Abstract
Eosinophils produce and release various pro-inflammatory mediators and also show immunomodulatory and tissue remodeling functions; thus, eosinophils may be involved in the pathophysiology of asthma and other eosinophilic disorders as well as host defense. Several major questions still remain. For example, how do human eosinophils become activated in diseased tissues or at the site of immune response? What types of host immunity might potentially involve eosinophils? Herein, we found that human eosinophils react vigorously to a common environmental fungus, Alternaria alternata, which is implicated in the development and/or exacerbation of human asthma. Eosinophils release their cytotoxic granule proteins, such as eosinophil-derived neurotoxin and major basic protein, into the extracellular milieu and onto the surface of fungal organisms and kill the fungus in a contact-dependent manner. Eosinophils use their versatile β2 integrin molecule, CD11b, to adhere to a major cell wall component, β-glucan, but eosinophils do not express other common fungal receptors, such as dectin-1 and lactosylceramide. The I-domain of CD11b is distinctively involved in the eosinophils’ interaction with β-glucan. Eosinophils do not react with another fungal cell wall component, chitin. Because human eosinophils respond to and kill certain fungal organisms, our findings identify a previously unrecognized innate immune function for eosinophils. This immune response by eosinophils may benefit the host, but in turn, it may also play a role in the development and/or exacerbation of eosinophil-related allergic human diseases, such as asthma.
Keywords: Eosinophils, Alternaria, Human, fungus
Introduction
Eosinophils were first described by Paul Ehrlich in 1879, and they are thought to have evolved as part of the innate/acquired immune response against parasitic helminths (1–3). Eosinophils are likely involved in the pathophysiology of various human diseases, such as bronchial asthma and atopic dermatitis (1–3). Eosinophils generate pro-inflammatory mediators, such as leukotrienes, reactive oxygen species and various cytokines and chemokines, and release pre-formed inflammatory mediators, such as major basic protein (MBP), eosinophil-derived neurotoxin (EDN) and eosinophil cationic protein (ECP) from granules, resulting in tissue damage and remodeling (4, 5). Deposition of released granule proteins has been demonstrated in diseased tissues, such as airway, skin, and heart, from patients with acute and chronic eosinophilic disorders (6–9). On the other hand, how eosinophils become activated and release inflammatory mediators is largely unknown.
The association between eosinophilic inflammation and infection or colonization with fungi has been long recognized. For example, in 1952, patients with allergic bronchopulmonary aspergillosis were reported, which demonstrate pulmonary eosinophilia and positive serological tests for aspergillus (10). The association between fungi and chronic eosinophilic airway inflammation has been described in patients with severe asthma and certain types of rhinosinusitis (11, 12). Furthermore, infiltration and degranulation of eosinophils were observed in lesions of patients with paracoccidiodomycosis (13). However, the immunological mechanisms underlying these relationships between fungi and eosinophilic inflammation have been poorly understood. Recently, major conceptual advances have been made in this regard. Chitin is a carbohydrate polymer, and it is found in the exoskeleton of insects and crustaceans (e.g. mites and cockroaches), the cell wall of fungi, as well as the pharynx, microfilarial sheath, and eggs of helminths. Importantly, in mice the intranasal administration of chitin induced a Th2-like airway response and eosinophilia, and these responses were inhibited by a vertebrate chitinase (14). Thus, immune responses to chitin-encased insects and fungi (15, 16) may be part of the normal innate immune response, and inappropriate regulation of the system may contribute to asthma and allergic diseases.
These relationships among eosinophilia, asthma, and fungi prompted us to investigate the direct response of eosinophils to fungi. First, do human eosinophils recognize fungi and produce pro-inflammatory mediators? Second, if eosinophils do react to these organisms, which eosinophil receptor(s) are involved? To this end, we used a ubiquitous, nonpathogenic airborne fungus, Alternaria alternata, as a pathologically-relevant model organism. Indeed, several epidemiological studies strongly implicate exposure or sensitization to A. alternata in the development and/or exacerbation of human asthma (17–19). We found that eosinophils exert a strong inflammatory response against germinating A. alternata and kill the fungus. A versatile β2 integrin adhesion molecule, CD11b, which is expressed by eosinophils, likely plays a key role in recognizing and/or interacting with β-glucan that is present on the surface of A. alternata.
