Allylic alcohols are integral subunits of a variety of biologically interesting natural products as well as key building blocks for a number of important synthetic transformations. Among the numerous strategies for the preparation of allylic alcohols, the reductive coupling of aldehydes and alkynes in either an inter- or intramolecular sense arguably provides the most direct access to this important substructure from simple precursors.1,2 Whereas several asymmetric approaches to the reductive coupling of aldehydes and alkynes have been reported,3 our recent studies involving the use of achiral N-heterocyclic carbene complexes of nickel illustrated several important features including broad scope with both internal and terminal alkynes, direct incorporation of a silyl protecting group, and the ability to tune alkyne regioselection in macrocyclizations based on ligand sterics.4 In order to capitalize upon these advantages, we have now examined the asymmetric coupling of aldehydes and alkynes using chiral N-heterocyclic carbene complexes.
Pioneering studies from Grubbs illustrated that N-heterocyclic carbenes derived from C-2 symmetric diamines and mono-ortho-substituted aryl halides were excellent participants in asymmetric ring-closing metathesis reactions.5 Members of this structural class of N-heterocyclic carbenes appeared to be promising candidates for asymmetric nickel-catalyzed reductive couplings. We thus examined the reductive coupling of benzaldehyde and 1-phenylpropyne under a variety of conditions to provide a lead ligand structure for further optimization. The known N-heterocyclic carbene ligands, generated in situ from 1a and 1b in THF with KO-t-Bu, allowed the production of the desired protected allylic alcohol 2a in modest yield and poor enantioselectivity (Table 1). New ligands 1c and 1d, which incorporate an ortho-phenyl or ortho-cyclohexyl substituent, were then prepared from the commercially available aryl bromides. A reaction involving ligand 1c afforded product 2a with slightly improved enantioselectivity, whereas a reaction with ligand 1d proceeded with significantly improved enantioselectivity, affording compound 2a in 76% ee in 60% yield. Despite the encouraging enantioselectivity with ligand 1d, examination of additional starting material combinations illustrated that yields were often poor to modest. Given the requirement of steric hindrance to stabilize free N-heterocyclic carbenes (by preventing dimerization), we next considered ortho,ortho-disubstituted carbene ligands. Ligand 1e was thus prepared, which did indeed allow improved chemical yields but with low enantioselectivities. Recognizing that ortho,ortho-disubstitution was optimal from the standpoint of chemical yield, whereas steric differentiation of the two ortho substituents was optimal from the standpoint of enantioselectivity, we next prepared ligand 1f. Under the same conditions described for the above experiments, chemical yields in couplings of benzaldehyde and 1-phenylpropyne improved to 98% with lower catalyst loading (2 mol%) in 78% ee. Whereas ligand 1f was primarily designed for enantioselectivity optimization, the catalytic activity of the nickel catalyst derived from this ligand surprisingly exceeded that of the commonly employed IMes and IPr N-heterocyclic carbene ligands in catalytic aldehyde/alkyne reductive couplings. We therefore anticipate that this new ligand may be useful in various metal-catalyzed5,6 or organocatalytic7 processes that rely on N-heterocyclic carbene species.
Table 1.
Examination of Ligand Structure.a
 |
Below each structure in the Table is the given the compound number of the imidazolium salt, % yield for the production of 2 using the ligand, and the % ee (in parentheses) of compound 2 produced with the ligand.
bThis entry employed 2 mol % of 1f, KO-t-Bu, and Ni(COD)2.
Upon identifying the excellent catalytic activity and promising enantioselectivity in a reaction with the catalyst derived from ligand 1f, we sought to explore its generality across a range of substrates. As illustrated (Table 2), the yields and enantioselectivities are relatively uniform across a broad range of substrates. Key functional groups cleanly tolerated in the procedure include aromatic as well as branched and unbranched aldehydes, internal alkynes that either possess or lack an aromatic substituent, terminal alkynes, and unprotected alcohols, wherein the trialkylsilyl group is regioselectively installed on the newly formed hydroxyl. Regioselection of alkyne insertion is high with the exception of internal alkynes that possess two aliphatic substituents (entries 4 and 11). Notably, the regioselectivity in one of these cases was found to undergo reversal with ligand 1d (compare entries 11 and 12). Therefore, ligand 1d may be useful in some applications due to this complementary regioselection. The reversal of regioselectivity is consistent with the steric-based model for regioselectivity reversal proposed in macrocyclizations involving achiral ligands.4b
Table 2.
