Abstract
We describe the nature and predictors of developmental trajectories of symptoms of DSM-IV nicotine dependence in adolescence following smoking initiation. Data are from a longitudinal cohort of 324 new smokers from grades 6 to 10 in the Chicago Public Schools, interviewed 5 times at 6 month intervals. Monthly data on DSM-IV symptoms of nicotine dependence were available for 36 months. Growth mixture modeling was applied to the monthly histories to identify trajectories of DSM-IV criteria of nicotine dependence. A four-class solution best fitted the data: No DSM criterion (47.7%); Early onset/Chronic course (19.8%); Early onset/Remission (17.3%); Late onset (15.2%). Blunt use prior to cigarette use was associated with the three symptomatic trajectories. Conduct disorder and prior heavy smoking were associated with Class 2 (Chronic). Conduct disorder differentiated Class 2 from Class 4 (Late onset), while pleasant initial sensitivity to the first tobacco experience was associated with Classes 2 and 3 (Remit) and differentiated Class 2 from Class 4. Novelty seeking characterized Class 3. Parental dependence differentiated chronicity (Class 2) from remission (Class 3) among those who developed symptoms early. Being Hispanic reduced membership in Classes 3 and 4, and being male for Class 3. The data highlight the importance of parental nicotine dependence as a risk factor for early and sustained nicotine dependence by the offspring, pleasant initial sensitivity and conduct disorder for early onset of dependence, and blunt use prior to smoking for all trajectories. The factors important for onset of dependence are not necessarily the same as those for sustained course.
Keywords: Nicotine dependence, Developmental trajectories, Risk factors, Adolescence
1.0 Introduction
Some progress has been made in our understanding of the development of nicotine dependence. While it has long been known that tobacco use is initiated in adolescence. It is only in the last decade that, following a brief report in the mid 90’s (Centers for Disease Control, 1994), adolescents have been seen also to experience symptoms of nicotine dependence (e.g., Colby et al., 2000). Going beyond the assessment of dependence at a particular point in time, several longitudinal studies have recently examined the timing and predictors of the transition from adolescent smoking to dependence. The latency between onset of smoking and dependence has not been clearly established. Differences in the behavior used to define onset of tobacco use and the definition of dependence across studies yield varying latency estimates from as short as 30 days or as long as 41 months for the appearance of the first dependence symptom and 23 to 46 months for the full syndrome. Thus, DiFranza et al. (2002, 2007; Scragg et al., 2008) concluded that symptoms developed very soon after brief intermittent use; 25% of 7th graders experienced one symptom as measured by the Hooked on Nicotine Checklist (HONC) within 30 days of inhaling from a cigarette for the first time (DiFranza et al., 2007). By contrast, O’Loughlin and her colleagues found that 25% of Canadian 7th graders smokers experienced withdrawal within 11 months and full ICD-10 nicotine dependence within 40.6 months after the first cigarette puffed (Gervais et al., 2006). Kandel et al. (2007) found that 25% of adolescents (mean age of 14.0 years at the initial interview) experienced the first symptom of dependence within 5 months of tobacco use onset and the full DSM-IV syndrome within 23 months.
Incidence data, however, do not inform about developmental patterns of symptoms following the onset of the first symptom. There is increasing recognition of the heterogeneity of the developmental course of substance use over time with variation in latency, level of escalation, chronicity, and remission (Jackson et al., 2005, p. 612). Statistical methods developed over the last ten years make it possible to identify heterogeneous developmental behavioral patterns over time. These methods, e.g., growth curve, latent class, growth mixture, hierarchical models (Duncan and Duncan, 2004; Muthén, 2004; Muthén and Schedden, l999; Nagin, 1999; Raudenbush and Bryk, 2002; Singer and Willet, 2003), characterize interindividual change and interindividual differences in intraindividual change, providing a dynamic identification of behavioral trajectories. As noted by Muthén and Muthén (2000), these models “analyze longitudinal data by relating an observed outcome variable to time or to a time-related variable such as age. Individual variation in growth is captured by the fact that coefficients in the growth model are random- that is, they vary across individuals.” (p. 885). The distinguishing feature of these models is the identification of heterogeneous developmental patterns that characterize individuals rather than variables. There has been an explosion of studies based on this approach with respect to smoking, with the outcome of interest being frequency/quantity of cigarettes smoked over the prior 30 days. We have identified 12 longitudinal studies of smoking trajectories, all published since 2000 (Abroms et al., 2005; Audrain-McGovern et al., 2004; Brook et al., 2006a,b; Chassin et al., 2000; Colder et al., 2001; Karp et al., 2005; Orlando et al., 2004; Stanton et al., 2004 ; Vitaro et al., 2004 ; White et al., 2000, 2002, 2004). Although Jackson et al. (2005) were concerned with concurrent trajectories of alcohol (drinking frequency) and tobacco (quantity cigarettes smoked), they also reported briefly on separate models for each substance. Across studies, measurement points vary from 4 to 10 over age intervals ranging from 3 to 31 years. A maximum of 7 trajectories have been identified, with most studies reporting three or four trajectory classes. These classes typically include a non-user class, and an early onset/rapid escalator/stable class, with varying intermediate patterns such as late onset stable smokers or quitters. In O’Loughlin’s five-year follow-up of Canadian 7th graders, the early onset rapid escalator trajectory was associated with the earlier development of full nicotine dependence (Karp et al., 2005). With the exceptions of Audrain-McGovern et al. (2007) and an analysis of joint alcohol and nicotine dependence disorders (Jackson et al., 2000), latent class growth modeling has not been applied to the development of symptoms of nicotine dependence. Measuring dependence with the mFTQ, Audrain-McGovern (2007) identified a single trajectory reflecting the development of increased number of symptoms in a cohort of high school students. Earlier onset age of smoking, pleasant initial experiences, lifetime marijuana use and number of friends who smoked predicted initial levels of dependence. Current smoking at the initial interview and normal metabolic activity (as per CYP2A6) predicted escalation. A latent growth modeling approach is presented here based on a DSM-IV assessment of dependence.
Our goals in this article are two-fold. We seek to identify (1) developmental trajectories (of criteria) of nicotine dependence in adolescence, and (2) predictors of the different trajectories. Although Audrain-McGovern et al. (2007) reported only one trajectory class for the mFTQ, in the absence of prior relevant work on DSM criteria of nicotine dependence, we assumed as an initial hypothesis that we would identify at least the two extreme classes observed with respect to changes in extensiveness of smoking, i.e., a non-smoking trajectory class and an early onset chronic class. The covariates of interest include significant psychosocial and selected biological predictors from studies of (1) dependence, and (2) of different trajectories of smoking intensity in latent growth models. The first set of studies includes longitudinal studies of predictors of dependence based on logistic regression models, predictors of the onset of dependence symptoms using survival analysis, and a recent study that examined the escalation of dependence symptoms using latent growth curve modeling (Audrain-McGovern et al., 2007).
A wider range of predictors have been examined with respect to smoking trajectories than incidence of nicotine dependence. Typically, predictors are measured at the initial point of the longitudinal follow-up. Differences may appear, however, in the relative importance of predictors at different points in the life course, whether early, mid or late adolescence or early adulthood. Factors examined both for smoking trajectories and dependence include sociodemographic characteristics, exposure to smokers in the proximate social environment (parents and peers), other substance use, in particular alcohol and marijuana, individual characteristics, such as academic performance, psychiatric disorders and personality characteristics, and exposure to prenatal maternal smoking. Consistent predictors of both heavy smoking trajectory membership and dependence include conduct problems and disorder, delinquency, depression, anxiety, novelty seeking, the use of alcohol and especially marijuana (Audrain-McGovern et al., 2004, 2007; Bardone et al., 1998; Breslau et al., 1993,1994; Brook et al., 2006a; Chassin et al., 2000; Dierker et al., 2001; DiFranza et al., 2004; Elkins et al., 2006; Fergusson et al., 1996, 2007; Hu et al., 2006; Isensee et al., 2003; Kandel et al., 2007; Karp et al., 2005, 2006; Orlando et al., 2004; Sonntag et al., 2000; Stanton et al., 2004; Storr et al., 2004; Vitaro et al., 2004; White et al., 2002).
