Abstract
An influential idea about memory and medial temporal lobe function suggests that hippocampal activity predicts subsequent recognition success only when decisions are based on recollection, whereas perirhinal activity predicts subsequent recognition success when decisions are based on familiarity. An alternative idea is that hippocampal and perirhinal activity are both sensitive to the level of overall memory strength. Using functional magnetic resonance imaging, we have tested the relationship between brain activity during learning and subsequent memory strength. Activity in a number of cortical regions (including regions within what has been termed the default network) was negatively correlated with subsequent memory strength, suggesting that this activity reflects inattention or mind wandering (and, as a result, poor memory). In contrast, activity in both hippocampus and perirhinal cortex positively correlated with the subsequent memory strength of remembered items. This finding suggests that both structures cooperate during learning to determine the memory strength of what is being learned and that there is not a sharp distinction between these structures with respect to recollection and familiarity.
Keywords: fMRI, hippocampus, memory, encoding, familiarity, recollection
Declarative memory depends on the hippocampus and adjacent medial temporal lobe structures (entorhinal, perirhinal, and parahippocampal cortices) (Squire et al., 2004). One of the most widely studied examples of declarative memory is recognition – that is, the ability to judge a recently encountered item as having been presented previously. Patients with damage to the medial temporal lobe, and even patients with damage limited to the hippocampus, have impaired recognition memory (Kopelman et al., 2007; Levy et al., 2004; Manns et al., 2003; Wais et al., 2006).
Functional magnetic resonance imaging (fMRI) has also been used to study recognition memory (Eichenbaum et al., 2007; Squire et al., 2007). In the subsequent memory paradigm (Paller and Wagner, 2002), participants study a list of items in the scanner and then later take a recognition memory test outside of the scanner. Activity associated with items that will later be remembered is then compared to activity associated with items that will later be forgotten. Because the hippocampus is necessary for recognition memory (as patients with hippocampal lesions have demonstrated), one might expect to find hippocampal activity during learning that is predictive of subsequent recognition. Yet, such hippocampal activity has been found only in some studies. The studies that have found hippocampal activity to be predictive of subsequent memory have typically involved tests of source memory or associative memory, not simple tests of recognition based on old/new judgments. For example, in studies of source memory, hippocampal activity was greater during learning when an item was later remembered together with some additional information about the study episode (e.g., the item was printed in red, not green) than when the item was later forgotten or remembered without source information (e.g., Davachi et al., 2003; Kensinger and Schacter, 2006; Ranganath et al., 2004, but see Gold et al., 2006).
A common interpretation of these findings is that hippocampal activity predicts subsequent recognition only when decisions are based on recollection and not when decisions are based on familiarity (Brown and Aggleton, 2001; Eichenbaum et al., 2007). Recollection and familiarity are two component processes thought to underlie recognition memory (Mandler, 1980). Recollection involves remembering specific contextual details about a prior learning episode (for example, source information). Familiarity involves knowing that an item was presented without having available any additional information about the learning episode. Whereas the hippocampus has been proposed to be important for recognition decisions based on recollection, the perirhinal cortex has been proposed to be important for recognition decisions based on familiarity (Brown and Aggleton, 2001; Eichenbaum et al., 2007).
Yet an alternative interpretation of these same findings is that hippocampal activity and perirhinal activity are sensitive to the level of overall memory strength. Recollection-based decisions and familiarity-based decisions generally reflect strong memories and weak memories, respectively (Squire et al., 2007; Wixted, 2007). For example, items that are recognized and also accompanied by source information typically reflect stronger memories than items that are recognized but not accompanied by source information (Gold et al., 2006; Slotnick and Dodson, 2005). If this idea has merit, then activity in hippocampus during learning, as well as activity in perirhinal cortex, should under appropriate conditions correlate positively with subsequent memory strength. This should occur even for simple tasks of recognition memory that involve no explicit recollective component and where participants make judgments of memory strength but make no distinction between recollection-based and familiarity-based decisions.
A different expectation about the relationship between brain activity and subsequent memory strength arises from findings in cortical regions (including prefrontal cortex, medial parietal cortex (posterior cingulate / precuneus), and inferior parietal cortex) of higher activity for subsequently forgotten information than for subsequently remembered information (Daselaar et al., 2004; Otten and Rugg, 2001b; Reynolds et al., 2004; Wagner and Davachi, 2001). Some of these regions are a part of what has been termed the default network, which comprises the medial prefrontal cortex, posterior cingulate and retrosplenial cortex, and inferior parietal lobe, as well as medial temporal lobe structures (Binder et al., 1999; Buckner et al., 2008; Gusnard et al., 2001; Gusnard and Raichle, 2001; Raichle et al., 2001). It has been suggested that activity in some of these brain regions, as well as other regions, signals mind-wandering or inattention (Mason et al., 2007; Weissman et al., 2006). From these findings, one might expect that activity within a number of regions, including but not limited to regions identified with the default network, might correlate negatively with subsequent memory strength.
We tested with fMRI how brain activity relates to subsequent memory strength, first examining the whole brain and then focusing on the medial temporal lobe. Participants were scanned as they studied a list of words, and then were given a test of recognition memory outside the scanner. To indicate memory strength, participants assigned confidence ratings to old and new words on a 6-point scale. They did not make any additional judgment as to whether their decision was based on recollection or familiarity.