Scope of Asymmetric Couplings.
 | |||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | % Yield (% ee)a | Regiosel. |
| 1 | Ph | Me | Ph | 98 (78)b | 10:1 |
| 2 | Ph | Et | Et | 82 (70) | -- |
| 3 | i-Pr | Me | Ph | 86 (70) | >19:1 |
| 4 | i-Pr | (CH2)3Ph | Me | 86 (75) | 3:1 |
| 5 | Cy | Et | Et | 84 (85)c | -- |
| 6 | (CH2)2Ph | Et | Et | 75 (78) | -- |
| 7 | Cy | Me | Ph | 78 (81) | >19:1 |
| 8 | Cy | H | n-hex | 64 (65) | >19:1 |
| 9 | n-hex | Me | Ph | 70 (73) | 10:1 |
| 10 | Cy | (CH2)4OH | Ph | 99 (79) | 9:1 |
| 11 | Cy | n-pent | Me | 79 (76) | 3:1e |
| 12 | Cy | Me | n-pent | 47 (79)d | 6:1 |
% Ee is given for the major regioisomer.
2 Mol% of 1f, Ni(COD)2, and KO-t-Bu were used.
Ligand (S,S)-1f, was used, and the enantiomer of the configuration shown for product 2 was obtained.
Ligand 1d was used.
Minor regioisomer of entry 11 is of the (S) configuration produced in 76% ee.
Given that macrocyclizations of ynal substrates provide an important entry to substructures found in many bioactive natural products, this new procedure was applied in an asymmetric macrocyclization of ynal 3 (eq 1). In this example, 14-membered macrocycle 4a and 13-membered macrocycle 4b were produced in 76% combined yield as an 86:14 mixture of regioisomers (79% ee for (S)-4a and 42% ee for (S)-4b). Notably, the regioselection is reversed in comparison to intermolecular examples (Table 2, entries 4 and 11), illustrating that ring size is a factor in determining regioselectivity.

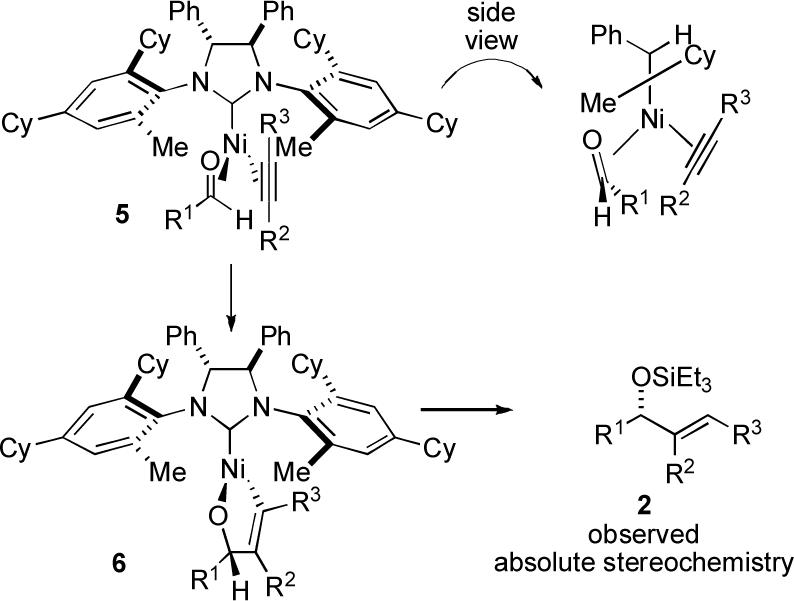
In analogy to the proposal from Grubbs in asymmetric ring-closing metathesis reactions involving members of the ligand class 1,5 we propose that the reaction proceeds via generation of a three coordinate complex 5 (Scheme 1).8 Tilting of the N-aryl ring of 5 relative to the imidazolidine ring would position the ortho-cyclohexyl substituent anti to the backbone phenyl group and distal to nickel as depicted. This orientation would then position the ortho-methyl substituent syn to the backbone phenyl group and proximal to nickel. It is the ortho-methyl substituent that thus dictates the selectivity of aldehyde binding according to this model. Oxidative cyclization of structure 5 to metallacycle 6 would then lead to the formation of 2, which is the major enantiomer observed.9
Scheme 1.
Model for Enantioselection.
In summary, an efficient approach to synthesis of allylic alcohols involving the catalytic asymmetric coupling of aldehydes and alkynes has been developed. A new chiral N-heterocyclic carbene ligand was prepared that provides improved reaction efficiencies and enantioselectivities compared with known, structurally-related N-heterocyclic carbene ligands. Although prior studies established good to excellent enantioselectivities with specific substrate combinations,3 the simple experimental protocol (fast reactions at rt with a stable reducing agent) provides significant preparative advantages of this new procedure, and the range of participating substrates is the broadest of any single method to date. The development of new generations of ligands, synthetic applications, and mechanistic studies are in progress.
Supplementary Material
Acknowledgment
The authors wish to acknowledge receipt of NIH grant GM57014 in support of this work. Dr. Scott Bader is kindly acknowledged for helpful suggestions.