Parental and peer smoking appear to be associated more consistently with smoking trajectory membership than with dependence. Five of eight trajectory studies reported positive associations for parents and seven of nine for peers (Abroms et al., 2005; Audrain-McGovern et al., 2004; Brook et al., 2006a; Chassin et al., 2000; Karp et al., 2005; Orlando et al., 2004; Stanton et al., 2004; Vitaro et al., 2004; White et al., 2002). Stanton et al. (2004) found maternal smoking to be significant when the child was 18 years old but not younger. Two out of five studies found an increased risk of offspring dependence when mothers had ever smoked, had smoked daily or had been dependent on nicotine (Hu et al., 2006; Lieb et al., 2003), while three studies found no association between parental smoking and child dependence (Audrain-McGovern et al., 2007; Fergusson et al., 1996; Rhode et al., 2004). Two studies that examined smoking by peers found that it increased the risk of dependence (Audrain-McGovern et al., 2007; Hu et al., 2006), whereas Patton et al. (2005) found no peer effects. Poorer academic performance, examined to a greater extent for smoking trajectories than dependence, predicts heavy involvement and may be more important in late than early adolescence (Audrain-McGovern et al., 2004; Karp et al., 2005; Orlando et al., 2004; White et al., 2002). Lower education is associated with higher rates of dependence (Hu et al., 2006). Prenatal maternal smoking predicts dependence (Buka et al., 2003; Lieb et al., 2003) but not heavy smoking trajectory membership (White et al., 2002).
Of the demographics, white ethnicity is consistently associated with heavy smoking trajectory and nicotine dependence (Andreski and Breslau, 1993; Breslau et al., 2001; Hu et al., 2006; Orlando et al., 2004; White et al., 2004), while female gender appears to be more important for dependence (DiFranza et al., 2002; Hu et al., 2006; Kandel et al., 2007; O’Loughlin et al., 2002) than smoking trajectory membership (Abroms et al., 2005; Brook et al., 2006a; Orlando et al., 2004; Vitaro et al., 2004). Karp et al. (2006) found no effects of age or gender on the incidence of symptoms of tobacco dependence.
Factors examined only for nicotine dependence include age of smoking onset and extensiveness of smoking, initial sensitivity to nicotine, and nicotine metabolism. Smoking history, in particular earlier age at onset of smoking a whole cigarette (Audrain-McGovern et al., 2007), shorter time since cigarette onset (Karp et al., 2006), and a shorter latency between onset and daily smoking, are associated with higher rates of dependence (Hu et al., 2006). Extensiveness of use predicts the onset and further increase of dependence symptoms (Audrain-McGovern et al., 2007; Kandel et al., 2007; Karp et al., 2006). Contradictory findings have been reported for nicotine metabolism: both slow (Karp et al., 2006) and normal CYP2A6 (Audrain-McGovern et al., 2007) rate of nicotine metabolism have been shown to increase the risk of dependence in adolescence.
In survival analysis of the onset of nicotine dependence (Kandel et al., 2007), conducted on a sample of tobacco users from the current study, we found that pleasant initial sensitivity to tobacco and number of cigarettes smoked the month prior to the first symptom increased the incidence of both the first DSM-IV criterion and the full syndrome. Parental dependence increased the incidence of the full syndrome. Hispanics were the least likely of the racial/ethnic groups to experience the first criterion and males were less likely than females to experience the full syndrome. Based on our prior work, and on work by others on the onset of dependence and on trajectories of smoking reviewed above, we focused in particular on gender, race/ethnicity, parent and peer smoking history, initial sensitivity to tobacco, depression, conduct problems, academic performance and other substance use as potential predictors of membership in different trajectories of nicotine dependence symptoms.
2.0 Methods
2.1 Sample
The analyses are based on five waves of interviews with a subsample from a multi-ethnic longitudinal cohort of 1,039 6th-10th graders from the Chicago Public Schools (CPS) and one parent, mostly mothers. A two-stage design was implemented to select efficiently the target sample for follow-up. In Phase I (spring 2003), 15,763 students in grades 6–10 were sampled from 43 public schools in the CPS. The sample was designed to provide approximately equal numbers of adolescents among the three major ethnic groups: non-Hispanic white, non-Hispanic African American, and Hispanic. Because of the ethnic distribution in the CPS, largely Hispanic schools were excluded and schools with large numbers of non-Hispanic white students were oversampled. The resulting sample is representative of each racial/ethnic group from the CPS, except for Hispanics from largely Hispanic high schools and whites with Polish speaking parents. Schools were divided into eight segments for staggered survey administration over four months. Students were given a brief self-administered questionnaire; the completion rate was 83.1%. Responses were used to select a target sample of 1,236 youths: 1,106 tobacco users who reported having started to use tobacco in the prior 12 months and 130 non-tobacco users susceptible to start smoking (as per Pierce et al., 1996), divided as evenly as possible among non-Hispanic whites, non-Hispanic African Americans and Hispanics. Whites and African Americans who had started to use tobacco 0–12 months earlier and Hispanics who had started 0–6 months earlier were selected with certainty; Hispanics who started 7–12 months earlier were sampled at a 25% rate, because there was a larger number of Hispanics than of other race/ethnic groups in the sample schools.
In Phase II, on average 9 weeks after each school survey, 1,039 (84.1%) of the 1,236 targeted youths and one parent agreed to participate in the longitudinal follow-up consisting of three annual computerized household interviews with youths and parents, each about 90 minutes long (Waves 1, 3, 5), and two short bi-annual interviews (20 minutes long) with youths six months after Waves 1 and 3 (Waves 2, 4). Data were collected from 2/03 – 10/05. The average interval between waves was 6.0–6.3 months, range=3–10 months. Completion rates at each successive wave were 96% of the Wave 1 sample. Hispanics who had started to use tobacco 7–12 months earlier were given a weight of 4, since they were sampled at the rate of 25%. All Hispanic tobacco users were rescaled to the unweighted number who was interviewed.
To maximize participants’ trust in the confidentiality of their responses, attempts were made to interview parent and child simultaneously by two different interviewers on the same day. At Wave 1, in 874 families, adolescents and parents were interviewed on the same day; of those, 510 were interviewed simultaneously by different interviewers. The field work was conducted by the National Opinion Research Center (NORC) at the University of Chicago.
2.2 Human subject procedures
Passive parental consent was obtained for the school survey and active consent for the household interviews; adolescent assent was obtained for the school and household interviews. The interviewers emphasized that all answers and results would be kept completely confidential and would not be communicated to anyone, including the adolescents’ parents or teachers. All procedures for obtaining parental consent and youth assent were approved by the Institutional Review Boards of the New York State Psychiatric Institute, Columbia University, and of NORC.
2.3 Data collection
Annual household computer assisted personal interviews were conducted with adolescents and one parent (mothers 86.8%). Tobacco use patterns were ascertained for cigarettes, cigars, pipes, bidis, kreteks, and smokeless tobacco (chewing tobacco, snuff, dip). The adolescent interview included a tobacco use history chart that obtained detailed monthly information on patterns of smoking (i.e., number of days had smoked and the average number of cigarettes smoked per day in each month) for the 12 months preceding the Wave 1 interview and for the intervals since each prior interview at Waves 2–5. A unique component of the chart included the ascertainment of specific DSM-IV dependence symptoms experienced with respect to tobacco use on a monthly basis in the intervals between successive waves as of Wave 1. At Wave 1, respondents were asked to report the onset month of each DSM-IV symptom experienced in the prior 12 months.
2.3.1 Inconsistencies in reporting
There were discrepancies between the school and household smoking reports (Griesler et al., in press). Of the youths who had reported lifetime cigarette smoking in school (N=832), 189 denied at Wave 1 in the household having ever smoked cigarettes. There were further discrepancies in the age of tobacco use onset. Only 269 (41.8%) of the 643 adolescents who had reported in school having started to smoke in the prior 12 months were identified as having started smoking cigarettes within the prior year based on the more precise (month/year of onset) ascertainment in the household interviews; 374 were estimated to have started to smoke more than 1 year before Wave 1. Of these, 53 started within 12 months prior to the school survey but more than 12 months prior to the household interview because of the time lag between the two data collections. The cumulative impact of non-participation and inconsistent reporting resulted in a sample that was biased toward the exclusion of tobacco users, especially heavier users.
