a mechanism could be applied to other leukocytes or non-lymphoid cells and what mechanisms are involved in achieving localized effects of TGF-β are important questions to be addressed in future studies.
TGF-β promotes the generation and function of Treg cells
Another mechanism by which TGF-β inhibits immune responses is through promoting the generation of Treg cells by inducing Foxp3 expression. Early studies demonstrated that TGF-β was necessary and sufficient for human CD8+ T cells to acquire suppressive activities (98). In addition, regulatory activity was induced in human naïve (CD45RA+RO−) CD4+ T cells by TGF-β following stimulation (99). TGF-β was subsequently demonstrated to induce the expression of Foxp3 in CD4+CD25− human T cells (100), and in activated murine CD4+ and CD8+ T cells. In the presence of TGF-β1, Staphylococcus enterotoxin B (SEB)-activated CD8+ T cells inhibited the proliferation and effector functions of CD4+ and CD8+ T cells. This inhibition was accompanied by elevated levels of IL-10 and TGF-β1 (101). TGF-β was later demonstrated to convert mouse CD4+CD25− into CD4+CD25+ T cells with elevated Foxp3 expression (100, 102).
Studies demonstrated that TGF-β is able to convert CD4+CD25− non-Treg cells into CD4+CD25+ Treg cells, and this conversion was accompanied with increased Foxp3 expression (100, 102). However, a substantial portion of Foxp3+ Treg cells are negative for CD25 (103, 104). In these studies, it was not distinguished whether such conversion is due to preferential expansion/survival of the existing Foxp3+CD25− population or due to de novo Foxp3 expression in the Foxp3−CD25− population. Unequivocal evidence for TGF-β conversion of Foxp3− cells into Foxp3+ cells came from our study using Foxp3-mRFP knockin mice, where Foxp3-expressing cells are marked by mRFP expression (104). TGF-β induced de novo Foxp3 expression in Foxp3− CD4+ T cells. Furthermore, only Foxp3+CD4+ cells but not Foxp3−CD4+ counterparts possessed regulatory activities (104).
Although TGF-β promotes Treg generation in vitro, it is controversial whether TGF-β is involved in the generation or maintenance of Foxp3-expressing Tregs under physiological conditions. Transient expression of TGF-β by a transgene specifically expressed in islets promotes the generation of CD4+CD25+ Tregs in situ with high Foxp3 expression in diabetes-predisposed NOD (nonobese diabetic) mice (105). This observation correlated with the suppression of diabetes. In addition, induced Treg cells suppressed the onset of diabetes following adoptive transfer of these cells into NOD mice (105). These findings demonstrated that TGF-β is sufficient to promote the generation of Tregs under physiological conditions. Conflicting results have been presented with regards to whether TGF-β is essential for the development and maintenance of nTregs. In one study, the CD4+CD25+ Treg population was shown to be decreased in adult mice transgenic for a dominant negative form of TGFβRII (106) under the control of the CD2 promoter (hCD2-ΔkTβRII). After being transferred into mice subjected to DSS (sodium 2,2-dimethyl-2-silapentane-5-sulphonate)-induced colitis, hCD2-ΔkTβRII transgenic CD4+CD25+ T cells proliferated poorly compared with wildtype CD4+CD25+ nTreg cells, thus suggesting that TGF-β signaling was required for the maintenance and expansion of CD4+CD25+ nTregs in vivo (107). However, in another transgenic model where a similar form of TGFDNR (dnTβRII) is expressed under the control of the CD4 promoter (56), CD4+CD25+ nTreg cells in CD4-dnTβRII transgenic mice developed normally. A slightly increased number of nTregs was found in the periphery of these mice compared to their wildtype counterparts (96, authors’ unpublished observations). Peripheral but not thymic nTregs were found to be reduced in 8–10-day-old TGF-β1−/− mice (94), suggesting an essential function for endogenous TGF-β1 in the maintenance of the peripheral population of Tregs. These results contrast those of an earlier study, where no defect of Treg development or maintenance was observed in TGF-β1−/− mice (108). A more recent definitive study demonstrated that Foxp3-expressing nTreg cells that lack TGFβRII developed normally in the thymus but were poorly maintained in the periphery (80). Interestingly, in the same study, TGFβRII-deficient Tregs were found to proliferate faster than the wildtype counterparts in the periphery, despite that lower numbers of TGFβRII-deficient Treg cells were found, suggesting that TGF-β signaling is required to promote the survival of peripheral Tregs (80). The reasons for these discrepancies are unknown but may be related to the different experimental systems and mouse genetic backgrounds used. In addition, because CD25 is also expressed by activated T cells, CD4+CD25+ Tregs identified in these studies may have been contaminated by activated T cells to varying degrees in the earlier studies. With the development of enhanced green fluorescence protein (EGFP)-Foxp3 and Foxp3-mRFP knockin mice and Foxp3 intracellular staining, these potential complications can be circumvented by identifying Treg based on Foxp3 expression (80, 103, 104). A consensus is forming that TGF-β is required for the maintenance of Tregs in the periphery. We investigated whether T-cell-produced TGF-β is required for Treg maintenance by studying mRFP-expressing Treg cells from mice lacking TGF-β1. We found that T-cell-produced TGF-β1 is dispensable for the development and maintenance of Treg cells (97), despite the fact that TGF-β1-deficient Treg cells lost their suppressive activities in a transfer model. Thus self-made TGF-β1 appears to be required for Treg function, while it remains an open question as what source of TGF-β is required for Treg maintenance.