Materials and methods
Reagents
Curdlan [linear β-(1,3)-glucan] was from Wako chemicals (Richmond, VA). Anti-dectin-1 antibody and mouse IgG1 control antibody were from R&D Systems (Minneapolis, MN). Anti-CD11b mAb, clone 2LPM19c, was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-CD11b mAbs, clones 5C6 and M1/70, were from Serotec (Raleigh, NC) and eBioscience (San Diego, CA), respectively. Anti-lactosylceramide mAb was from Ancell (Bayport, MN). Anti-β-glucan antibody (mouse IgM) was a generous gift from Dr. Jonathan Reichner (Brown University). Control Mouse IgM and chitin from crab shells were from Sigma Aldrich (St. Louis, MO). Human cytokine antibody array V kit was from RayBiotech, Inc (Norcross, GA). Anti-CD32 mAb, clone IV3, was from StemCell Technologies (Vancouver, BC) and C3-depleted serum was from Calbiochem (San Diego, CA). Rhodamine (TRITC)-conjugated AffiniPure Goat Anti-Rabbit IgG (H+L)(minimal cross-reaction to human serum proteins) was from Jackson ImmunoResearch Laboratories (West Grove, PA). Alternaria alternata (ATCC 11680) was cultured on potato dextrose agar (Sigma-Aldrich, St. Louis, MO) at 25 °C for 10 days before harvesting spores. After harvesting the spores by flooding the agar dishes with sterile water, they were counted by using a hemacytometer.
Cell isolation
Eosinophils were isolated from the blood of 29 normal and mildly atopic volunteers using negative selection with anti-CD16 microbeads (Miltenyi Biotec, Aubum, CA) as previously described with only one slight modification (20). Granulocytes were incubated with an equal volume of anti-CD16-conjugated magnetic beads on ice for 30 minutes. This protocol consistently yielded >96% eosinophil purity. Neutrophils were isolated from the blood of the same donors used for eosinophil isolation. The eosinophil isolation protocol referenced above was followed with 10 μl of the granulocyte pellet being re-suspended in buffer and then counted using Randolph’s stain. This consistently yielded ≥95% neutrophil purity and allowed us to do parallel experiments on neutrophils and eosinophils on the same day from the same donor. Dendritic cells (DCs) were generated by culturing CD14-positive monocytes, which were isolated from the blood of healthy volunteers, with GM-CSF (10 ng/ml) and IL-4 (10 ng/ml) (R&D systems) for 6 days in RPMI 1640 medium supplemented with 10% calf serum. The study was approved by the Institutional Review Board at the Mayo Clinic, Rochester, MN; all volunteers provided informed consent.