2.4 Definitions of variables
2.4.1 Nicotine dependence
Nicotine dependence was measured as per the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV, American Psychiatric Association, 1994), based on an instrument developed for adolescents and young adults by the Tobacco Etiology Research Network (Dierker et al., 2007; Sledjeski et al., 2007). The scale has high internal reliability and concurrent, predictive and incremental validity (Sledjeski et al., 2007). The 11-item scale measured specific symptoms that defined the seven DSM-IV dependence criteria experienced in connection with the specific tobacco products used by respondents (Kandel et al., 2005, 2007). Four criteria were indexed by two alternate symptoms; three were indexed by a single symptom. The computerized interviews personalized the questions to specify the products used by each respondent (see supplementary material for additional details of the methodology).1 The seven criteria were tolerance, withdrawal, impaired control, unsuccessful attempts to quit, great deal of time spent using tobacco, neglect important activities, and use despite physical or psychological problems (α=.84). Full dependence was defined when at least three criteria were met within a 12-month period. The question about withdrawal asked about 12 specific symptoms; 3 were sham items included to check on the reliability of respondents’ answers. These three items and a fourth item about craving were excluded from the scoring. The withdrawal criterion was met when respondents checked at least four of the eight valid symptoms. The terms symptoms and criteria are used interchangeably, although care is taken to specify criteria when referring to criteria for the full dependence syndrome.
2.4.2 Covariates
All covariates, except depressive symptoms among those who started to smoke after Wave 2, were measured at Wave 1. With the exceptions of two parental variables, the data were reported by adolescents.
2.4.2.1 Sociodemographic Variables
Race/ethnicity
non-Hispanic white; non-Hispanic African American; and Hispanic.
Gender
male; female.
2.4.2.2 Tobacco and Drug Use History
Onset age of smoking cigarettes
10–17 years. Based on birth date, and month and year first smoked cigarette.
Number of cigarettes smoked the month prior to the onset of the first DSM criterion
Pack or more: ≥ 22.5 cigarettes (90th percentile). Calculated as the product of the number of days smoked in that month (frequency) and number of cigarettes smoked on days smoked (quantity). Both variables were recoded to mid-point values before multiplication. Dichotomized at 22.5 cigarettes. Frequency of cigarette use: Smokers who did not smoke in that particular month were coded 0; smoked 1–2 days=1.5; 3–5 days=4; 6–9 days=7.5; 10–19 days=15; 20–29 days=25; 30 days=30. Daily quantity smoked on days smoked: One or two puffs=0.5; 1 cigarette=1; 2–5 cigarettes=3; 6–15 cigarettes=10; 16–25 cigarettes=20; 26–35 cigarettes=30; more than 35 cigarettes=40 per day smoked in the month. Coded as the last month smoked for those without DSM symptoms.
Initial sensitivity to tobacco use (modified Pomerleau et al., 1998). Measured experiences associated with first tobacco use. Dizziness and rush/buzz symptoms were differentiated into pleasurable and unpleasurable experiences. Two scales averaged the scores of component items: (1) Pleasant symptoms (pleasant sensations, relaxation, pleasurable dizziness, pleasurable rush or buzz (α=.77)); (2) Unpleasant symptoms (unpleasant sensations, nausea, unpleasurable dizziness, unpleasurable rush or buzz, coughing, heart pounding, headache, bad taste (α=.80)). Each symptom coded 1=none to 4=intense experience.
Alcohol use before cigarette use onset
1=yes; 0=no.
Marijuana use before cigarette use onset
1=yes; 0=no.
Blunt (hollowed cigar filled with marijuana) use before cigarette use onset
1=yes; 0=no.
2.4.2.3 Smoking by parents, siblings and peers
Participating parent’s lifetime smoking and dependence
Self-reported by interviewed parent (284 mothers, 18 fathers, 22 others): never smoked cigarettes; ever smoked but never DSM-IV nicotine dependent; lifetime DSM-IV nicotine dependent.
Biological mother’s prenatal smoking
Reported by interviewed parent: 1=yes; 0=no. A dummy variable was included for 22 missing cases.
Perceived siblings’ smoking
1=any sibling ever smoked cigarettes; 0=none.
Perceived peer smoking
1=at least one close friend currently smoked cigarettes; 0=none.
3.4.2.4 Psychosocial Variables
Measured at Wave 1 except for depressive symptoms. Depressive symptoms scale (measured at Wave 1 or at Wave 2 for smokers who started to smoke at Wave 3): Average of 12 4-point items (Gadow et al., 2002). The scale measures DSM-IV criteria for major depressive disorder and dysthymia (American Psychiatric Association, 1994) in the last 30 days. Youths rated their behaviors (trouble falling or staying asleep, trouble concentrating, tired/no energy, eating a lot, sleeping a lot, skipped meals/ate little) and feelings (grouchy/cranky, unhappy/sad, did not feel like doing anything, did not act like self, felt things never work out right, felt could not do things as well as others) in the last 30 days on a 4-point scale (0=never, 1=sometimes, 2=often, 3=very often). The suicide ideation item was removed from the original 13-item scale because of human subject concerns. Average score ranged from 0–3 (α=.87). A dummy variable indexing high depression was created for those who scored in the upper 25% of the distribution (scores >0.90).
Anxiety disorder diagnostic screen
Scored positive in the last 12 months for DSM-IV social phobia (2 symptoms) or generalized anxiety disorder (3 out of 4 symptoms) on the DISC predictive scale (DPS v4.32, Lucas et al., 2001) (α=.63).
Conduct disorder diagnostic screen
Scored positive for DSM-IV conduct disorder on the DISC predictive scale if met at least 3 out of 8 symptoms in the last 12 months (DPS v4.32, Lucas et al., 2001) (α=.62).
Novelty seeking
Based on Cloninger’s Tridimensional Personality Questionnaire (Wills et al., 1998). Average score of nine 5-point items: 1=not at all true; 2=a little true; 3=somewhat true; 4=pretty true; 5=very true. The items were: try things just for fun, look for something exciting, can get people to believe lies, do things based on how feel at the moment, get excited and lose control, like when people can do whatever they want, follow instincts, can stretch the truth and change interests a lot (α=0.87).
Academic performance
Grades on last report card: 1=D or lower; 2=half C or mostly C; 3=half B or mostly B; 4= half A or mostly A.
2.5 Analytical sample
The analytical sample included 324 new cigarette smokers, who were identified in the household survey as having started to smoke within 12 months preceding Wave 1 (N=284) or between Waves 1 and 3 (N=40), and provided reports on symptoms for at least 12 months preceding Wave 5, in order to meet DSM requirements (see below). The 284 cases included 269 adolescents who reported having started to smoke within the last 12 months in the household and in school plus 38 who only reported onset prior to W1 in the household, less 23 with missing data on DSM symptoms. Smoking only one or two puffs qualified cigarette smokers for inclusion in the sample. Of those who had started smoking within the 12 months prior to Wave 1, 15.9% had started within the last 3 months, 26.8% 4–6 months earlier, 29.2% 7–9 months earlier, and 28.2% had started 10–12 months earlier.
2.6 Statistical analysis
The number of DSM-IV nicotine dependence criteria experienced over 36 months in 25 successive 12-months periods was analyzed among new cigarette smokers. Dependence was defined when three or more criteria were experienced within each period. Growth mixture models (GMM) were used to identify trajectories of nicotine dependence. By combining conventional growth modeling with latent class analysis (LCA), GMM estimates the mean of the growth curve for each trajectory class and allows for individual variation around the mean (Muthén and Asparouhov, 2007). Allowing for individual variation within classes is an advantage over latent class analysis (Nagin, 1999). Using the newly released version of Mplus5, a series of unconditional GMMs were estimated by fitting one to five classes, with linear and quadratic parameters. The optimal number of classes was partially determined by two fit indices: (a) a smaller Bayesian information criterion, i.e., a high log likelihood ratio with a small number of free parameters (Schwarz, 1978; Sclove, 1987); (b) a significant Lo-Mendell-Rubin adjusted likelihood ratio test for k versus k-1 classes (Lo et al., 2001). Substantive and theoretical considerations were also taken into account in the selection of a particular solution (Audrain-McGovern et al., 2004; Muthén, 2004).