The downstream signaling through which TGF-β mediates the generation of Foxp3-expressing Tregs remains unclear. R-Smad3−/− mice developed by two different groups showed distinct phenotypes in the immune system. While no apparent abnormality was observed when exon1 of the R-Smad3 gene was knocked out in mice (109), impaired mucosal immunity associated with activated T cells was found when the exon8 of the R-Smad3 gene was targeted (60). However, there is no evidence to suggest that the phenotype found in the latter study is due to perturbed Treg compartments. A recent study proposed a cooperative role for TGF-β in upregulating CD25 expression following TCR stimulation, and this function of TGF-β appeared to be mediated through the R-Smad3/Co-Smad4 pathways (110). Thus R-Smad3/Co-Smad4 regulation of the generation and/or maintenance of Tregs may be through cooperation with IL-2 signaling. However, because the Treg population appeared to be normal when Co-Smad4 is deficient in T cells (60, 110, authors’ unpublished observation), it is questionable that Co-Smad4 and R-Smad-dependent TGF-β signaling is critically involved in Treg generation under physiological conditions.
TGF-β also promotes immune responses
TGF-β is prevalently viewed as an immune suppressive cytokine. However, as a pleiotropic cytokine, TGF-β has been found to exhibit immune-promoting properties, which has recently attracted much attention. In early studies, TGF-β was found to enhance the proliferation of mouse CD8+ T cells under certain conditions (111) and to increase TNF-α production by both CD4+ and CD8+ T cells (112). TGF-β accelerates T-cell death in some studies (113, 114), while an anti-apoptotic role for TGF-β has also been documented (115, 116).
TGF-β was recently identified to be important for the induction of IL-17-producing cells under inflammatory conditions (117–120). The Th17 cell is a new class of effector T cells that is gaining increasing attention. Th17 cells produce IL-17 and IL-22, and they are critical for the induction of experimental autoimmune encephalitis (EAE) in mice. Under culture conditions, TGF-β in combination with IL-6 promotes Th17 differentiation. In addition, TGF-β is important for the generation of Th17 cells and the induction of EAE in mice (118, 121). By deleting the TGF-β1 gene specifically in T cells, we found that T-cell-produced TGF-β1 is required for the induction of pathogenic Th17 cells in the intestines as well as in the spinal cord. Myelin oligodendrocyte glycoprotein (MOG)-induced EAE is drastically ameliorated when T cells cannot generate TGF-β1 (97). Considering that TGF-β1 can be generated by conventional T as well as Treg cells, it would be important to discern whether either type of cells is required for the generation of Th17 cells and the induction of EAE in mice.
Lack of TGF-β signaling is often associated with increased proliferation and effector function of immune cells. However, two recent independent studies demonstrated that TGF-β might promote proliferation and survival of immune cells under certain conditions. The numbers of canonical CD1d-dependent NK (NK1.1+) T cells and CD8+ T cells were decreased upon TGF-βRII deletion, suggesting a critical role for TGF-β signaling in their development (80). In the absence of TGF-β signaling, OT-II CD4+ T cells, which only responded to ovalbumin (OVA) peptide from chicken OVA, were prone to apoptosis in the periphery and failed to differentiate into effector cells. This phenotype correlates with reduced expression of CD122 on these cells. This finding suggests that TGF-β signaling is required for the survival of some cells. However, it remains to be addressed as to what is the relationship among TGF-β-promoted T-cell survival, CD122 expression, and Th1 differentiation.
Collectively, these studies highlight the multi-faceted effects of TGF-β on various immune functions and emphasize the importance of cellular and environmental contexts in directing the discrete roles of TGF-β. What triggers and mediates TGF-β to perform such a broad spectrum of functions needs to be elucidated in the years to come.
Tregs are critical in mediating immune suppression
Early studies showing that thymectomy of neonatal mice within three days after birth led to lethal autoimmunity suggested a set of thymus-derived cells are important to maintain self-tolerance in the periphery (122, 123). Upon identification of CD4 and CD25 as markers for Treg cells, Sakaguchi’s group (30) used anti-CD25 antibody to deplete these cells, and such manipulation led to lethal autoimmunity due to the loss of peripheral tolerance in adult mice. In 2003, an X-linked forkhead family transcription factor named Foxp3 was identified to be expressed specifically in nTregs among the lymphocyte populations (41, 124, 125). Spontaneous monogenetic mutation in the Foxp3 gene results in systemic autoimmunity in Scurfy mice and IPEX (X-linked neonatal diabetes mellitus, enteropathy, and endocrinopathy syndrome) patients (126–130). The disease manifested in Scurfy mice was attributed to Treg deficiency. However, T-cell extrinsic elements were reported to contribute to the Scurfy phenotype (131), while counter-evidence was also presented (132). In addition, targeted deletion of Foxp3 gene in mice led to a phenotype reminiscent of Scurfy mice, with undetectable CD4+CD25+ T cells (133–135). Moreover, ectopically overexpressed the Foxp3 gene endowed immune suppressive activities to conventional CD4+ and CD8+ T cells (127, 128, 135). Therefore, Foxp3 controls the development and function of nTreg cells, a type of suppressor T cells that are essential in maintaining self-tolerance and immune homeostasis (Fig. 2).