Eosinophil and neutrophil degranulation assay
To test whether eosinophils respond to live A. alternata and release inflammatory mediators, A. alternata spores (5×103/well or numbers indicated in the figures) were suspended in Hanks balanced salt solution (HBSS) supplemented with 25mM HEPES, 0.01% gelatin, and heat inactivated 10% α-Calf Serum (α-CS) and allowed to germinate and to form hyphae in 24-well tissue culture plates overnight at 20 °C. On the next day, freshly isolated eosinophils were suspended in HBSS with 25 mM HEPES and 10% α-CS, and 1×105 cells were added into each A. alternata well and incubated 18 h at 37 °C. In some experiments, to examine the kinetics of the eosinophil response, the eosinophil and fungal mixture was cultured from 1 h to 24 h. After incubation, cell-free supernatants from the plates were collected and stored at −20 °C to quantitate EDN released into supernatants. For the EDN ELISA, 96-well flat-bottom plates (Immulon® 4HXB; Thermo Electron Corporation, Milford, MA) were coated with 100 μl of murine anti-human EDN mAb (5 μg/ml in PBS, clone 167-6C5) and incubated at 4 °C overnight; anti-human EDN mAb (clones 167-6C5 and 167-2G4) were made at Mayo Clinic Rochester. The plates were then washed with wash buffer (0.01% Tween in PBS) using a microplate washer (BioTek Instruments, Winooski, VT). The wells were blocked with 200 μl 1% bovine serum albumin in assay buffer (0.05% Tween in PBS) for 30 minutes at room temperature and washed. Dilutions of the EDN standard or test sample (100 μl) were added to the wells and incubated at room temperature for 2 h. The plates were then washed and incubated with 100 μl of horseradish peroxidase-labeled anti-human EDN detection mAb (1:15,000 dilution in PBS, clone 167-2G4) for 30 minutes at room temperature. After washing, 100 μl of freshly made 3,3′,5,5′-tetramethylbenzidine substrate (Pierce Protein Research Products; Thermo Fischer Scientific, Rockford, IL) was added and incubated for 5 to 10 minutes. Immediately after stopping the reaction with 50 μl of 1M H2SO4, the absorbance at 450 nm was measured with a microplate reader (SpectraMax Plus; Molecular Devices, Sunnyvale, CA). The lowest point of the standard curve was 0.09 ng/ml. All assays were conducted in duplicate. To detect cytokines and chemokines produced by activated eosinophils, cell-free supernatants were analyzed by the Human cytokine antibody array V kit, following the procedure recommended by the manufacturer.
To examine whether physical contact between A. alternata and eosinophils is necessary to activate eosinophils, 24-well Transwell® inserts (pore size 0.4 μm, Costar, Corning, NY) were used to prevent contact between A. alternata and eosinophils. In other experiments, eosinophils were incubated with supernatants from cultured A. alternata. To examine whether eosinophils respond to fungal cell wall components, serial dilutions of β-glucan particles (Curdlan) or chitin particles (10–1,000 μg/ml) were used as stimuli. To examine the involvement of a β2 integrin, CD11b, in eosinophil activation in response to live A. alternata or β-glucan, eosinophils were preincubated with various anti-CD11b mAb or isotype matched control mouse Ig (5~10 μg/ml) for 30 min at room temperature and then exposed to stimulus.
To examine whether neutrophils respond to β-glucan and whether CD11b is involved in their interaction, neutrophils were preincubated with anti-CD11b mAbs or control mouse Ig and they were exposed to A. alternata by using the same conditions as described above for the eosinophil experiments. To quantitate neutrophil degranulation, the concentrations of myeloperoxidase (MPO) in cell-free supernatants were measured by using an MPO detection ELISA kit (Hycult Biotechnology, Uden, The Netherlands) according to the manufacturer’s directions.
Morphological assessment of eosinophil and A. alternata interaction
To monitor the interaction between eosinophils and A. alternata in detail, we used immunohistochemistry. After eosinophils were cultured 18 h with A. alternata, they were mounted on positively charged slides by cytospin, and stained with rabbit anti-human MBP or control rabbit IgG (21). All specimens were incubated in 10% normal goat serum and 1% chromotrope 2R to block nonspecific binding by the second-stage antibody. Rhodamine-conjugated goat anti-rabbit IgG was used as the secondary antibody. The slides were observed using an Axiophot fluorescence microscope (Carl Zeiss, Inc, Oberkochen, Germany) and recorded by AxioCam HR digital camera and AxioVision 4.0 software (Carl Zeiss, Inc.). The same camera settings (e.g. exposure time) were used to record images for both control IgG and anti-MBP, and the images presented have not been manipulated.