Results
Behavioral Performance
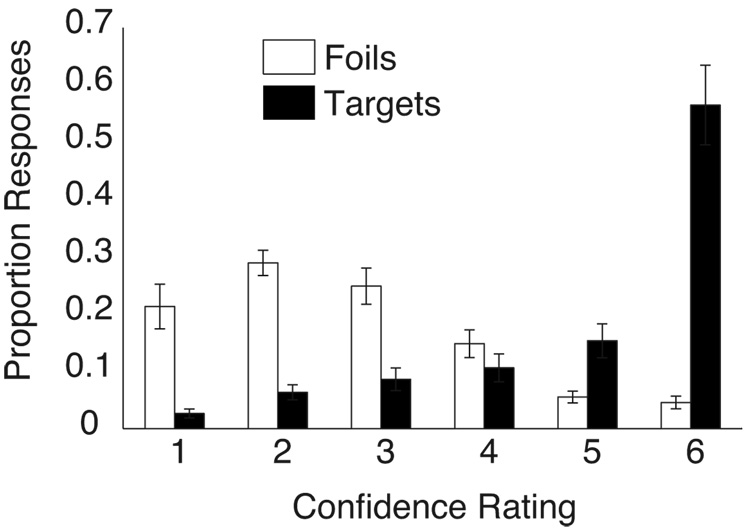
The distribution of responses on the recognition memory test (Figure 1) is presented in Figure 2. Overall, participants scored 78.8 ± 9.6% correct (hit rate = 82.4 ± 12.7%; false alarm rate = 24.9 ± 10.6%; d' = 1.79 ± 0.72). Study words were back-sorted according to the memory confidence rating subsequently assigned to each word on the recognition memory test. Despite a high overall miss rate on the recognition memory test, there were relatively few high-confidence miss trials (mean = 9.4, range = 0–31). Accordingly, we combined memory strengths 1 and 2 into a single memory strength bin for fMRI analyses. This procedure resulted in a mean of 32.0 ± 6.5, 30.6 ± 7.1, 37.8 ± 8.6, 54.8 ± 10.6, and 202.4 ± 25.1 words in memory strength bins 1&2, 3, 4, 5, and 6, respectively. Mean reaction times (RTs) during the study trials were 915 ± 52, 956 ± 48, 980 ± 50, 1005 ± 49, and 1046 ± 40 ms for memory strengths 1&2, 3, 4, 5 and 6, respectively. Longer RTs at study were associated with higher memory strengths at test (linear trend, F(1,11) = 4.93, p < 0.05).
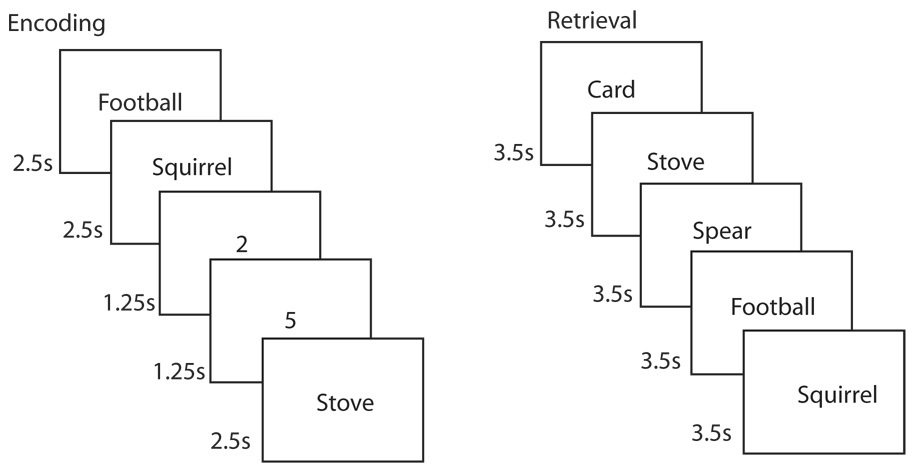
Figure 1.
Recognition memory task. In the scanner, participants rated (pleasant/unpleasant) 360 words (2.5 s/word). Words were intermixed with 864 1.25-s baseline trials in which participants indicated whether a digit was odd or even. At test (about 15 min later, out of the scanner), participants made confidence ratings (1–6, 1 = “definitely new”, 6 = “definitely old”) for the 360 studied words and 360 novel words (3.5 s/word).
Figure 2.
Behavioral performance. Proportion of targets (black bars) and foils (white bars) endorsed at each confidence level (1 = “definitely new” and 6 = “definitely old”). Error bars indicate SEM.
fMRI Results
First, we identified regions in which activity varied, either positively or negatively, during study as a function of subsequent memory strength. Specifically, we conducted a linear trend analysis on the whole brain data with the coefficients −2, −1, 0, 1, and 2 assigned to memory strengths 1&2, 3, 4, 5 and 6, respectively. The resulting statistical map was thresholded at a voxel-wise p-value of p < 0.002 and a spatial-extent threshold of 32 contiguous voxels (256 mm3, p < 0.005). Table 1 lists regions that demonstrated a significant linear trend relating fMRI activity at study to memory strength at test. In each case, fMRI activity and memory strength were negatively correlated. That is, fMRI activity was higher in these functionally defined regions during presentation of words that would subsequently be forgotten than during presentation of words that would subsequently be remembered.
Table 1.