In order to reduce model misspecification and identify significant predictors of trajectory class membership, the models were reestimated with the inclusion of selected covariates in multinomial logistic regressions within growth mixture modeling (GMM). The covariates were selected on the basis of a newly available procedure in Mplus 5 in which potentially significant class predictors were selected on the basis of a Wald test of mean equality across the latent trajectory classes using posterior probability based multiple imputations (Muthén and Muthén, 1998–2007). With control for covariates on class membership and growth factors in GMM, the estimation of class membership will be more correctly specified (Muthén, 2004). Thirteen cases were excluded from the growth mixture modeling because of missing data on covariates.
All analyses used weighted samples that corrected for the under sampling of Hispanics who had started to use tobacco 7 to 12 months prior to the school survey. To handle missingness of the outcome variables, the missing at random (MAR) estimation procedure provided by Mplus was implemented (Muthén and Muthén, 1998–2007).
3.0 Results
3.1 Descriptive results
At Wave 1, adolescents in the analytical sample were on average 14.0 years old (S.D.=1.3), range 11 to 17 years; 43.2% were male, 56.8% female. The racial/ethnic distribution was non-Hispanic white (30.4%), non-Hispanic African American (26.0%), and Hispanic (43.6%). In addition to cigarettes, 23.3% had also used other tobacco products: cigars (21.0 %), smokeless tobacco (2.1 %), kreteks (0.5%), bidis (0.5 %), and pipes (0.2%). The average age of cigarette smoking onset was 13.7 years (S.D. =1.3), range 10 to 17 years. These youths were light smokers. By Wave 5, 27.2% had ever smoked less than one cigarette, 8.9% had ever smoked a whole cigarette, 17.0% had smoked 2–5 cigarettes, 10.9% 6–15, 7.2% 16–25, 8.2% 26–99 and 20.6% 100 or more cigarettes; 12.6% had ever smoked daily. In any one month, a very small percentage of smokers (6.8%) reported smoking 30 days in that month; 6.0% reported smoking 20–29 days, 10.4% 11–20 days, 19.3% 6–10 days, 9.5% 3–5 days, and 48.0% 1–2 days. Those who smoked daily smoked an average of 8.3 cigarettes per day.
By Wave 5, 54.7% of cigarette smokers had experienced at least one criterion of DSM-IV nicotine dependence, 42.9% two or more criteria, 28.8% three or more. Of those who had experienced one criterion, 39.8% had already done so by Wave 1; 23.1% of those who met three criteria had also done so by Wave 1. As is to be expected, levels of smoking varied greatly across levels of dependence. Among smokers who never experienced any symptoms of dependence. 50.6% had only ever smoked only 1 puff of cigarette, 1.2% had ever smoked 100 cigarettes. The percentages among those who reported 1–2 criteria were 15.2% and 16.2%, respectively. The percentages among those who met criteria for full dependence were 1.1% and 55.1%, respectively. The percentages who smoked daily or near daily in any one month were 0% among those with no symptoms, 6.1% among those with 1–2 criteria, and 24.8% among those with full dependence. Observed rates of the dependence syndrome were similar among males and females, and lower among Hispanics (20.7%) than non-Hispanic African Americans (39.8%, p< 0.01) and whites (30.9%, p<0.08).
3.2 Growth Mixture Models: criteria of nicotine dependence
Five models were estimated sequentially through GMM (in Mplus5) starting with the single class model. Having determined that the quadratic growth factor was significant, this factor was applied to all subsequent models. In addition, models were tested assuming nonzero versus zero inflated Poisson distribution, non random versus random intercept, and class-equal versus class-varying variances. The model fit statistics for GMM are shown in Table 1. The BIC and LRT statistics all favored the 5-trajectory model. However, the size of one of the classes was very small, and the growth shape for that class was very skewed, with a high number of symptoms at the beginning and end points of observation. Thus, the four-class solution, with quadratic growth factor, random intercept, and class-equal variances on the intercept, was chosen. Since the zero-inflated Poisson model for the two-class solution (BIC=9,780) did not fit better than the non-zero inflated Poisson (BIC=9,763), the zero-inflated model was not applied to the final four-class solution. To ensure the generalizability of the models, the best log likelihood was replicated. For the four-class solution, the best log likelihood was replicated three times after 500 random starting values were generated in the initial stage and 100 optimizations with the highest log likelihood were used in the final stage.
Table 1.
Test statistics for trajectories of nicotine dependence in unconditional and conditional Growth Mixture Models (new cigarette smokers by Wave 3, N=324)
| Trajectories | Log Likelihood | BIC | BICa | Free parameters | LRT( P) | Class sizeb |
|---|---|---|---|---|---|---|
| Unconditional | ||||||
| 1 | −5107 | 10236 | 10223 | 4 | NA | 324 |
| 2 | −4858 | 9763 | 9738 | 8 | 0.00 | 190,134 |
| 3 | −4663 | 9396 | 9358 | 12 | 0.03 | 144,126,53 |
| 4 | −4438 | 8969 | 8918 | 16 | 0.00 | 152,68,58,46 |
| 5 | −4373 | 8856 | 8796 | 19 | 0.00 | 148,63,46,44,22 |
| Conditional | ||||||
| 4 classes with 12 covariatesc | −4130 | 8592 | 8408 | 58 | 149,61,54,47 |
Sample-size adjusted BIC.
Respondents assigned to a class on the basis of weighted posterior probabilities.
Excludes 13 cases with missing data.
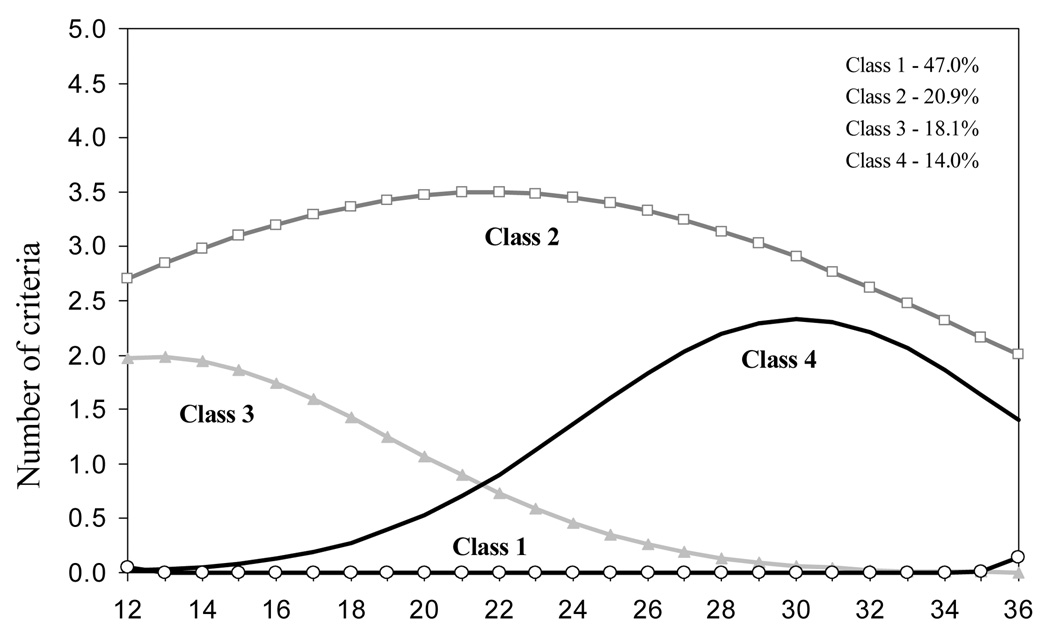
The four latent classes were: (1) No dependence criteria; (2) Early onset of full DSM-IV dependence, chronic course; (3) Early onset of symptoms, remission; (4) Late onset of symptoms (See Figure 1). The class proportions were 47.0%, 20.9%, 18.1%, and 14.0%, respectively.
Figure 1.