Eosinophils fungicidal activity
A. alternata spores (5×103/well) were allowed to germinate and to form hyphae in HBSS with 25mM HEPES, 0.01% gelatin, and heat inactivated 10% α-CS overnight at 20 °C in 24-well tissue culture plates. The next day, 1×105 freshly isolated eosinophils were suspended in HBSS buffer with 25 mM HEPES and 10% α-CS and added into the wells with or without A. alternata and incubated 18 h at 37 °C. Cytospin preparations of eosinophils and A. alternata were stained with propidium iodide (PI) for 1 min at room temperature, following a previously published method with minor modifications (22). Images were recorded immediately by fluorescence microscopy. The viability of A. alternata hyphae was determined using fluorescence microscopy to observe ≥ 50 hyphae with low magnification (×100). Hyphae with a length ≥ half of the field were evaluated. Because fungal hyphae are elongated, they were judged PI-positive if roughly > 75% of the entire visible length of the hyphae was stained with PI. They were judged PI-negative if < 5% was stained with PI. No equivocal staining with PI (i.e. less than 75% but more than 5% of the piece is stained with PI) was observed.
FACS analysis
To examine the expression of dectin-1, lactosylceramide, and CD11b by eosinophils, neutrophils and monocyte-derived DCs, freshly isolated eosinophils (1×106 cells), eosinophils primed with IL-5 (1 ng/ml) for 1 h at 37 °C, or freshly isolated neutrophils or cultured DCs were incubated with FcγR blocking reagent (Miltenyi Biotech, Auburn, CA) and stained with anti-dectin-1, anti-lactosylceramide, anti-CD11b mAbs (10 μg/ml) or appropriate control mouse Ig for 30 min, followed by FITC-conjugated goat F(ab′)2 anti-mouse IgG antibody (Sigma Aldrich) for 1 h on ice. To detect intracellular proteins, cells were first fixed and permeabilized using afixation/permeabilization kit (Caltag, Burlingame, CA) followed by primary and secondary antibodies, as described above. Cells were fixed with 1% paraformaldehyde and were analyzed using FACScan (Becton Dickinson, Mountain View, CA) and Becton Dickinson lysis II software.
Immunofluorescence microscopy of A. alternata hyphae
To detect the expression of β-glucan on the surface of Alternaria, the spores were allowed to germinate in HBSS with 25mM HEPES, 0.01% gelatin, and heat inactivated 10% αCS overnight at 20 °C, as described above. After cytospin, the A. alternata was fixed with 3.7% formaldehyde for 20 min at room temperature. A. alternata was then incubated with anti-β-glucan mAb or control antibody for 1 h at room temperature, followed by Texas-red conjugated goat F(ab′)2 anti-mouse IgM antibody (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA). The images were recorded by using a fluorescence microscope, as described above.
Statistical analysis
All the error bars represent SEM. Two-sided differences between two samples were analyzed with the Student t-test. A P value < 0.05 was considered significant.
Results
Human eosinophils recognize the fungus, Alternaria alternata, and produce inflammatory responses
We first examined whether human eosinophils recognize A. alternata organisms and release inflammatory mediators. A. alternata spores (5000/well) were allowed to germinate and to form hyphae overnight. Freshly isolated eosinophils (1×105/well) were added to the culture and incubated for up to 24 h. Eosinophil degranulation was observed after 3 h of incubation and increased in a time-dependent manner (Figure 1A); at 24 h, approximately 16% of total cellular EDN was released into supernatants. No EDN was released when eosinophils were incubated with medium alone. Dose-response experiments showed that even 625 spores/well were sufficient to induce EDN release at 18 h (Figure 1B). Morphologically, eosinophils clustered around and bound to A. alternata fungal hyphae (Figure 1C). Because eosinophils appeared bound to A. alternata hyphae, we examined whether adhesion between eosinophils and A. alternata is necessary for eosinophil activation. Indeed, separation of eosinophils and A. alternata by a Transwell® system significantly inhibited eosinophil degranulation induced by A. alternata (p<0.05, n=3) (Figure 1D). Furthermore, eosinophils incubated with culture supernatants of A. alternata released minimal EDN (8.4±2.9 and 9.9±1.7 ng/105 cells with medium alone and with A. alternata supernatants, respectively, mean±SEM, n=3).