Regions where activity during learning correlated negatively with subsequent memory strength (confidence ratings 1 – 6).
| Peak activation | Linear trend | |||||
|---|---|---|---|---|---|---|
| (ROI #) | Region | X | Y | Z | F value | |
| 1 | R | Inferior parietal cortex | 57 | −43 | 14 | 27.3 |
| 2 | R | Posterior prefrontal cortex | 37 | 31 | 32 | 39.4 |
| 3 | R | Anterior prefrontal cortex | 29 | 59 | 16 | 37.7 |
| 4 | B | Medial parietal cortex | −1 | −59 | 42 | 18.5 |
| 5 | L | Inferior parietal cortex | −57 | −45 | 38 | 20.5 |
| 6 | L | Superior parietal cortex | −47 | −57 | 46 | 23.5 |
| 7 | L | Superior temporal gyrus | −43 | −53 | 18 | 22.3 |
| 8 | R | Middle frontal gyrus | 43 | 49 | 14 | 19.6 |
| 9 | R | Precentral gyrus | 51 | 11 | 4 | 20.0 |
| 10 | R | Superior parietal cortex | 43 | −47 | 52 | 18.0 |
| 11 | R | Superior parietal cortex | 35 | −41 | 60 | 17.5 |
| 12 | L | Lateral temporal cortex | −47 | −19 | −10 | 17.2 |
| 13 | R | Precentral gyrus | 39 | −17 | 58 | 16.3 |
| 14 | R | Superior frontal gyrus | 17 | 63 | 22 | 19.2 |
Note: all df for the linear trend = (1,65). All p’s < 0.001.
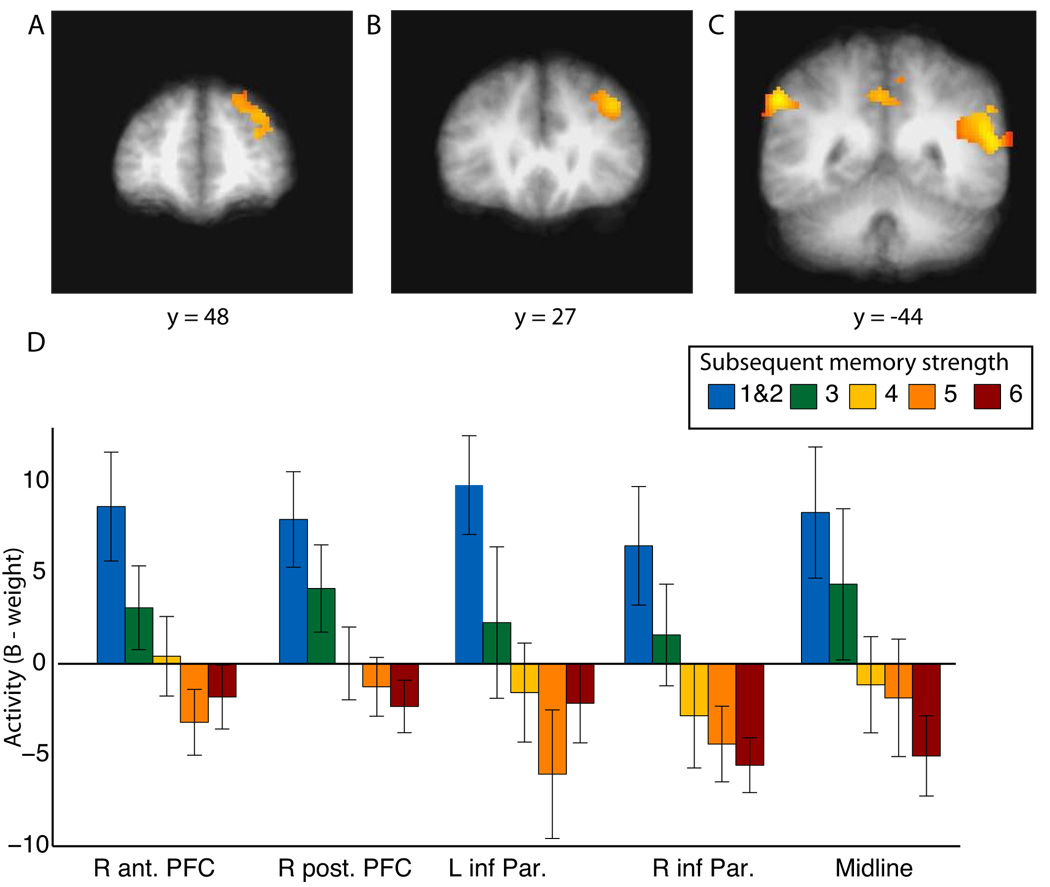
Figure 3A–C shows the five largest regions: right anterior prefrontal cortex, right posterior prefrontal cortex, bilateral inferior parietal cortex, and a posterior midline cortical region. Figure 3D shows the activity associated with each memory strength for each of these five functionally defined regions. Previous studies have also observed this inverse subsequent memory effect (i.e., greater activity during study for subsequently forgotten items than subsequently remembered items) in these same regions or closely adjacent regions: dorsolateral prefrontal cortex, posterior midline regions, and inferior parietal cortex (Daselaar et al., 2004; Otten and Rugg, 2001b; Reynolds et al., 2004; Wagner and Davachi, 2001). Some of these regions (bilateral inferior parietal cortex and the posterior midline) have been identified as belonging to the “default network,” i.e., a set of brain regions that are active during rest conditions (Gusnard et al., 2001; Gusnard and Raichle, 2001; Raichle et al., 2001). Related to this idea, activity in these and other regions has also been found to correlate with mind wandering (Mason et al., 2007) and lapses of attention (Weissman et al., 2006). Thus, the increased fMRI activity associated with subsequently forgotten words may represent task-irrelevant mental activity that leads to encoding failures.