Trajectories of DSM-IV criteria of nicotine dependence in growth mixture modeling (new cigarette smokers by W3, N=324)a
aClass membership based on weighted posterior probabilities, without covariates in the model.
bEach time point represents the observed number of DSM criteria in a 12-month period (e.g. 12=months 1–12 and 14=months 3–14).
BIC=8969, Free Parameters =16, Lo-Mendell-Rubin test=0.0009
3.3 Growth Mixture Models with covariates: nicotine dependence and covariates
To reduce model misspecification and identify significant predictors of the various trajectories, the models were reestimated with the inclusion of selected covariates. In a first step, adolescent smokers were assigned class membership on the basis of posterior probabilities from the unconditional GMM estimation. To select a restricted number of potentially significant covariates among the 18 factors of interest, the differences between conditional class specific means for each variable were evaluated from the four-class growth mixture model through a Wald test. Eleven variables were significantly different among the four classes. Seven variables were not statistically significant: gender, unpleasant initial sensitivity, maternal prenatal smoking, perceived sibling smoking, depressive symptoms, being positive on the anxiety disorder screen, and academic performance. In a second step, the 11 significant covariates plus gender were included in a re-estimation of the latent growth models in growth mixture modeling (GMM). We examined the impact of these covariates on the trajectories and on the growth parameters (intercept, linear and quadratic trends).
3.3.1 Trajectories of nicotine dependence criteria
The resulting four trajectories were very similar to those identified originally without covariates, although there were small discrepancies in the proportions of individuals assigned to each class. On the basis of weighted posterior probabilities, 47.7% of these recent smokers were assigned to Class 1, No DSM criterion; 19.8% to Class 2, Early onset/Chronic course; 17.3 % to Class 3, Early Onset/Remission; and 15.2% to Class 4, Late onset. The No DSM criterion class (Class 1) smoked for a very short period of time: 64.1% smoked only one month, and 20.0% two to three months after starting to smoke. Of those in the Early onset/Remission class (Class 3), 73.9% quit smoking within two years after initiating smoking. Among those in Class 4, Late DSM onset, 48.0% experienced their first symptom within 20 months after onset of smoking. Of cases in Class 2, the chronic trajectory class, 47.5% experienced full DSM dependence in the first 12 months after onset of smoking, 59.3% in months 13 to 24, and 53.0% in months 25 to 36; of those who experienced the full dependence syndrome in the first 12 months after onset of smoking, 69.0% still did so in months 25–36. Nineteen percent (18.6%) did not meet full criteria for DSM-IV nicotine dependence at any time in the 36 months following the onset of smoking, but they experienced one or two criteria throughout the entire period of observation. Of those in Class 3, the Early onset/Remission trajectory, 30.0%, 5.1%, and 0% met full DSM criteria in months 1–12, 13–24 and 25–36, respectively. Of cases in Class 4, 22.1% experienced full DSM within months 13–24 and 26.3% within months 25–36 following onset of smoking.
3.3.2 Predictors of trajectories membership
The prevalences of the 12 covariates in each trajectory are displayed in Table 2. At the univariate level, all variables except gender were statistically significant based on a Wald test of mean equality across classes. In order of significance, the risk factors were number of cigarettes smoked the month before the first DSM criterion, higher pleasant symptoms at the initial tobacco use experience, positive screen on conduct disorder, race/ethnicity, onset age of smoking, the use of blunts, parental nicotine dependence, novelty seeking, alcohol and marijuana before smoking onset, and perceived peer smoking.
Table 2.
Distribution of means of covariates across latent trajectories of nicotine dependence using posterior probabilitybased multiple imputations in 4-class GMM (new cigarette smokers by Wave 3, N=324)
| Class 1 No DSM (N=152) | Class 2 Early DSM/Chronic (N=68) | Class 3 Early DSM/Remission (N=58) | Class 4 Late DSM (N=46) | ||
|---|---|---|---|---|---|
| Covariatesa | % or Mean/(S.D.) | % or Mean/(S.D) | % or Mean/(S.D) | % or Mean/(S.D) | Wald Test (p-value) |
| Male (vs. female) (%) | 46.1 | 46.8 | 34.6 | 39.3 | 0.387 |
| Race/ethnicity | |||||
| White (%) | 26.9 | 32.5 | 26.3 | 44.0 | 0.176 |
| African American (%) | 18.7 | 30.9 | 41.7 | 23.1 | 0.008** |
| Hispanic (%) | 54.4 | 36.6 | 32.0 | 32.9 | 0.002** |
| Onset age of smoking cigarettesb(M(S.D.)) | 13.6(1.3) | 13.9(5.2) | 14.2(0.8) | 13.3(0.8) | 0.002** |
| Pack or more of cigarettes month before 1st DSMc | 3.7 | 26.5 | 20.0 | 15.4 | 0.000*** |
| Alcohol before cigarette onsetb(%) | 31.1 | 48.3 | 48.1 | 39.7 | 0.034* |
| Marijuana before cigarette onsetb(%) | 16.3 | 29.4 | 34.1 | 19.0 | 0.025* |
| Blunt before cigarette onsetb(%) | 9.5 | 30.5 | 25.7 | 19.0 | 0.001** |
| Pleasant initial sensitivity to tobaccob(M(S.D.)) | 1.2(0.4) | 1.6(0.4) | 1.8(0.5) | 1.3(0.2) | 0.000*** |
| Parent self-reported DSM dependence | |||||
| Never smoked (%) | 34.6 | 19.8 | 31.7 | 28.9 | 0.115 |
| Smoked, not dependent (%) | 51.7 | 50.9 | 43.4 | 38.6 | 0.357 |
| Ever dependent (%) | 13.7 | 29.2 | 24.8 | 32.4 | 0.008** |
| Perceived peer smoking (%) | 40.7 | 60.7 | 54.8 | 46.4 | 0.029* |
| Conduct disorder positive screen (%) | 4.1 | 25.4 | 20.1 | 8.4 | 0.000*** |
| Novelty seeking (M(S.D.)) | 2.5(0.7) | 2.7(0.6) | 2.9(0.5) | 2.6(0.5) | 0.003** |
Measured at Wave 1, except as noted.
Measured when first reported using tobacco or other drugs (W1-W3).
From smoking history chart.
p<0.10
p <0.05
p<0.01
p<0.001
To identify unique predictors of trajectory class membership, the model was reestimated with the inclusion of the 11 covariates, plus gender, which was included because of its theoretical interest. Rates of dependence are generally higher among females than males. The most important comparisons are those of the symptomatic classes with the non-symptomatic class (see Table 3).
Table 3.