Figure 1.
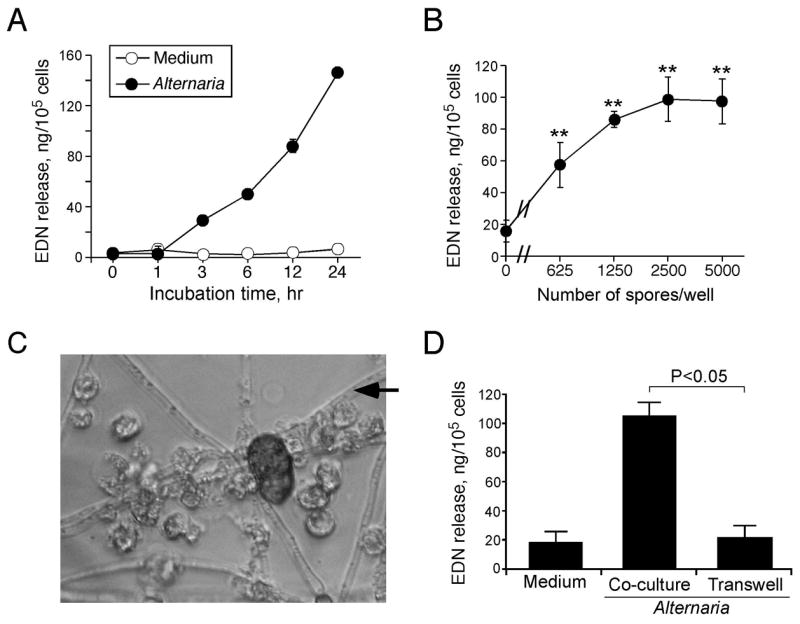
Human eosinophils are activated by whole Alternaria alternata organisms and release granule protein. A, Isolated human eosinophils were cultured with germinating A. alternata spores (5000/well) for different time periods up to 24 h. After culture, the cell-free supernatants were harvested, and EDN released in the supernatants was measured by ELISA. Results show means±range of duplicate samples from an experiment, which is representative of 2 independent experiments. B, Isolated human eosinophils were cultured with different numbers of germinating A. alternata spores for 18 h, and EDN released in the cell-free supernatants was measured by ELISA. Results show means±SEM from 3 independent experiments. ** p<0.01, compared to medium alone (i.e. no A. alternata spores). C, Eosinophils were cultured with germinating A. alternata spores (5000/well) for 18 h. Photomicrograph was taken using an inverted microscope (original magnification, ×400); black arrow indicates the thread-like A. alternata hyphae; the eosinophils are translucent ovals; and an A. alternata spore appears as a dark oval. D, Physical contact between eosinophils and A. alternata was blocked with a Transwell® system during 18 h of eosinophil-A. alternata culture; co-culture wells are like those in (A); and EDN released in the cell-free supernatants was measured by ELISA. Results show means±SEM from 3 independent experiments.
To examine the interaction between eosinophils and A. alternata more closely, we used immunohistochemistry to look for eosinophil degranulation in response to the fungal spores and hyphae. As shown in bright field images (Figures 2A, 2C,2E), eosinophils appear to be aggregated on the hyphae and spores. When viewed with a Rhodamine filter, the field in 2C shows the intense anti-MBP staining (Figure 2D) that appears localized to the eosinophils and to the hyphae and spores; staining with control rabbit IgG showed no fluorescence (Figure 2B). Interestingly, using higher magnification, deposition of extracellular MBP is clearly apparent on the fungal hyphae (Figures 2F). Thus, human eosinophils recognize A. alternata in vitro and release pro-inflammatory and cytotoxic granule proteins, such as EDN and MBP, in a contact-dependent manner.
Figure 2.