Figure 3.
Regions where activity correlated negatively with subsequent memory strength. A linear trend in fMRI activation that predicted subsequent memory strength was observed in (A) right anterior prefrontal cortex (R ant. PFC), (B) right posterior prefrontal cortex (R post. PFC), (C) bilateral inferior parietal cortex (L inf. Par. and R inf. Par.), and medial parietal cortex (midline). Regions of activation are shown superimposed on the averaged T1-weighted scans of all 14 participants. (D) Activation in each of the five regions as a function of the subsequent strength of recognition memory. Error bars indicate SEM.
Also in the whole brain, several regions exhibited activity that was higher for the study task than for the baseline task and also varied positively with subsequent memory strength (thresholded at a voxel-wise p-value of 0.005 and a spatial-extent threshold of 38 continuous voxels; 304mm3, p < 0.005, see Supplemental Table). Among these regions was the left inferior frontal gyrus. This finding is consistent with previous reports that the left inferior frontal gyrus exhibits higher activity during orienting tasks that predict subsequent memory success and that activity is also higher in this region for subsequently remembered items than for subsequently forgotten items (e.g., Reynolds et al., 2004; Wagner et al., 1998).
Next, we identified regions in the medial temporal lobe where there was significant activity associated with the experimental task and also where activity predicted subsequent memory. Thus, we identified the overlapping regions that emerged from two separate analyses (i.e., we carried out a conjunction analysis, sometimes termed “inclusive mask”; Cabeza et al., 2004; Weissman et al., 2006). In the first analysis, we identified regions of the medial temporal lobe where activity associated with the study task was different than activity associated with the baseline (digit) task. In the second analysis, we identified regions where activity varied with the memory strength of subsequently remembered items. Initially, we looked for regions where activity varied across all memory strengths but did not find any regions where the relationship was positive. We then reasoned that participants might not have been fully engaged in the study task in the case of items that were subsequently forgotten (items with memory strengths 1&2 or 3) and were instead engaged in task-irrelevant activity. Indeed, this reasoning was consistent with the finding that activity in a number of regions, including regions where activity has been related to inattention and mind wandering, was high for the subsequently forgotten items. Accordingly, we next restricted our analysis to subsequently remembered items (items with memory strengths of 4, 5, or 6). Specifically, we conducted a linear trend analysis on the medial temporal lobe data for the subsequently remembered items using coefficients −1, 0, and 1 for memory strengths 4, 5, and 6, respectively.
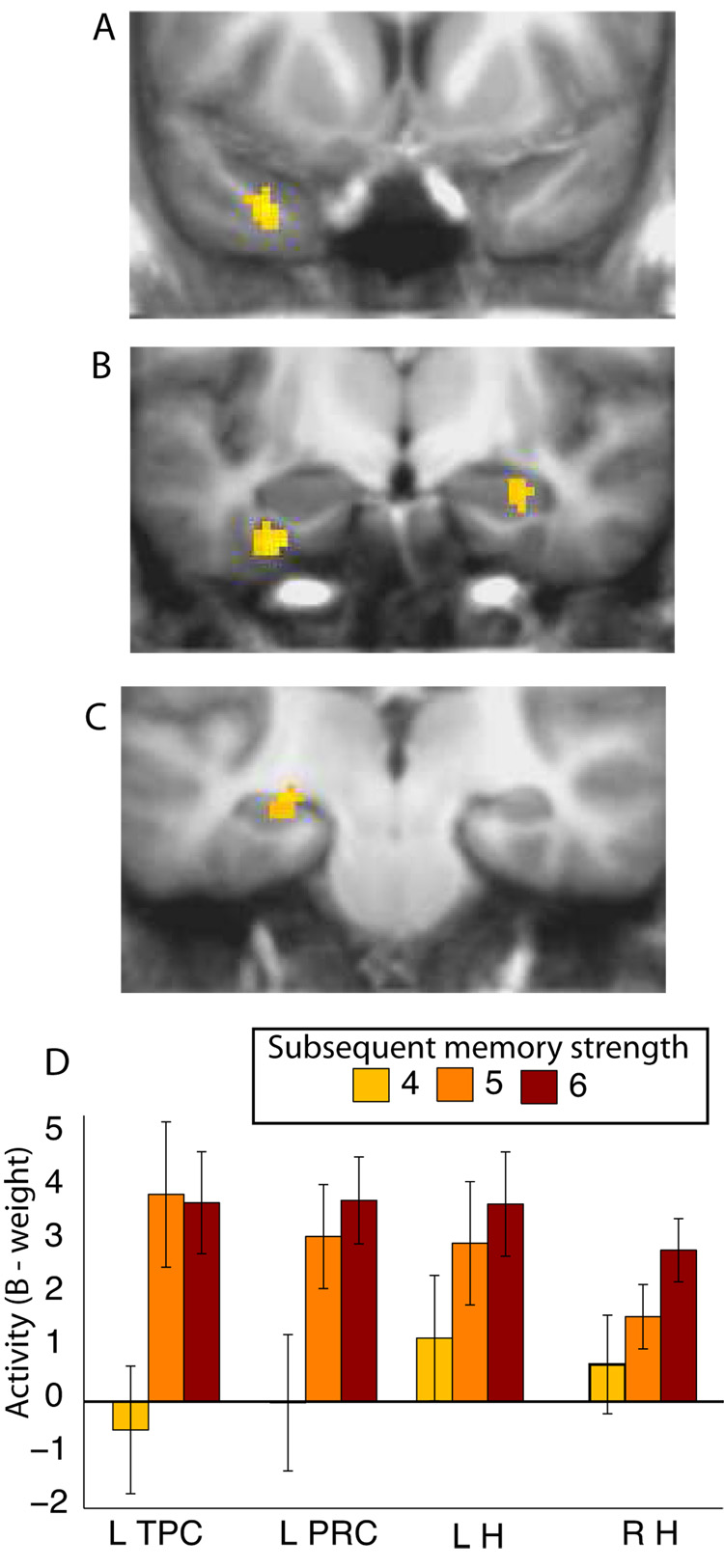
The overlapping regions that resulted from these two analyses yielded a statistical map that we thresholded at a voxel-wise p-value of p < 0.005 and a spatial-extent threshold of 10 contiguous voxels (80 mm3, p < 0.005). Table 2 lists the four regions within the medial temporal lobe that resulted from this procedure: left temporopolar cortex, left perirhinal cortex, right anterior hippocampus, and left hippocampus (Figure 4A–C). These functionally defined regions exhibited activity during learning that was positively correlated with subsequent memory strength (Figure 4D). An ANOVA revealed no Region X Memory Strength interaction (F(6,78) = 1.3, p > 0.2), indicating that the relationship between memory strength and activity was similar across regions. Two additional ANOVAs, comparing the left or right hippocampus with left perirhinal cortex, also revealed no difference in the relationship between activity and memory strength (Fs < 1.5, ps > 0.2). Time courses of the activity in each of the four regions are presented in Supplemental Figure 1.
Table 2.
Regions in the medial temporal lobe where activity during learning correlated positively with the memory strength of subsequently remembered items (confidence ratings 4 – 6).