Fig. 2. Yin-Yang roles for Foxp3-expressing cells in immune regulation.
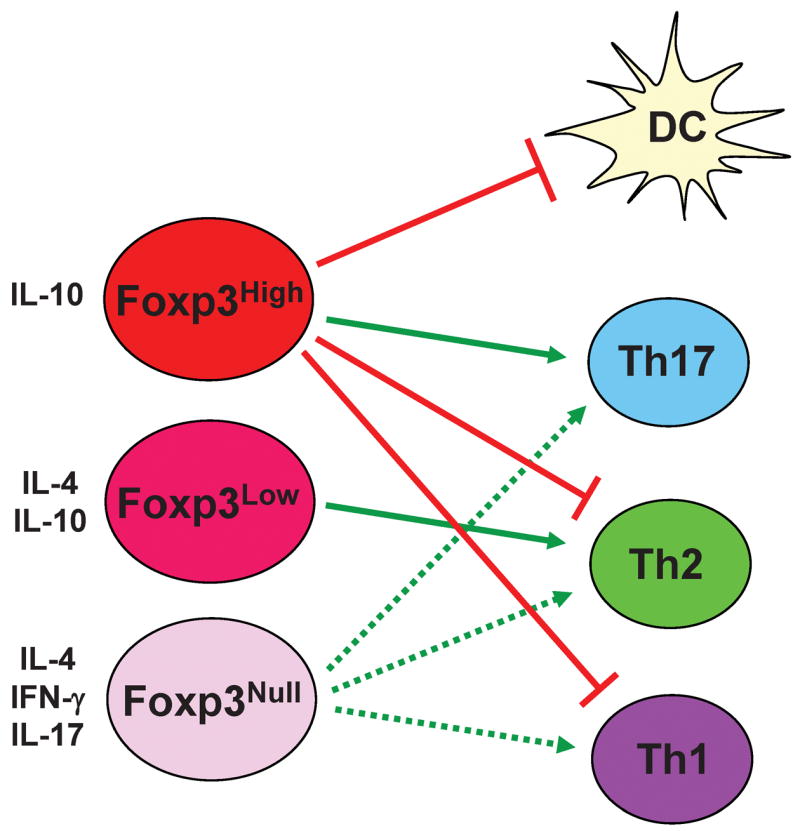
High levels of Foxp3 expression endow T-cell immune suppressive activities to suppress the function of effector T cells and DCs through cell contact-dependent and -independent mechanisms. T cells with high levels of Foxp3 expression promote Th17 cell differentiation, potentially through TGF-β. With decreasing Foxp3 expression, T cells can be converted into different types of effector T cells and direct the differentiation of conventional T cells towards different lineages.
Tregs function through cell contact-dependent and -independent mechanisms
The essential role of Tregs in immune suppression is indisputable. Yet, the mechanisms by which Treg cells carry out their function remain ill-defined. Nevertheless, it is generally agreed that Tregs suppress immune responses through multiple mechanisms, including cell contact-dependent and -independent mechanisms. Several surface molecules preferentially expressed by Treg cells are proposed to be important for their function. For example, CD25, a high affinity IL-2-binding receptor, is highly expressed by the nTreg cells (136). In addition, the peripheral maintenance of nTregs is dependent on IL-2 signaling (137–139), which is also important for the proliferation and survival of activated effector T cells. It is therefore hypothesized that one mechanism of nTreg suppression of conventional T-cell activation is through competition for IL-2 consumption (140, 141). However, studies showing that CD25-deficient nTreg cells possess intact suppressive activity question the validity of this hypothesis (138). CTLA-4, another surface molecule preferentially expressed by nTregs, is important for inhibiting immune activation by competing for costimulatory ligands on T cells (142) as well as inhibiting the function of antigen-presenting cells (143, 144). Thus, it is suggested that CTLA-4 is important for nTreg mediated immune suppression. Genetic evidence argues against the critical roles for CTLA-4 in nTreg function, as the function of nTregs deficient in CTLA-4 appeared to be normal in vitro and in vivo (145). However, antibody-mediated blockage of CTLA-4 abrogated nTreg function (145), but it remains unknown whether this effect is due to a non-specific effect or due to a simultaneous block of CTLA-4 and its potential homolog with redundant functions by the antibodies.
Recent studies have revreveled important roles of cytokines in nTreg function. TGF-β appears to be critical in mediating nTreg function, as T cells from CD4-dnTβRII mice that are unresponsive to TGF-β are refractory to nTreg-mediated suppression in vitro and in vivo (12, 95, 96). Despite the fact that TGF-β mRNA is not elevated in nTreg cells, it is suggested that the membrane-bound form of TGF-β is increased in nTreg cells and is important for their function (12, 40). IL-10 is another immune suppressive cytokine preferentially expressed in Tregs (146, 147), and IL-10 is important in mediating the functions of these cells (147, 148). Besides suppressing effector T-cell function directly, a recent study suggests that nTregs dampen immune responses through regulating an innate component, such as DC. nTregs can potentially induce tolerogenic DCs through CTLA-4 engagement-induced tryptophan catabolism (149). In addition, it appears that nTregs destabilize the interaction between antigenic DCs with conventional T cells to prevent the activation of T cells (150). It is clear now that multiple mechanisms are involved in Treg-mediated immune suppression. Cell contact-dependent and -independent mechanisms critical for controlling Treg function remain to be identified.