Predictors of developmental trajectoriesa of DSM-IV criteria of nicotine dependence in the 4-class conditional GMM model (new cigarette smokers by Wave 3, N=311b)
| Covariates c | Class 2 (Chronic) AOR | vs. | Class 1 (None) 95%CI | Class 3 (Remit) AOR | vs. | Class 1 (None) 95%CI | Class 4 (Late) AOR | vs. | Class 1 (None) 95%CI | Class 2 (Chronic) AOR | vs. | Class 3 (Remit) 95% | Class 2 (Chronic) AOR | vs. | Class 4 (Late) 95% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race/ethnicity (vs. white) | |||||||||||||||
| African American | 1.18 | 0.43– 3.26 | 1.83 | 0.68– 4.92 | 0.89 | 0.32– 2.47 | 0.64 | 0.20– 2.09 | 1.34 | 0.39– 4.62 | |||||
| Hispanic | 0.50 | 0.19– 1.32 | 0.30* | 0.11– 0.87 | 0.34* | 0.15– 0.79 | 1.66 | 0.50– 5.44 | 1.48 | 0.51– 4.30 | |||||
| Male(vs. female) | 0.70 | 0.32– 1.52 | 0.40* | 0.17– 0.97 | 0.63 | 0.28– 1.45 | 1.73 | 0.65– 4.59 | 1.10 | 0.44– 2.78 | |||||
| Onset age of smoking cigarettesd | 1.04 | 0.69– 1.56 | 1.23 | 0.86– 1.74 | 0.77† | 0.59– 1.02 | 0.85 | 0.54– 1.32 | 1.34 | 0.85– 2.14 | |||||
| Pack or more of cigarettes in the month before 1st DSMe | 4.21* | 0.99–17.86 | 4.26† | 0.96–18.96 | 3.92† | 0.77–19.80 | 0.99 | 0.30– 3.28 | 1.08 | 0.32– 3.66 | |||||
| Alcohol before cigarette onsetd | 2.16 | 0.78– 5.99 | 1.85 | 0.76– 4.54 | 1.69 | 0.79– 3.64 | 1.17 | 0.38– 3.63 | 1.28 | 0.42– 3.86 | |||||
| Marijuana before cigarette onsetd onset | 0.42 | 0.14– 1.24 | 0.76 | 0.31– 1.88 | 0.71 | 0.21– 2.40 | 0.55 | 0.17– 1.80 | 0.59 | 0.16– 2.16 | |||||
| Blunt before cigarette onsetd | 7.02** | 2.01–24.52 | 5.87** | 1.74–19.79 | 5.24* | 1.17–23.37 | 1.20 | 0.39– 3.71 | 1.34 | 0.36– 4.94 | |||||
| Pleasant initial sensitivity to tobaccod | 2.96* | 1.22– 7.19 | 4.85*** | 2.03–11.55 | 1.20 | 0.45– 3.23 | 0.61 | 0.30– 1.25 | 2.46* | 1.08– 5.64 | |||||
| Parent self-reported DSM (vs. never smoked) | |||||||||||||||
| Smoked, not dependent | 1.70 | 0.60– 4.87 | 0.55 | 0.22– 1.37 | 0.55 | 0.22– 1.34 | 3.10† | 0.91–10.51 | 3.10† | 0.94–10.22 | |||||
| Ever dependent | 3.13† | 0.92–10.70 | 0.71 | 0.19– 2.63 | 2.25 | 0.77– 6.57 | 4.43* | 1.00–19.64 | 1.39 | 0.39– 4.95 | |||||
| Perceived peer smoking | 1.70 | 0.77– 3.74 | 1.03 | 0.44– 2.40 | 1.26 | 0.57– 2.80 | 1.65 | 0.61– 4.49 | 1.35 | 0.52– 3.48 | |||||
| Conduct disorder positive screen | 8.22** | 2.18–31.03 | 4.24† | 0.80–22.49 | 2.21 | 0.48–10.17 | 1.94 | 0.50– 7.45 | 3.72* | 1.08–12.79 | |||||
| Novelty seeking | 1.23 | 0.74– 2.06 | 1.81* | 1.03– 3.18 | 1.22 | 0.71– 2.09 | 0.68 | 0.36– 1.29 | 1.01 | 0.55– 1.84 | |||||
Class1= no DSM (N=149), class 2= Early DSM/Chronic (N=61), class 3= Early DSM/Remission (N=54), class 4= Late DSM (N=47). BIC=8591.97 (Free Parameters=58) Log likelihood=–4129.53 (Replicated 5 times at starts=500,100)
Excludes 13 cases with missing data.
Measured at W1, except as noted.
Measured when first reported using tobacco or other drugs (W1–W3).
From smoking history chart.
p<0.10
p<0.05
p<0.01
p<0.001
Early onset/Chronic course (Class 2) versus No DSM criteria (Class 1)
Compared with adolescents with no DSM criteria, those in the early onset/full DSM/chronic trajectory were more likely to have used blunts before the onset of smoking, to be positive on the conduct disorder screen, to have smoked at least a pack of cigarettes in the month prior to the first criterion, and to experience pleasant experiences at the initial tobacco use.
Early onset/Remission (Class 3) versus No DSM criteria (Class 1)
Compared with adolescents with no DSM criteria, those with early onset who remitted were more likely to have experienced more pleasant symptoms at their initial tobacco use experience, to have used blunts before the onset of smoking, to be higher on novelty seeking, to be female and to be white rather than Hispanic. Being positive on the conduct disorder screen almost reached significance.
Late DSM onset (Class 4) versus No DSM criteria (Class 1)
Compared with adolescents with no DSM criteria, those with late DSM onset were more likely to have used blunts before the onset of smoking and to be white rather than Hispanic. Earlier onset of smoking almost reached significance.
Early onset/Chronic course (Class 2) versus Early onset/Remission (Class 3)
Compared with adolescents with early onset of DSM followed by remission, adolescents with chronic course were more likely to have one parent who smoked, especially if the parent was ever dependent on nicotine.
Early onset/Chronic course (Class 2) versus late DSM onset (Class 4)
Adolescents with pleasant initial sensitivity to tobacco and those positive on the conduct disorder screen were more likely to experience the Early onset/Chronic course of full DSM than late DSM onset. Parental smoking almost reached statistical significance.
3.3.3 Effects of covariates on growth factors
We were unable to estimate the effects of the covariates on any of the growth factors (i.e. intercept, linear and quadratic terms) either because the models would not converge or the best log likelihood was not replicated even for a single growth factor.
4.0 Conclusion
To the best of our knowledge, this is the first report in the literature of the development of trajectories of DSM-IV criteria for nicotine dependence following the onset of smoking. The data are from a general population sample of new adolescent smokers (mean age 14.0 years at the initial interview), with histories of nicotine dependence symptoms over 36 months following smoking initiation (at mean age of 13.7 years). Growth mixture models were estimated with the inclusion of covariates to reduce model misspecification and to identify the optimum number of trajectory classes. Four classes provided the best fitting model: Class 1, No DSM symptoms (47.7 %); Class 2, Early onset of full DSM/Chronic course (19.8 %); Class 3, Early onset of DSM criteria/Remission (17.3 %); Class 4, Late onset of DSM criteria (15.2 %). The most nicotine dependent state was represented by the Early onset of full DSM/Chronic course trajectory.
Our understanding of the time course of symptoms following the onset of smoking is limited by the 36-month follow-up available to date. Within this limitation, it appears that the trajectory characterized by early onset of full DSM/chronic course experienced full dependence shortly after the onset of smoking and maintained the symptoms for the duration of the follow-up, i.e., as long as three years. The Early onset/Remission class no longer had symptoms after 24 months. The Late onset class developed dependence symptoms 20 months after the onset of smoking. The persistence of nicotine dependence for this class is undetermined.
Compared with those without symptoms, the Early onset/Chronic course trajectory is characterized by blunt use before smoking onset, conduct problems, smoking at least a pack of cigarettes in the prior month and higher pleasant initial sensitivity to tobacco use. The Early onset/Remission class is similarly characterized by higher initial pleasant sensitivity at the first tobacco experience and blunt use prior to the onset of smoking. However, membership in that class is also predicted by higher novelty seeking, being female, and being white rather than Hispanic. One factor differentiates the Early onset/Chronic course from Early onset/Remission, namely parental dependence. The Late onset class is characterized by blunt use prior to smoking initiation and being white. Pleasant initial sensitivity and conduct disorder were higher among smokers assigned to the early chronic class than the late onset class. Unlike prior research on nicotine dependence, this research did not find any multivariate effects of prenatal smoking, depression, or peer smoking on trajectories of dependence.
The predictors of trajectory class, identified through growth mixture modeling of continuous symptom histories, provide novel understanding of the role over time of predictors of the onset of symptoms identified in prior survival analysis (Kandel et al., 2007). Not only does extensiveness of smoking predict earlier onset of symptoms of dependence, as documented by survival analysis, but relatively heavy smoking (for the age group) prior to the first symptom predicts subsequent course and membership in the early onset/chronic class. The other two significant predictors of symptom onset, pleasant initial sensitivity and parental nicotine dependence, predict selected trajectories. Initial sensitivity characterizes those who develop symptoms early whether they experience symptoms chronically or they remit, while parental dependence differentiates adolescents with early onset of symptoms who persist from those who remit. The effects of parental dependence include both a socialization and a genetic component that cannot be differentiated from each other given the present research design. The search for novel experiences as indexed by novelty seeking is associated with transient dependence. This factor may facilitate the initial development of dependence but may not be sufficient to sustain chronicity of use and persistence of dependence following early onset of symptoms. Conduct problems, which were not significant predictors of the onset of dependence in survival analysis with control for other covariates, are highly significant predictors of early and chronic course.