| Peak activation | Linear trend | ||||
|---|---|---|---|---|---|
| Region | X | Y | Z | F value | |
| L | Temporopolar cortex | −33 | 8 | −30 | 11.9 |
| L | Perirhinal cortex | −31 | −9 | −26 | 9.8 |
| L | Hippocampus | −25 | −21 | −8 | 12.0 |
| R | Hippocampus | 26 | −11 | −14 | 8.6 |
Note: all df for the linear trend = (1,39). All p’s < 0.01.
Figure 4.
fMRI activation in the medial temporal lobe as a function of subsequent memory strength. Activation in (A) left temporopolar cortex (L TPC), (B) left perirhinal cortex (L PRC), right hippocampus (R H), and (C) left hippocampus (L H) varied as a function of the subsequent strength of remembered items (4, 5, or 6). Regions of activation are shown superimposed on the averaged T1-weighted scans of all 14 participants. (D) Activation in each of the four regions as a function of the subsequent strength of remembered items. Error bars indicate SEM.
To explore further the relationship between brain activity and memory strength in these four medial temporal lobe regions, we also extracted for each region the signal for subsequent memory strengths 1&2 and 3 (Supplemental Figure 2). In all four regions, activity was numerically lowest for items receiving a subsequent rating of 4. And in three of the four regions, the numerical values of the five memory strengths (1&2, 3, 4, 5, 6) conformed to a U-shape, such that activity was numerically highest for items receiving a subsequent rating of 1&2 or 6, numerically lower for items receiving a subsequent rating of 3 or 5, and numerically lowest for items receiving a subsequent rating of 4.
Discussion
We measured brain activity with fMRI during an incidental learning task and later collected confidence judgments during a post-scan recognition memory test. There were two main findings. First, in regions within what has been termed the default network, as well as in other regions, activity negatively correlated with subsequent memory strength. Second, in the medial temporal lobe, activity in both the hippocampus and perirhinal cortex positively correlated with the subsequent memory strength of remembered items.
The finding that activity in prefrontal cortex, inferior parietal cortex, and the posterior midline (which include regions of the default network) decreased with increasing subsequent memory strength (Figure 3) is consistent with previous fMRI reports. Thus, in earlier studies, activity in these structures for items that were subsequently forgotten was greater than for items that were subsequently remembered with high confidence (Daselaar et al., 2004; Otten and Rugg, 2001b). We have extended these results by showing that activity was negatively associated with subsequent memory across five levels of memory strength. The default network was originally identified as consisting of areas that were more active during resting states than during cognitive tasks of interest (Gusnard et al., 2001; Gusnard and Raichle, 2001; Raichle et al., 2001; Shulman et al., 1997). Interestingly, activity within these regions, as well as other regions, was subsequently linked to momentary lapses in attention and mind wandering (Mason et al., 2007; Weissman et al., 2006). Our finding of a negative association between subsequent memory strength and activity in default network structures as well as other regions, is consistent with these ideas. Participants may have varied from trial to trial in how attentive they were to the words being presented, and this variation affected how successful they later were at recognizing the words.
The second finding was that activity in hippocampus, perirhinal cortex, and temporopolar cortex increased with the subsequent memory strength of remembered items (i.e., items with memory strengths of 4, 5, or 6) (Figure 4). Interestingly, even though activity in these structures did increase during learning in relation to the memory strength of subsequently remembered items, this activity was no higher than activity associated with subsequently forgotten items (i.e., items with memory strengths of 1&2 and 3). Indeed, the activity across all five memory strengths tended toward a U-shaped function (Supplemental Figure 2).