Treg function is controlled by Foxp3
As a single molecule that controls the development and function of Tregs, Foxp3 has been under close scrutiny since its discovery. We and others have developed knockin mouse models to track Foxp3-expressing cells with fluorescent proteins (103, 104). Foxp3 can be expressed in thymic derived Treg cells; its expression can also be induced in conventional T cells in vitro by TGF-β, regardless of their proliferation status (104). In addition, Foxp3 expression can be induced in vivo in conventional T cells under sub-optimal stimulation (151). More importantly, in these studies, Foxp3 expression has always been associated with the immune suppressive function. Thus, it is generally thought that Foxp3 serves as an on-and-off switch to positively regulate Treg function in a binary fashion. However, a transient increase of Foxp3 expression in human CD4+ T cells did not result in suppressive function. In addition, in many cases of human IPEX patients, functional Foxp3 protein is made but at much reduced levels. Moreover, emerging evidence associates decreased Foxp3 expression in Treg cells with various human autoimmune disorders, such as graft-versus-host disease (152), autoimmune myasthenia gravis (153), and multiple sclerosis (154). We have also found that intra-islet Treg cells expressed lower levels of Foxp3 than Treg cells from other peripheral lymphoid organs in diabetic NOD mice, while the frequencies of Foxp3-expressing Treg cells among the different compartments were comparable. Thus, we hypothesized that Foxp3 controls Treg function in a dose-dependent, non-binary fashion, and decreased Foxp3 expression could lead to impaired Treg function and be causal for immune disorders.
Support for our hypothesis came from the generation and analysis of a knockin mouse model in our laboratory. In these mice, a gene cassette co-expressing luciferase and EGFP, whose translation is under the control of two tandem internal ribosomal entry sites (IRES), was inserted into the 3′-untranslated region (UTR) of the endogenous Foxp3 locus of C57BL/6 mice to generate Foxp3-IRES-Luciferase-IRES-eGFP (FILIG) allele. Our initial intention was to co-mark Foxp3-expressing cells with GFP and luciferase, facilitating the isolation and in vivo tracking of these cells. Nevertheless, the resulting animal expressed decreased levels of Foxp3. The exact mechanism on how the attenuated Foxp3 expression is achieved is not entirely clear. It is likely that the four AU-rich elements (AREs) located in the luciferase cDNA that was inserted into 3′ UTR region of endogenous Foxp3 gene destabilized Foxp3 mRNA resulting in the lower expression, because AREs localized in the 3′ UTR are known to destabilize mRNA. Such serendipitously generated mice allowed us to investigate the effects of decreased Foxp3 expression on the physiology of Foxp3-expressing T cells.
While heterozygous FILIG female mice (FILIG/+) were fertile and phenotypically normal, hemizygous FILIG male mice (FILIG/Y) were infertile and runted compared to age-matched wildtype mice. Over 50% of FILIG/Y mice developed scaly skin, and nearly all of them developed eyelid defects resembling blepharitis, a Th2 disorder, around four weeks of age. By three months of age, all the FILIG/Y mice succumbed to an aggressive lymphoproliferative autoimmune syndrome, manifested by extremely enlarged spleens and peripheral lymph nodes, infiltration of leukocytes into non-lymphoid organs, drastically increased levels of auto-antibodies in the serum, and activated peripheral CD4+ and CD8+ T cells. Overall, FILIG/Y mice displayed phenotypes reminiscent of Scurfy mice (155) and T-cell-specific Foxp3 knockout mice (135). The transcription of the IRES-Luciferase-IRES-eGFP gene cassette was normal, as luciferase expression was detected in the lymphoid as well as the non-lymphoid organs of sick FILIG/Y mice, and GFP expression was only detected in CD4+ T cells but not other cell types. By intracellular Foxp3 staining, Foxp3 was found to be expressed in GFP+ FILIG T cells but at a 5–10 fold lower level. Compared with wildtype Treg cells, the surface expression of ‘signature genes’ for Treg cells, such as CD25, CTLA-4, and GITR (38, 156, 157), were decreased in GFP+ CD4+ T cells. mRNA levels of Foxp3, Cd25, Ctla4, and Gitr were also decreased, suggesting that the downregulation of these genes was at the mRNA level. These results demonstrated that decreased Foxp3 expression altered the properties of Treg cells and resulted in an aggressive lymphoproliferative autoimmune syndrome in hemizygous male mice. Thus, Foxp3 programs the gene expression of Tregs in a tunable and dose-dependent fashion (158).