The role of blunts used prior to cigarettes warrants discussion. Twenty percent of blunt users have also used other forms of tobacco, such as pipes or plain cigars. It is striking that blunt use significantly predicts each of the symptomatic trajectories, and especially the two characterized by early onset of symptoms, even with control for extensiveness of cigarette consumption. Since blunts deliver nicotine in large doses, prior blunt smoking would be expected to be associated with nicotine dependence 1. These findings emphasize the role that nicotine consumption and multiple drug use plays in the process of nicotine dependence.
The marginal effect of age of smoking initiation on the late onset trajectory provides potential new insights into the process of nicotine dependence. Younger age of smoking initiation is associated with higher rates of dependence (e.g., Audrain-McGovern et al., 2007; Kandel, 2003). This association is also observed in the total school sample from which the longitudinal cohort was drawn (Data not presented). The data suggest the additional insight that youths who start smoking at an earlier age are more likely to experience dependence after a longer latency than those who start smoking at a later age. Thus, early smoking initiators may be more likely to belong to the late onset trajectory class. Furthermore, there is a suggestion that if those who initiate smoking early also experience symptoms of dependence early, they are less likely to remit. We classified adolescents into three groups according to their age of initiation into smoking; 26.7% of those who started smoking at age 12 or below were in trajectory Class 4, the late onset class, compared with 8.5% of those who started at age 15 or over. By contrast, 6.4%, versus 41.0%, respectively, were classified in Class 3, the Early onset/Remit class. Similarly, we had found earlier in a national sample of adolescents that the younger the age of onset into smoking, the slower the transition to daily smoking. Two years after onset of smoking, half as many adolescents who started smoking at age 10 or younger had become daily smokers as among those who started smoking at ages 15–17 (Kandel, 2003). One interpretation for this finding is that the availability of tobacco and opportunities for smoking are much fewer for younger than older adolescents.
The four trajectories of nicotine dependence are similar to the trajectories commonly observed for extensiveness of smoking (See review above). Typically, these trajectories include a non-smoking class (parallel to the non symptomatic class of dependence), in addition to early-stable, late-stable, and experimenter classes (Brook et al., 2006a,b; Chassin et al., 2000; Orlando et al., 2004). A striking difference between the predictors of trajectories of smoking and those of dependence symptoms is the lack of significance of perceived peer smoking in distinguishing among the later trajectories. Although peer effects reflect both selection and socialization processes, they can be conceptualized as measuring social influence and social reinforcement. The results suggest that the social context of smoking, especially that of same age peers, is less important for dependent smoking than for onset and sustained smoking.
Limitations of the study need to be highlighted. Although based on a longitudinal sample, the reports of symptoms are retrospective for the 6 months intervals between the follow up interviews and may be subject to an unknown degree of unreliability. In addition, the follow-up interval covers only a short period of the natural history of smoking and nicotine dependence. Follow ups over longer intervals over the life course are necessary to obtain a full understanding of the natural history of nicotine dependence. In addition, due to left-censoring, the present sample excluded cases that started smoking earlier than 12 months preceding the initial interview. Thus, the results may not apply to youths who start smoking at younger ages than those represented by the present sample.
However, the findings document clearly that the development of symptoms of nicotine dependence follow alternate trajectories. The predictors of the onset of dependence may differ from those that explain different developmental course over time, which vary by timing of onset, level of escalation, duration, and remission of symptoms. These insights, in turn, make it possible to develop prevention and intervention programs that can be better tailored with respect to the nature of the program, the attributes of the target, and the period in the life cycle when these programs are administered.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional details of the methodology can be obtained with the online version of this paper at http://dx.doi.org by entering doi:xxxxxxxx.
Supplementary material available with the online version of this paper at http://dx.doi.org by entering doi:xxxxxxx)
We are indebted for one of the reviewers of the article for suggesting this interpretation.
References
- Abroms L, Simons-Morton B, Haynie DL, Chen R. Psychosocial predictors of smoking trajectories during middle and high school. Addiction. 2005;100:852–861. doi: 10.1111/j.1360-0443.2005.01090.x. [DOI] [PubMed] [Google Scholar]
- Andreski P, Breslau N. Smoking and nicotine dependence in young adults: Differences between blacks and whites. Drug Alcohol Depend. 1993;32:119–125. doi: 10.1016/0376-8716(93)80004-x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington: American Psychiatric Association; 1994. [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Cuevas J, Rodgers K, Patterson F. Identifying and characterizing adolescent smoking trajectories. Cancer Epidemiol. Biomarkers Prev. 2004;13:2023–2034. [PubMed] [Google Scholar]
- Audrain-McGovern J, Nael AK, Rodriguez D, Wileyto EP, Shields PG. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–e274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- Bardone MS, Moffitt TE, Caspi A, Dickson N, Stanton WR, Silva PA. Adult physical health outcomes of adolescent girls with conduct disorder, depression, and anxiety. J. Am. Child Adolesc. Psychiatry. 1998:595–601. doi: 10.1097/00004583-199806000-00009. [DOI] [PubMed] [Google Scholar]
- Breslau N, Johnson EO, Hiripi E, Kessler RC. Nicotine dependence in the United States. Arch. Gen. Psychiatry. 2001;58:810–816. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence and major depression: new evidence from a prospective investigation. Arch. Gen. Psychiatry. 1993;50:31–35. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. DSM-III-R nicotine dependence in young adults: prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89:743–754. doi: 10.1111/j.1360-0443.1994.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Brook JS, Ning Y, Brook DW. Personality risk actors associated with trajectories of tobacco use. Am. J. Addict. 2006a;15:426–433. doi: 10.1080/10550490600996363. [DOI] [PubMed] [Google Scholar]
- Brook JS, Pahl K, Ning Y. Peer and parental influences on longitudinal trajectories of smoking among African Americans and Puerto Ricans. Nicotine Tob. Res. 2006b;5:639–651. doi: 10.1080/14622200600789627. [DOI] [PubMed] [Google Scholar]
- Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am. J. Psychiatry. 2003;160:1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Reasons for tobacco use and symptoms of nicotine withdrawal among adolescents and young tobacco users: United States, 1993. MMWR Morb. Mortal. Wkly. Rep. 1994;43:745–750. [PubMed] [Google Scholar]
- Chassin LA, Presson CC, Rose JS, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a midwestern community sample: multiple trajectories and their psychosocial correlates. Health Psychol. 2000;19:223–231. [PubMed] [Google Scholar]
- Colby SM, Tiffany ST, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend. 2000;59:S83–S95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Colder CR, Mehta P, Balanda K, Campbell RT, Mayhew KP, Stanton WR, Pentz MA, Flay BR. Health Psychol. 2001;20:127–135. doi: 10.1037//0278-6133.20.2.127. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Merikangas KR, Flaherty BP, Stolar M. Association between psychiatric disorders and the progression of tobacco use behaviors. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:1159–1167. doi: 10.1097/00004583-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Donny E, Tiffany S, Colby SM, Perrine N, Clayton RR. The association between cigarette smoking and DSM-IV nicotine dependence among first year college students. Drug Alcohol Depend. 2007;86:106–114. doi: 10.1016/j.drugalcdep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Ockene JK, McNeill AD, Coleman M, Wood C. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob. Control. 2002;11:228–235. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, O’Loughlin J, Pbert L, Ockene JK, McNeil AD, Hazelton J, Friedman K, Dussault G, Wood C, Wellman RJ. Symptoms of tobacco dependence after brief intermittent use: the development and assessment of nicotine dependence in youth-2 study. Arch. Pediatr. Adolesc. Med. 2007:161–167. doi: 10.1001/archpedi.161.7.704. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Ockene JK, McNeill AD, Coleman M, Wood C. Trait anxiety and nicotine dependence in adolescents: a report from the DANDY study. Addict. Behav. 2004;29:911–919. doi: 10.1016/j.addbeh.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC. Special series: advances in latent variable analysis: applications to clinical research. An introduction to latent growth curve modeling. Behav. Ther. 2004;35-2:333–363. [Google Scholar]