We suggest that the U-shaped pattern of activity in these structures reflects variation in attention to the study words at the time of word presentation. Thus, on trials where the study words were later least well remembered (i.e., study words later given ratings of 1&2), the high activity in the medial temporal lobe may indicate that participants gave strong attention to, and subsequently would have had good memory for, mental activity unrelated to the word task, and/or that participants were retrieving task-irrelevant information from memory. Correspondingly, on trials where the study words were later best remembered (i.e., study words later given a rating of 6), high activity in the medial temporal lobe indicates that participants gave strong attention to, and subsequently had good memory for, the study words themselves.
These ideas lead one to expect that in regions where fMRI activity decreased with increasing subsequent memory strength (1 – 6) (presumably because of task-irrelevant mental activity), fMRI activity would be more strongly correlated with activity in the medial temporal lobe for subsequently forgotten items (memory strengths 1&2, 3) than for subsequently remembered items (memory strengths 4, 5, 6). And conversely, in regions where fMRI activity increased with subsequent memory strength (1 – 6) (presumably because of task-relevant mental activity), fMRI activity would be more strongly correlated with activity in the medial temporal lobe for subsequently remembered items (memory strengths 4, 5, 6) than for subsequently forgotten items (memory strengths 1&2, 3). The results of a functional connectivity analysis, correlating activity in the medial temporal lobe with regions exhibiting a negative correlation between activity and memory strengths 1 – 6 and also with regions exhibiting a positive correlation between activity and memory strengths 1 – 6, were consistent with this idea (Supplemental Figure 3).
It is important to emphasize that the U-shaped pattern of findings would be expected to depend on the extent to which a study task demands full attention. In the present study, the task was relatively undemanding and even tedious (a pleasant/unpleasant judgment was made for each of 360 words), and participants could have made their responses early during the 2.5-second period when each word was presented. The rest of the time was available for mind wandering. In studies where the learning task is more demanding, or more time consuming, participants would be less likely to engage in task-irrelevant activity. In such circumstances, the relation between neural activity and subsequent memory performance can be expected to be different than what was observed here, and activity in the default network and other regions where activity correlated negatively with memory strength, might not be apparent during learning (Kirwan et al., submitted; Ranganath et al., 2004).
Our findings raise a caution about the interpretation of comparisons between activity related to subsequently remembered and subsequently forgotten items. At first glance, our finding that activity for subsequently remembered items was similar to the activity for subsequently forgotten items might suggest that medial temporal lobe activity does not predict subsequent memory. Yet, predictive activity was revealed when the analysis focused specifically on the relative memory strength of subsequently remembered items. Some previous studies also did not report a difference in medial temporal lobe activity between subsequently remembered items and subsequently forgotten items (Baker et al., 2001; Buckner et al., 2001; Otten and Rugg, 2001a). One possible explanation for such an outcome is that, for items that were subsequently forgotten, there was substantial mnemonic activity unrelated to the task and that an analysis restricted to the relative strength of subsequently remembered items might have revealed predictive activity in the medial temporal lobe.
Another issue raised by our findings concerns possible functional differentiation within the medial temporal lobe. One suggestion is that the hippocampus and perirhinal cortex differ in their contributions to recognition memory decisions. Specifically, the hippocampus has been suggested to support recollection-based decisions, and the perirhinal cortex has been suggested to support familiarity-based decisions (Brown and Aggleton, 2001; Eichenbaum et al., 2007). Some fMRI studies have been taken in support of this distinction (e.g., Davachi et al., 2003; Davachi and Wagner, 2002; Kensinger and Schacter, 2006; Ranganath et al., 2004; Uncapher et al., 2006; Uncapher and Rugg, 2005). Yet, it has also been pointed out that the distinction between recollection and familiarity has frequently been confounded with memory strength and that hippocampal activity and perirhinal activity may be sensitive to different levels of memory strength rather than qualitatively different memory processes (Squire et al., 2007).
We tested whether subsequent memory strength would correlate with activity in the hippocampus and perirhinal cortex, and we found that activity in both these structures exhibited a similar linear relationship with the subsequent memory strength of remembered items. Our study did not distinguish explicitly between the effects of memory strength and the effects of recollection and familiarity, so it remains possible that the perirhinal and hippocampal activity reflects familiarity-based and recollection-based decisions, respectively. Nevertheless, given that the relationship between activity and memory strength was similar in hippocampus and perirhinal cortex, it seems parsimonious to interpret the finding in each structure in similar ways. Thus, if increasing activity in hippocampus is thought to predict increasing numbers of recollection-based decisions, then it seems reasonable to suggest that increasing activity in perirhinal cortex also predicts recollection-based decisions. Conversely, if increasing activity in perirhinal cortex is thought to predict increasing numbers of familiarity-based decisions, then it seems reasonable to suggest that increasing activity in hippocampus also predicts familiarity-based decisions. We suggest that activity in both hippocampus and perirhinal cortex during learning predicts the subsequent memory strength of remembered items, regardless whether memory is based on recollection or familiarity. In any case, we suggest that the possible importance of memory strength in fMRI studies of recognition memory deserves consideration.