Further investigation revealed interesting phenotypic changes in the functions of Treg cells that express decreased Foxp3. Foxp3 is required for the development and maintenance of Treg cells (135). However, decreased Foxp3 expression in FILIG T cells did not lead to defective thymic development of Treg cells. This result agrees with those from a separate study, where GFP cDNA was inserted into the endogenous Foxp3 coding region to mark those T cells with Foxp3 promoter activity but that failed to produce functional Foxp3 protein (159). Substantial numbers of GFP-expressing thymocytes were also detected in these mice (159). By adoptive transfer assays, we found that attenuated Foxp3 expression did not result in intrinsic defects in the homeostatic expansion/maintenance of Treg cells in the periphery. However, in the presence of Treg cells with normal levels of Foxp3 expression, Foxp3 low-expressing cells poorly repopulated the periphery. Decreased CD25 expression on GFP+ FILIG CD4+ T cells compared to wildtype Treg cells could account for this phenomenon, since Treg maintenance is dependent on IL-2 signaling (137–139). Extra-thymic generation of Foxp3-expressing T cells can be promoted in vitro by TGF-β (102, 104, 121). TGF-β induced de novo Foxp3 expression in GFP+ FILIG CD4+ T cells to a similar extent as in wildtype Treg cells. Therefore, these results demonstrated that decreased Foxp3 expression did not affect the development, homeostatic expansion/maintenance, or TGF-β-driven de novo generation of Foxp3-expressing T cells (158). These findings present a possibility that defective and even ablated Foxp3 expression might not result in the total elimination of the ‘Treg lineage’. Indeed, Foxp3-null mice generated Treg lineage cells in the thymus and can be maintained in the periphery (159). Thus, in Scurfy mice and in IPEX human patients, Foxp3-expressing T-cell subsets are likely to be generated in the thymus and maintained in the periphery. Further studies are warranted to identify and characterize these Treg lineage cells lacking functional Foxp3.
In vitro, hypoproliferative (‘anergic’) and immunosuppressive activities are two defining properties for Foxp3-expressing Treg cells that are thought to go hand-in-hand (39). Intriguingly, upon TCR stimulation in vitro, while GFP+ FILIG cells remained anergic, their immunosuppressive activities were greatly impaired. Thus, anergy and immunosuppression are two separable properties of Treg cells that are affected differentially by the expression levels of Foxp3 (158). In addition, using a transfer approach, we demonstrated that the intrinsic immunosuppressive activities of Foxp3+ FILIG CD4+ T cells were also abolished in vivo, although adoptively transferred GFP+ FILIG cells infiltrated efficiently into lymphoid as well as non-lymphoid organs. Thus, the suppressive function of Tregs requires high levels of Foxp3 (158). Interestingly, Foxp3-deficient Treg lineage cells remain anergic but without immunosuppressive function (159). Therefore, unlike what has been recognized, Foxp3 may not be the ‘grand-master’ of Treg development, as its expression is only required for stabilizing ‘regulatory function’ of already differentiated Treg lineage cells. The molecular master-switch controlling the commitment of Treg lineage remains to be discovered. In addition, despite the fact that multiple molecules, such as CTLA-4 and CD25, have been suggested to contribute to the suppressive activities of Tregs, it remains unsolved whether any of them may be sufficient to control Treg suppressive function. It would be interesting to investigate whether any of these genes are able to reconstitute the Treg function in the aforementioned mouse models. Genome-scaled DNA-binding profiling studies have been performed to identify Foxp3-specific target genes that might be crucial for Treg function. Over 700 potential targets including promoters, intragenic regulatory elements and smRNA/siRNA have been identified (160). Future efforts are needed to sift through these candidates to pinpoint the critical Foxp3 target genes for controlling the immunosuppressive function of Treg cells.
Positive roles for Tregs in immune responses
Tregs contribute to inflammation by inducing Th17 cells indirectly through TGF-β. TGF-β appears to be critical for the induction of Th17 cells, according to our and others’ studies (120). High levels of TGF-β, probably existing as a membrane-bound form, are found on Treg cells. Therefore, Treg cells have been identified as an important inducer for Th17 cells in vitro. Using TGF-β1 knockout mice, future studies need to be performed to address whether Tregs are the critical source of TGF-β in Th17 cell induction and thus the onset of EAE.
Foxp3-expressing cells can convert into effector cells. Analysis of Foxp3+ FILIG cells revealed that these cells developed effector cell functions that likely contribute to these immune disorders. GFP+ (Foxp3+) cells from healthy FILIG/+ mice did not exhibit an effector cell phenotype, suggesting that they did not spontaneously activate and convert into effector cells when substantial numbers of wildtype Treg cells are present (158). Surprisingly, however, a large portion of GFP+ cells from diseased FILIG/Y mice produced IL-4, while the percentages of the cells expressing IL-2, IFN-γ, or IL-17 were only modestly increased compared to wildtype cells. The percentage of IL-4-producing cells also increased in Foxp3−CD4+ T cells from FILIG/Y mice, consistent with the Th2 disorder observed in these mice. Similarly, upon transfer, GFP+ FILIG cells preferentially converted into Th2-type effectors, despite the fact that the coexisting Foxp3− cells produced large quantities of IFN-γ and therefore provided Th1-polarizing conditions (158). Thus, Foxp3-expressing cells with decreased Foxp3 expression preferentially converted into Th2-type effector cells in vivo, even in the presence of IFN-γ-producing Th1 cells. Upon conversion into Th2-type effectors, GFP+ FILIG cells potently promoted the Th2 differentiation of conventional T cells in vivo and in vitro regardless of the Th1-skewing conditions imposed by the cytokine milieu (158). In another study, Treg lineage cells without functional Foxp3 were able to express Th1, Th2, as well as Th17 effector cytokines (159). These findings present an interesting possibility that Foxp3-expressing cells might not always suppress but rather in some cases might foster the immune response, e.g. under inflammatory conditions. In fact, TNF-α, a pro-inflammatory cytokine, has been shown to repress the expression of Foxp3 in human Treg cells (161). It is reasonable to believe that in a highly inflammatory micro-environment, resident Tregs decrease their Foxp3 expression, subsequently lose their suppressive function, and might even be converted into effector cells to contribute to the immune responses. Upon resolution of infection, inflammatory cytokines would subside, and Foxp3 levels, as well as suppressive activity, would be restored.