- Elkins IJ, King SM, McGue M, Iacono WG. Personality traits and the development of nicotine, alcohol, and illicit drug disorders: prospective links from adolescence to young adulthood. J. Abnorm. Psychol. 2006;115:26–39. doi: 10.1037/0021-843X.115.1.26. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM. Conduct and attentional problems childhood and adolescence and later substance use, abuse, and dependence: results of a 25-year longitudinal study. Drug Alcohol Depend. 2007;88:14–26. doi: 10.1016/j.drugalcdep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Comorbidity between depressive disorders and nicotine dependence in a cohort of 16-year-olds. Arch. Gen. Psychiatry. 1996;53:1043–1047. doi: 10.1001/archpsyc.1996.01830110081010. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J, Carlson G, Schneider J, Nolan EE, Mattison RE, rundberg-rivera V. A DSM-IV-referenced, adolescent self-report rating scale. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:671–679. doi: 10.1097/00004583-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Gervais A, O’Loughlin J, Meshefedjian G, Bancej C, Tremblay M. Milestones in the natural course of cigarette use onset in adolescents. Can. Med. Assoc. J. 2006;175:255–261. doi: 10.1503/cmaj.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesler PC, Kandel DB, Schaffran C, Hu M-C, Davies M. Inconsistencies in self-reported smoking among adolescents: a comparison of reports in school and in household settings. Public Opinion Quarterly. doi: 10.1093/poq/nfn016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M-C, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am. J. Pub. Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee B, Wittchen H-U, Stein MB, Höfler M, Lieb R. Smoking increases the risk of panic. Arch. Gen. Psychiatry. 2003;60:692–700. doi: 10.1001/archpsyc.60.7.692. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Schulenberg JE. Cojoint developmental trajectories of young adult alcohol and tobacco use. J. Abnorm. Psychol. 2005;114-4:612–626. doi: 10.1037/0021-843X.114.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Wood PK. Trajectories of conjoint substance use disorders: a developmental, typological approach to comorbidity. Alcohol Clin. Exp. Res. 2000;24:902–913. [PubMed] [Google Scholar]
- Kandel DB. In proceedings of Symposium of Addictions: Impact on Canada. Ottawa, Ontario, Canada: Royal Society of Cananda; 2003. The natual history of smoking and nicotine dependence. [Google Scholar]
- Kandel DB, Hu M-C, Greisler PC, Schaffran C. On the development of nicotine dependence in adolescence. Drug Alcohol Depend. 2007;91:26–39. doi: 10.1016/j.drugalcdep.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Schaffran C, Griesler P, Samuolis J, Davies M, Galanti MR. On the measurement of nicotine dependence in adolescence: comparisons of the FTND and a DSM-IV based scale. J. Pediatr. Psychol. 2005;30:319–332. doi: 10.1093/jpepsy/jsi027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp I, O’Loughlin J, Paradis G, Hanley J, DiFranza J. Smoking trajectories of adolescent novice smokers in a longitudinal study of tobacco use. Ann. Epidemiol. 2005;15:445–452. doi: 10.1016/j.annepidem.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Karp I, O’Loughlin J, Hanley J, Tyndale RF, Paradis G. Risk factors for tobacco dependence in adolescent smoking. Tob. Control. 2006;15:199–204. doi: 10.1136/tc.2005.014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb R, Schreier A, Pfister H, Wittchen H-U. Maternal smoking and smoking in adolescents: a prospective community study of adolescents and their mothers. Eur. Addict. Res. 2003;9:120–130. doi: 10.1159/000070980. [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Muthén BO. Latent variable analysis: growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Nebury Park, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- Muthén B, Asparouhov T. Growth mixture modeling: analysis with non-Gaussian random effects. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, editors. Advances in Longitudinal Data Analysis. Chapman & Hall/ CRC Press; 2007. Forthcoming in. [Google Scholar]
- Muthén B, Muthén L. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin. Exp. Res. 2000;24:882–891. [PubMed] [Google Scholar]
- Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Muthén L, Muthén B. Mplus user’s guide. Los Angeles: Author; 1998–2007. [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: a semiparametric group based approach. Psychol. Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- O’Loughlin J, DiFranza J, Tarasuk J, Meshefedjian G, McMillan-Davey E, Paradis G, Tyndale RF, Clarke P, Hanley J. Assessment of nicotine dependence symptoms in adolescents: a comparison of five indicators. Tob. Control. 2002;11:354–360. doi: 10.1136/tc.11.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando M, Tucker JS, Ellickson PL, Klein DJ. Developmental trajectories of cigarette smoking and their correlates from early adolescence to young adulthood. J. Consult. Clin. Psychol. 2004;72:400–410. doi: 10.1037/0022-006X.72.3.400. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways. Addiction. 2005;100:1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Choi WS, Gilpin EA, Farkas AJ. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol. 1996;15:355–361. doi: 10.1037//0278-6133.15.5.355. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Namenek RJ. Early experience with tobacco among women smokers, ex-smokers, and never smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Byrk AS. Hierarchial linear models: Applications and data analysis methods. 2nd ed. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Rhode P, Kahler CW, Lewinsohn PM, Brown RA. Psychiatric disorders, familial factors, and cigarette smoking. Nicotine Tob. Res. 2004;1:119–132. doi: 10.1080/14622200310001656948. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann. Stat. 1978;6:461–464. [Google Scholar]
- Sclove SL. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;52:333–343. [Google Scholar]
- Scragg R, Wellman RJ, Laugensen M, DiFranza JR. Diminished autonomy over tobacco can appear with the first cigarettes. Addict. Behav. 2008;33:689–698. doi: 10.1016/j.addbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willet JB. Applied longitunal data analysis: modeling change and event occurence. New York: Oxford University Press; 2003. [Google Scholar]
- Sonntag H, Wittchen H-U, Höfler M, Kessler RC, Stein MB. Are social fears and DSM-IV social anxiety disorder associated with smoking and nicotine dependence in adolescents and young adults? Eur. Psychiatry. 2000;15:67–74. doi: 10.1016/s0924-9338(00)00209-1. [DOI] [PubMed] [Google Scholar]
- Sledjeski EM, Dierker LC, Costello D, Shiffman S, Donny E, Flay BR, Tobacco Etiology Research Network (TERN) Predictive validity of four nicotine dependence measures in a college sample. Drug Alcohol Depend. 2007;87:10–19. doi: 10.1016/j.drugalcdep.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Stanton WR, Flay BR, Colder CR, Mehta P. Identifying and predicting adolescent smokers’ developmental trajectories. Nicotine Tob. Res. 2004;6:843–852. doi: 10.1080/14622200410001734076. [DOI] [PubMed] [Google Scholar]
- Storr CL, Reboussin BA, Anthony JC. Early childhood misbehavior and the estimated risk of becoming tobacco-dependent. Am. J. Epidemiol. 2004;160:126–130. doi: 10.1093/aje/kwh184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaro F, Wanner B, Brendgen M, Gosselin C, Gendreau PL. Differential contribution of parents and friends to smoking trajectories during adolescence. Addict. Behav. 2004;29:831–835. doi: 10.1016/j.addbeh.2004.02.018. [DOI] [PubMed] [Google Scholar]
- White HR, Johnson V, Buyske S. Parental modeling and parenting behavior effects on offspring alcohol and cigarette use. A growth curve analysis. J. Subst. Abuse. 2000;12:287–310. doi: 10.1016/s0899-3289(00)00056-0. [DOI] [PubMed] [Google Scholar]
- White HR, Pandina RJ, Chen P-H. Developmental trajectories of cigarette use from early adolescence into young adulthood. Drug Alcohol Depend. 2002;65:167–178. doi: 10.1016/s0376-8716(01)00159-4. [DOI] [PubMed] [Google Scholar]
- White HR, Nagin D, Replogle E, Stouthamer-Loeber M. Racial differences in trajectories of cigarette use. Drug Alcohol Depend. 2004;76:219–227. doi: 10.1016/j.drugalcdep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Wills TA, Windle M, Cleary SD. Temperament and novelty seeking in adolescent substance sue: convergence of dimensions of temperament with constructs from Coloninger’s theory. Pers. Soc. Psychol. 1998;74:387–406. doi: 10.1037//0022-3514.74.2.387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.