It is worth indicating how our study differs from an earlier study that also correlated activity during learning with subsequent memory (Ranganath et al., 2004). We used a single orienting question (is the word pleasant or unpleasant?) and later tested memory strength for the items. In contrast, Ranganath et al. used one of two orienting questions on each trial and later tested memory strength for the items as well as source memory about information related to the orienting question. This difference was probably important. Our relatively tedious and undemanding study task likely resulted in task-irrelevant activity during study, whereas the more demanding task used by Ranganath et al. (2004) likely encouraged participants to remain on task. We found that activity in the hippocampus and perirhinal cortex varied positively with memory strengths 4 – 6 (and across all memory strengths 1 – 6, activity conformed to a U-shape, see Discussion above), while Ranganath and colleagues found that activity in the perirhinal cortex varied positively with memory strengths 1 – 5.
It is also worth mentioning that reaction times during study varied positively with subsequent memory strength. It is important to note that this pattern was qualitatively different from the U-shaped relationship between activity and subsequent memory strength in the medial temporal lobe. Thus, our findings in the medial temporal lobe cannot be attributed to the effect of reaction times during study.
In summary, activity during learning in regions known to be active during momentary lapses of attention and mind wandering was negatively correlated with subsequent memory strength. This finding shows that activity during learning might sometimes reflect processes unrelated to the task of interest. Further, in both hippocampus and perirhinal cortex, activity was positively correlated with the subsequent memory strength of remembered items. This finding does not point to a sharp distinction between these structures with respect to recollection and familiarity. At the same time, the data do not count against the idea that the functions of these structures are distinct in other important ways (Squire et al., 2007; Suzuki and Eichenbaum, 2000).
Methods
Participants
Fourteen right-handed volunteers (6 female; mean age = 27.3; range = 18 – 34) recruited from the University community gave written informed consent prior to participation.
Materials
The stimuli were 720 nouns with a mean frequency of 27 (range 1 – 198) and concreteness ratings greater than 500 (mean = 573; Wilson, 1988). Half the words were assigned to six 60-word study lists, and half the words served as foils for the retrieval test. The assignment of words to the study and test conditions was counterbalanced across participants.
Procedure
Participants were scanned in 6 separate runs (~2 minute delay between runs), during which the 360 target words were presented (Figure 1). Participants made a pleasant/unpleasant rating for each word (2.5-second presentation time). Responses were collected via an MR-compatible button box. Participants were not informed that their memory for words would be tested. An odd/even digit task was intermixed with the word presentation, and served as a baseline against which the hemodynamic response was estimated. For the digit task, participants saw a digit (1–8) for 1.25 seconds and indicated by button press whether the digit was odd or even. Digit task trials (144 trials per scan run) were pseudorandomly intermixed with the encoding trials with the following constraints: each scan run began and ended with at least 12 digit trials, and all digit trials occurred in groups of 2, 4, or 6 so as to fit within the 2.5-second repetition time (TR; see below). Participants were given a short practice block prior to scanning to ensure that they understood the task and the button assignments.
Following scanning (~15 min delay), participants took a surprise post-scan recognition memory test. They saw all 360 words from the scan session (targets) and 360 novel foils one at a time (3.5 seconds per word) in a random order. The recognition memory test was divided into 12 blocks with a short break between blocks. For each word, participants made a recognition confidence judgment on a scale from 1 to 6 (1 = “definitely new”, 2 = “probably new”, 3 = “maybe new”, 4 = “maybe old”, 5 = “probably old”, and 6 = “definitely old”). No explicit judgment was made regarding whether decisions were based on recollection and familiarity. Before testing, participants completed a short practice block to ensure that they understood the instructions and the confidence rating scale.
fMRI Imaging
Imaging was carried out on a 3T GE scanner at the Center for Functional MRI (University of California San Diego). Functional images were acquired using a gradient-echo, echo-planar, T2*-weighted pulse sequence (TR = 2500 ms; 132 TRs per run; TE = 30 ms; flip angle 90°; matrix size = 64 × 64; field of view 22 cm). The first five TRs acquired were discarded to allow for T1 equilibration. Forty-two oblique coronal slices (slice thickness = 5 mm) were acquired perpendicular to the long axis of the hippocampus and covering the whole brain. Following the 6 functional runs, high-resolution structural images were acquired using a T1-weighted, fast spoiled gradient-echo (FSPGR) pulse sequence (flip angle = 12°; TE = 3.1 ms; 172 slices; 1 mm slice thickness; matrix size = 256 × 256; field of view = 25 cm).
fMRI Data Analysis
fMRI data were analyzed using the AFNI suite of programs (Cox, 1996). Functional data were coregistered in three dimensions to the whole-brain anatomical data and coregistered through time to reduce effects of head motion. Motion events, defined as TRs in which there was more than 0.3 degrees of rotation or 0.6 mm of translation in any direction were eliminated from the analysis (as well as the TR immediately preceding and following the motion contaminated TR). Behavioral vectors were created that coded each study trial for subsequent recognition confidence rating (i.e. memory strengths 1 – 6). Trials in which there was no response for either the pleasantness rating task or for the subsequent recognition memory test (mean = 5 per participant) were excluded from further analysis. Due to low rates of high confidence misses (see Behavioral Results), memory strengths 1 and 2 were combined into a single vector. The five behavioral vectors and six vectors that coded for motion (three for translation and three for rotation) were used in a deconvolution analysis of the fMRI time series data (3dDeconvolve; http://afni.nimh.nih.gov/pub/dist/doc/manuals/ 3dDeconvolve.pdf). The resultant fit coefficients (β coefficients) represent activity versus baseline in each voxel for a given time point and each of five trial types (memory strength 1&2, 3, 4, 5, and 6). This activity was summed over the expected hemodynamic response (0 – 15 seconds after trial onset) and taken as the estimate of the response to each trial type (relative to the digit task baseline).