Conclusions
The importance of active immune suppression is widely acknowledged. Studies on TGF-β and Tregs have shed light on how immune suppression applications. Advances in these areas have been and are being translated into clinical benefits. Further investigations are warranted to elucidate the mechanism through which TGF-β and Tregs control immune responses. In addition, we are starting to realize that under certain conditions, TGF-β and Tregs can both serve as promoting factors to direct immune responses. To facilitate designing immunotherapies against inflammatory diseases and cancers, more studies are needed to reveal alternative functions of TGF-β and Tregs. Moreover, as TGF-β and possibly Foxp3 function in the non-lymphoid system, further studies on the roles for TGF-β and Foxp3 in the non-lymphoid systems and on the interaction between lymphoid and non-lymphoid systems are certainly needed to achieve a global comprehensive view of our immune system.
Acknowledgments
We are grateful to L. Zenewicz for critical reading and helpful comments. We thank F. Manzo for secretarial assistance. Y.Y.W. is a Cancer Research Institute (CRI) fellow. R.A.F. is an investigator of the Howard Hughes Medical Institute. Work from this laboratory quoted in this review is supported by grants from the NIH and American Diabetes Association (ADA).
References
- 1.Gershon RK. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–185. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 2.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 3.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 4.Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther. 2003;98:257–265. doi: 10.1016/s0163-7258(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 5.Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor beta 1 precursor by human furin convertase. J Biol Chem. 1995;270:10618–10624. doi: 10.1074/jbc.270.18.10618. [DOI] [PubMed] [Google Scholar]
- 6.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 8.Crawford SE, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 9.Munger JS, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 10.Yehualaeshet T, O’Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol. 1999;155:841–851. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 12.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 14.Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massague J. The TGF beta receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell. 2001;8:671–682. doi: 10.1016/s1097-2765(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 15.Inman GJ, Nicolas FJ, Hill CS. Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-beta receptor activity. Mol Cell. 2002;10:283–294. doi: 10.1016/s1097-2765(02)00585-3. [DOI] [PubMed] [Google Scholar]
- 16.Johnson K, Kirkpatrick H, Comer A, Hoffmann FM, Laughon A. Interaction of Smad complexes with tripartite DNA-binding sites. J Biol Chem. 1999;274:20709–20716. doi: 10.1074/jbc.274.29.20709. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, Wang YF, Jayaraman L, Yang H, Massague J, Pavletich NP. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell. 1998;94:585–594. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- 18.Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 19.Nakao A, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 20.Ebisawa T, et al. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem. 2001;276:12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- 21.Kavsak P, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. 2000;6:1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 22.Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- 23.Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 25.Blanchette F, Rivard N, Rudd P, Grondin F, Attisano L, Dubois CM. Cross-talk between the p42/p44 MAP kinase and Smad pathways in transforming growth factor beta 1-induced furin gene transactivation. J Biol Chem. 2001;276:33986–33994. doi: 10.1074/jbc.M100093200. [DOI] [PubMed] [Google Scholar]
- 26.Funaba M, Zimmerman CM, Mathews LS. Modulation of Smad2-mediated signaling by extracellular signal-regulated kinase. J Biol Chem. 2002;277:41361–41368. doi: 10.1074/jbc.M204597200. [DOI] [PubMed] [Google Scholar]
- 27.Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choy L, Derynck R. The type II transforming growth factor (TGF)-beta receptor-interacting protein TRIP-1 acts as a modulator of the TGF-beta response. J Biol Chem. 1998;273:31455–31462. doi: 10.1074/jbc.273.47.31455. [DOI] [PubMed] [Google Scholar]
- 29.Griswold-Prenner I, Kamibayashi C, Maruoka EM, Mumby MC, Derynck R. Physical and functional interactions between type I transforming growth factor beta receptors and Balpha, a WD-40 repeat subunit of phosphatase 2A. Mol Cell Biol. 1998;18:6595–6604. doi: 10.1128/mcb.18.11.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGonigle S, Beall MJ, Pearce EJ. Eukaryotic initiation factor 2 alpha subunit associates with TGF beta receptors and 14-3-3 epsilon and acts as a modulator of the TGF beta response. Biochemistry. 2002;41:579–587. doi: 10.1021/bi011407z. [DOI] [PubMed] [Google Scholar]
- 31.Chen CH, et al. Transforming growth factor beta blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J Exp Med. 2003;197:1689–1699. doi: 10.1084/jem.20021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773–4777. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 34.Lin JT, Martin SL, Xia L, Gorham JD. TGF-beta1 uses distinct mechanisms to inhibit IFN-gamma expression in CD4+ T cells at priming and at recall: differential involvement of Stat4 and T-bet. J Immunol. 2005;174:5950–5958. doi: 10.4049/jimmunol.174.10.5950. [DOI] [PubMed] [Google Scholar]
- 35.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–914. [PMC free article] [PubMed] [Google Scholar]
- 37.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–737. [PMC free article] [PubMed] [Google Scholar]
- 38.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 39.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 42.Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 43.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 44.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 45.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 46.Vieira PL, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 47.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 49.Stassen M, et al. Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol. 2004;34:1303–1311. doi: 10.1002/eji.200324656. [DOI] [PubMed] [Google Scholar]