Footnotes
Supporting Information Available: Full experimental details and copies of NMR spectral data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1. For nickel-catalyzed aldehyde / alkyne reductive couplings or cyclizations. [Google Scholar]; a Oblinger E, Montgomery J. J. Am. Chem. Soc. 1997;119:9065. [Google Scholar]; b Tang X-Q, Montgomery J. J. Am. Chem. Soc. 1999;121:6098. [Google Scholar]; c Tang X-Q, Montgomery J. J. Am. Chem. Soc. 2000;122:6950. [Google Scholar]; d Huang W-S, Chan J, Jamison TF. Org. Lett. 2000;2:4221. doi: 10.1021/ol006781q. [DOI] [PubMed] [Google Scholar]; e Takai K, Sakamoto S, Isshiki T. Org. Lett. 2003;5:653. doi: 10.1021/ol0272996. [DOI] [PubMed] [Google Scholar]; f Montgomery J. Angew. Chem. Int. Ed. 2004;43:3890. doi: 10.1002/anie.200300634. For a review, see. [DOI] [PubMed] [Google Scholar]
- 2. For aldehyde / alkyne reductive couplings using other metals. [Google Scholar]; a Srebnik M. Tetrahedron Lett. 1991;32:2449. [Google Scholar]; b Kataoka Y, Miyai J, Oshima K, Takai K, Utimoto K. J. Org. Chem. 1992;57:1973. [Google Scholar]; c Wipf P, Xu W. Tetrahedron Lett. 1994;35:5197. [Google Scholar]; d Crowe WE, Rachita MJ. J. Am. Chem. Soc. 1995;117:6787. [Google Scholar]; e Chen YK, Walsh PJ. J. Am. Chem. Soc. 2004;126:3702. doi: 10.1021/ja0396145. [DOI] [PubMed] [Google Scholar]; f Bahadoor AB, Flyer A, Micalizio GC. J. Am. Chem. Soc. 2005;127:3694. doi: 10.1021/ja050039+. [DOI] [PubMed] [Google Scholar]
- 3.a Oppolzer W, Radinov RN. Helv. Chem. Acta. 1992;75:170. [Google Scholar]; b Oppolzer W, Radinov RN. J. Am. Chem. Soc. 1993;115:1593. [Google Scholar]; c Oppolzer W, Radinov RN, El-Sayed E. J. Org. Chem. 2001;66:4766. doi: 10.1021/jo000463n. [DOI] [PubMed] [Google Scholar]; d Wipf P, Ribe S. J. Org. Chem. 1998;63:6454. [Google Scholar]; e Miller KM, Huang W-S, Jamison TF. J. Am. Chem. Soc. 2003;125:3442. doi: 10.1021/ja034366y. [DOI] [PubMed] [Google Scholar]; f Colby EA, Jamison TF. J. Org. Chem. 2003;68:156. doi: 10.1021/jo0264123. [DOI] [PubMed] [Google Scholar]; g Komanduri V, Krische MJ. J. Am. Chem. Soc. 2006;128:16448. doi: 10.1021/ja0673027. [DOI] [PubMed] [Google Scholar]; h Kong J-R, Ngai M-Y, Krische MJ. J. Am. Chem. Soc. 2006;128:718. doi: 10.1021/ja056474l. [DOI] [PubMed] [Google Scholar]; i Rhee JU, Krische MJ. J. Am. Chem. Soc. 2006;128:10674. doi: 10.1021/ja0637954. [DOI] [PubMed] [Google Scholar]
- 4.a Mahandru GM, Liu G, Montgomery J. J. Am. Chem. Soc. 2004;126:3698. doi: 10.1021/ja049644n. [DOI] [PubMed] [Google Scholar]; b Knapp-Reed B, Mahandru GM, Montgomery J. J. Am. Chem. Soc. 2005;127:13156. doi: 10.1021/ja054590i. [DOI] [PubMed] [Google Scholar]
- 5.a Seiders TJ, Ward DW, Grubbs RH. Org. Lett. 2001;3:3225. doi: 10.1021/ol0165692. [DOI] [PubMed] [Google Scholar]; b Funk TW, Berlin JM, Grubbs RH. J. Am. Chem. Soc. 2006;128:1840. doi: 10.1021/ja055994d. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Berlin JM, Goldberg SD, Grubbs RH. Angew. Chem. Int. Ed. 2006;45:7591. doi: 10.1002/anie.200602469. [DOI] [PubMed] [Google Scholar]
- 6.a Herrmann WA. Angew. Chem. Int. Ed. 2002;41:1290. doi: 10.1002/1521-3773(20020415)41:8<1290::aid-anie1290>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]; b Perry MC, Burgess K. Tetrahedron Asymm. 2003;14:951. [Google Scholar]
- 7.Marion N, Díez-González S, Nolan SP. Angew. Chem. Int. Ed. 2007;46:2988. doi: 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]
- 8.Dorta R, Stevens ED, Scott NM, Costabile C, Cavallo L, Hoff CD, Nolan SD. J. Am. Chem. Soc. 2005;127:2485. doi: 10.1021/ja0438821. Trigonal Ni(CO)2(NHC) complexes have been fully characterized. [DOI] [PubMed] [Google Scholar]
- 9. Absolute stereochemistry of twelve of the fourteen examples (Table 1 and eq 1) were established by Mosher's ester analysis. See supporting information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