Initial spatial normalization was accomplished using each participant’s structural MRI scan to transform the data to the atlas of Talairach and Tournoux (Talairach and Tournoux, 1988). Statistical maps were also transformed to Talairach space, resampled to 2 mm3, and smoothed using a Gaussian filter (4 mm FWHM) that respected the anatomical boundaries of the several MTL regions defined for each individual participant (see below). In short, the blurring was carried out within the set of anatomically defined MTL regions, but the blur was cut off at the edges of the regions to prevent activity from one region (e.g. parahippocampal cortex) from being blurred into another, adjacent region (e.g. hippocampus). The transformed data were then used in the initial whole-brain analyses.
To achieve better alignment and increase statistical power for the analysis of medial temporal lobe activity (Miller et al., 2005), the ROI-LDDMM alignment technique was used (Kirwan et al., 2007). Anatomical regions of interest were manually segmented in 3D on the Talairach-transformed anatomical images for the hippocampus, temporopolar, entorhinal, perirhinal, and parahippocampal cortices. Temporopolar, entorhinal, and perirhinal cortices were defined according to the landmarks described by Insausti et al. (Insausti et al., 1998b). The parahippocampal cortex was defined bilaterally as the portion of the parahippocampal gyrus caudal to the perirhinal cortex and rostral to the splenium of the corpus callosum (Insausti et al., 1998a). The anatomically defined ROIs for each individual participant were then used to calculate a vector field to warp each set of ROIs onto a previously defined model using ROI-LDDMM (Kirwan et al., 2007). This transformation was then applied to the statistical maps, and all MTL analyses were performed on the ROI-LDDMM transformed data.
Supplementary Material
Acknowledgments
Supported by the Medical Research Service of the Department of Veterans Affairs, NIMH Grant 24600, the Metropolitan Life Foundation, NIMH Training Grant MH 20002 (C.B.K.), and a National Science Foundation Predoctoral Fellowship (Y.S.). We thank Jennifer Frascino, Christine Smith, Mark Starr, and John Wixted for advice and assistance. We also thank the Center for Imaging Science at the Johns Hopkins University for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker JT, Sanders AL, Maccotta L, Buckner RL. Neural correlates of verbal memory encoding during semantic and structural processing tasks. Neuroreport. 2001;12:1251–1256. doi: 10.1097/00001756-200105080-00039. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna J, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals New York Academy Sciences. 2008 doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA. Encoding processes during retrieval tasks. J Cogn Neurosci. 2001;13:406–415. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Smith CN, Bayley PJ, Shrager Y, Brewer JB, Stark CE, Hopkins RO, Squire LR. Item memory, source memory, and the medial temporal lobe: concordant findings from fMRI and memory-impaired patients. Proc Natl Acad Sci U S A. 2006;103:9351–9356. doi: 10.1073/pnas.0602716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Insausti R, Insausti AM, Sobreviela MT, Salinas A, Martinez-Penuela JM. Human medial temporal lobe in aging: anatomical basis of memory preservation. Microsc Res Tech. 1998a;43:8–15. doi: 10.1002/(SICI)1097-0029(19981001)43:1<8::AID-JEMT2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998b;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J Neurosci. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CE. High-resolution fMRI investigation of the medial temporal lobe. Hum Brain Mapp. 2007;28:959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Wixted JT, Squire LR. Activity In The Medial Temporal Lobe Predicts Memory Strength. Whereas Activity In The Prefrontal Cortex Predicts Recollection. doi: 10.1523/JNEUROSCI.3456-08.2008. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD, Bright P, Buckman J, Fradera A, Yoshimasu H, Jacobson C, Colchester AC. Recall and recognition memory in amnesia: patients with hippocampal, medial temporal, temporal lobe or frontal pathology. Neuropsychologia. 2007;45:1232–1246. doi: 10.1016/j.neuropsychologia.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Levy DA, Hopkins RO, Squire LR. Impaired odor recognition memory in patients with hippocampal lesions. Learn Mem. 2004;11:794–796. doi: 10.1101/lm.82504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler G. Recognizing: the judgment of previous occurrence. Psychol Rev. 1980;87:252–271. [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proc Natl Acad Sci U S A. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb Cortex. 2001a;11:1150–1160. doi: 10.1093/cercor/11.12.1150. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001b;11:1528–1530. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, Donaldson DI, Wagner AD, Braver TS. Item- and task-level processes in the left inferior prefrontal cortex: positive and negative correlates of encoding. Neuroimage. 2004;21:1472–1483. doi: 10.1016/j.neuroimage.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Dodson CS. Support for a continuous (single-process) model of recognition memory and source memory. Mem Cognit. 2005;33:151–170. doi: 10.3758/bf03195305. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Eichenbaum H. The neurophysiology of memory. Ann N Y Acad Sci. 2000;911:175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Encoding and the durability of episodic memory: a functional magnetic resonance imaging study. J Neurosci. 2005;25:7260–7267. doi: 10.1523/JNEUROSCI.1641-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Davachi L. Cognitive neuroscience: forgetting of things past. Curr Biol. 2001;11:R964–R967. doi: 10.1016/s0960-9822(01)00575-9. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49:459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wilson MD. The MRC Psycholinguistic Database: Machine Readable Dictionary, Version 2. Behavioural Research Methods, Instruments and Computers. 1988;20:6–11. [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychol Rev. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.