- 50.Kehrl JH, Roberts AB, Wakefield LM, Jakowlew S, Sporn MB, Fauci AS. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986;137:3855–3860. [PubMed] [Google Scholar]
- 51.Kehrl JH, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kulkarni AB, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cazac BB, Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–451. doi: 10.1016/s1074-7613(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 55.Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 57.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 58.Lucas PJ, Kim SJ, Melby SJ, Gress RE. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor beta II receptor. J Exp Med. 2000;191:1187–1196. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakao A, Miike S, Hatano M, Okumura K, Tokuhisa T, Ra C, Iwamoto I. Blockade of transforming growth factor beta/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med. 2000;192:151–158. doi: 10.1084/jem.192.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 62.Brabletz T, Pfeuffer I, Schorr E, Siebelt F, Wirth T, Serfling E. Transforming growth factor beta and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol Cell Biol. 1993;13:1155–1162. doi: 10.1128/mcb.13.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKarns SC, Schwartz RH, Kaminski NE. Smad3 is essential for TGF-beta 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J Immunol. 2004;172:4275–4284. doi: 10.4049/jimmunol.172.7.4275. [DOI] [PubMed] [Google Scholar]
- 64.Tzachanis D, et al. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat Immunol. 2001;2:1174–1182. doi: 10.1038/ni730. [DOI] [PubMed] [Google Scholar]
- 65.Coffey RJ, Jr, Bascom CC, Sipes NJ, Graves-Deal R, Weissman BE, Moses HL. Selective inhibition of growth-related gene expression in murine keratinocytes by transforming growth factor beta. Mol Cell Biol. 1988;8:3088–3093. doi: 10.1128/mcb.8.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 68.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 69.Ruegemer JJ, et al. Regulatory effects of transforming growth factor-beta on IL-2- and IL-4-dependent T cell-cycle progression. J Immunol. 1990;144:1767–1776. [PubMed] [Google Scholar]
- 70.Wolfraim LA, Walz TM, James Z, Fernandez T, Letterio JJ. p21Cip1 and p27Kip1 act in synergy to alter the sensitivity of naive T cells to TGF-beta-mediated G1 arrest through modulation of IL-2 responsiveness. J Immunol. 2004;173:3093–3102. doi: 10.4049/jimmunol.173.5.3093. [DOI] [PubMed] [Google Scholar]
- 71.Cottrez F, Groux H. Regulation of TGF-beta response during T cell activation is modulated by IL-10. J Immunol. 2001;167:773–778. doi: 10.4049/jimmunol.167.2.773. [DOI] [PubMed] [Google Scholar]
- 72.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 73.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 74.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 77.Gorham JD, Guler ML, Fenoglio D, Gubler U, Murphy KM. Low dose TGF-beta attenuates IL-12 responsiveness in murine Th cells. J Immunol. 1998;161:1664–1670. [PubMed] [Google Scholar]
- 78.Heath VL, Murphy EE, Crain C, Tomlinson MG, O’Garra A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol. 2000;30:2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 79.Ludviksson BR, Seegers D, Resnick AS, Strober W. The effect of TGF-beta1 on immune responses of naive versus memory CD4+ Th1/Th2 T cells. Eur J Immunol. 2000;30:2101–2111. doi: 10.1002/1521-4141(200007)30:7<2101::AID-IMMU2101>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 80.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 81.Sad S, Mosmann TR. Single IL-2-secreting precursor CD4 T cell can develop into either Th1 or Th2 cytokine secretion phenotype. J Immunol. 1994;153:3514–3522. [PubMed] [Google Scholar]
- 82.Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. 2003;198:1179–1188. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahmadzadeh M, Rosenberg SA. TGF-beta1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174:5215–5223. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonig H, Banning U, Hannen M, Kim YM, Verheyen J, Mauz-Korholz C, Korholz D. Transforming growth factor-beta1 suppresses interleukin-15-mediated interferon-gamma production in human T lymphocytes. Scand J Immunol. 1999;50:612–618. doi: 10.1046/j.1365-3083.1999.00635.x. [DOI] [PubMed] [Google Scholar]
- 85.Ranges GE, Figari IS, Espevik T, Palladino MA., Jr Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987;166:991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smyth MJ, Strobl SL, Young HA, Ortaldo JR, Ochoa AC. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8+ T lymphocytes. Inhibition by transforming growth factor-beta. J Immunol. 1991;146:3289–3297. [PubMed] [Google Scholar]
- 87.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 88.Rudner LA, Lin JT, Park IK, Cates JM, Dyer DA, Franz DM, French MA, et al. Necroinflammatory liver disease in BALB/c background, TGF-beta 1-deficient mice requires CD4+ T cells. J Immunol. 2003;170:4785–4792. doi: 10.4049/jimmunol.170.9.4785. [DOI] [PubMed] [Google Scholar]
- 89.Letterio JJ, Geiser AG, Kulkarni AB, Dang H, Kong L, Nakabayashi T, Mackall CL, et al. Autoimmunity associated with TGF-beta1-deficiency in mice is dependent on MHC class II antigen expression. J Clin Invest. 1996;98:2109–2119. doi: 10.1172/JCI119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 91.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 92.Leveen P, et al. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 2002;100:560–568. doi: 10.1182/blood.v100.2.560. [DOI] [PubMed] [Google Scholar]
- 93.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 94.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fahlen L, et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 98.Gray JD, Hirokawa M, Horwitz DA. The role of transforming growth factor beta in the generation of suppression: an interaction between CD8+ T and NK cells. J Exp Med. 1994;180:1937–1942. doi: 10.1084/jem.180.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 100.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 101.Rich S, Seelig M, Lee HM, Lin J. Transforming growth factor beta 1 costimulated growth and regulatory function of staphylococcal enterotoxin B-responsive CD8+ T cells. J Immunol. 1995;155:609–618. [PubMed] [Google Scholar]
- 102.Chen W, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 104.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schramm C, et al. Impairment of TGF-beta signaling in T cells increases susceptibility to experimental autoimmune hepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284:G525–535. doi: 10.1152/ajpgi.00286.2002. [DOI] [PubMed] [Google Scholar]
- 107.Huber S, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–6531. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 108.Mamura M, et al. CD28 disruption exacerbates inflammation in Tgf-beta1-/-mice: in vivo suppression by CD4+CD25+ regulatory T cells independent of autocrine TGF-beta1. Blood. 2004;103:4594–4601. doi: 10.1182/blood-2003-08-2897. [DOI] [PubMed] [Google Scholar]
- 109.Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- 110.Kim HP, Kim BG, Letterio J, Leonard WJ. Smad-dependent cooperative regulation of interleukin 2 receptor alpha chain gene expression by T cell receptor and transforming growth factor-beta. J Biol Chem. 2005;280:34042–34047. doi: 10.1074/jbc.M505833200. [DOI] [PubMed] [Google Scholar]
- 111.Lee HM, Rich S. Differential activation of CD8+ T cells by transforming growth factor-beta 1. J Immunol. 1993;151:668–677. [PubMed] [Google Scholar]
- 112.Gray JD, Liu T, Huynh N, Horwitz DA. Transforming growth factor beta enhances the expression of CD154 (CD40L) and production of tumor necrosis factor alpha by human T lymphocytes. Immunol Lett. 2001;78:83–88. doi: 10.1016/s0165-2478(01)00233-4. [DOI] [PubMed] [Google Scholar]
- 113.Chung EJ, Choi SH, Shim YH, Bang YJ, Hur KC, Kim CW. Transforming growth factor-beta induces apoptosis in activated murine T cells through the activation of caspase 1-like protease. Cell Immunol. 2000;204:46–54. doi: 10.1006/cimm.2000.1694. [DOI] [PubMed] [Google Scholar]
- 114.Sillett HK, Cruickshank SM, Southgate J, Trejdosiewicz LK. Transforming growth factor-beta promotes ‘death by neglect’ in post-activated human T cells. Immunology. 2001;102:310–316. doi: 10.1046/j.1365-2567.2001.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen W, Jin W, Tian H, Sicurello P, Frank M, Orenstein JM, Wahl SM. Requirement for transforming growth factor beta1 in controlling T cell apoptosis. J Exp Med. 2001;194:439–453. doi: 10.1084/jem.194.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Genestier L, Kasibhatla S, Brunner T, Green DR. Transforming growth factor beta1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J Exp Med. 1999;189:231–239. doi: 10.1084/jem.189.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 118.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 119.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 120.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 121.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 122.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982;156:1577–1586. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med. 1982;156:1565–1576. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6:327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- 126.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 127.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 128.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 129.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 130.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 131.Chang X, et al. The Scurfy mutation of FoxP3 in the thymus stroma leads to defective thymopoiesis. J Exp Med. 2005;202:1141–1151. doi: 10.1084/jem.20050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liston A, Farr AG, Chen Z, Benoist C, Mathis D, Manley NR, Rudensky AY. Lack of Foxp3 function and expression in the thymic epithelium. J Exp Med. 2007;204:475–480. doi: 10.1084/jem.20062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 134.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 135.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 136.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 138.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 139.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barthlott T, et al. CD25+ CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. Int Immunol. 2005;17:279–288. doi: 10.1093/intimm/dxh207. [DOI] [PubMed] [Google Scholar]
- 141.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 142.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 143.Slavik JM, Hutchcroft JE, Bierer BE. CD28/CTLA-4 and CD80/CD86 families: signaling and function. Immunol Res. 1999;19:1–24. doi: 10.1007/BF02786473. [DOI] [PubMed] [Google Scholar]
- 144.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 145.Boden E, Tang Q, Bour-Jordan H, Bluestone JA. The role of CD28 and CTLA4 in the function and homeostasis of CD4+CD25+ regulatory T cells. Novartis Found Symp. 2003;252:55–63. doi: 10.1002/0470871628.ch5. discussion 63-56, 106-114. [DOI] [PubMed] [Google Scholar]
- 146.Uhlig HH, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 148.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 150.Tadokoro CE, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 152.Miura Y, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 153.Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–741. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huan J, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 155.Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc Natl Acad Sci USA. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein(high), Foxp3-expressing CD25+ and CD25− regulatory T cells. J Immunol. 2006;176:4748–4756. doi: 10.4049/jimmunol.176.8.4748. [DOI] [PubMed] [Google Scholar]
- 157.Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 159.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 160.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 161.Valencia XSG, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]