Morphology of eosinophils and A. alternata organisms after culture. A. alternata hyphae and spores were incubated with eosinophils for 18 h. Specimens were fixed and stained with control rabbit IgG (B) or anti-MBP Ab ( D, F) followed by Rhodamine-conjugated secondary Ab, as described in Materials and Methods. A, C, The bright field photomicrographs show eosinophils clustered around the hyphae. B, staining with control rabbit IgG shows no fluorescence signal. D, staining with anti-MBP Ab shows intense MBP staining. E, F, Higher magnification view (from same field in C,D) shows clusters of eosinophils containing MBP and also extracellular MBP (arrowheads) coating the hyphae. Original magnification, x400. Figures are representative of 2 independent experiments.
Eosinophils damage A. alternata
To examine the outcome of the eosinophils’ interaction with A. alternata, the hyphae, which had been cultured with medium alone or with eosinophils for 18 h, were stained with PI and examined by bright-field and fluorescence microscopy. A. alternata hyphae incubated with medium alone were refractile and appeared healthy (Figure 3A); these hyphae did not stain with PI, indicating minimal damage to the cell wall (Figure 3B). In addition, eosinophils incubated without A. alternata hyphae did not stain with PI (Figures 3C, 3D). In contrast, A. alternata hyphae incubated with eosinophils stained strongly with PI (Figures 3E, 3F), suggesting that the integrity of the fungal cell walls has been compromised. The eosinophils, which were incubated with A. alternata hyphae, also stained positive with PI (Figure 3F), consistent with previous observations in degranulated eosinophils (23). Four separate experiments showed that damage to fungal cell wall (e.g. PI-positive hyphae) was observed in 95±3 % of A. alternata incubated with eosinophils, compared to only 5±2 % in Alternaria incubated with medium alone (mean±SEM, n=4). Thus, eosinophils likely possess fungicidal activity against A. alternata.
Figure 3.

Eosinophils damage A. alternata organisms. Photomicrographs show germinating A. alternata organisms after culture with medium alone (A,B), eosinophils cultured with medium alone (C,D) or A. alternata organisms after culture with isolated eosinophils (E,F) for 18 h. The preparations of the A. alternata in medium (B), of the eosinophils in medium (D), and of the eosinophil-A. alternata mixture (F) were stained with PI and observed using fluorescence microscopy. Original magnification, x100. Figures are representative of 6 independent experiments.
β-glucan, not chitin, is recognized by eosinophils
The fungal cell wall is composed of various carbohydrates, including manno-glycoprotein, β-glucan, and chitin (24). In particular, the immune recognition of chitin has been recently implicated in the pathogenesis of allergic airway inflammation in mouse models and in patients with asthma (25, 26). Therefore, to identify the fungal cell surface molecule(s) recognized by human eosinophils without the confounding presence of other cell wall molecules, we examined whether isolated chitin or β-glucan can activate eosinophils. After cells were incubated with serial dilutions of chitin or β-glucan (Curdlan) particles (~100 μm diameter), the cell-free supernatants were analyzed for EDN and chemokines. Eosinophils did not degranulate in response to chitin particles up to 1000 μg/ml (Figure 4A). In contrast, eosinophils responded vigorously to β-glucan particles at 100 μg/ml and released EDN into the extracellular milieu in a concentration-dependent manner (p<0.05 and <0.01, n=4). Morphologically, eosinophils adhered to the glucan particles (Figure 4A, inset), similarly to the observations with fungal hyphae (vide supra Figure 1C). Furthermore, several chemokines, including MCP-1, IL-8, and MIP-1α, were clearly detectable by protein microarray in cell-free supernatants of eosinophils after their incubation with β-glucan particles (Figure 4B). Thus, eosinophils appear to release granule proteins and chemokines, when exposed to β-glucan alone in the absence of other fungal cell wall molecules; in other words, β-glucan is sufficient to activate eosinophils. Furthermore, there is likely a selectivity in the eosinophils’ responses to different fungal carbohydrate structures, such as β-(1,3)-linked glucose polymers (i.e. β-glucan) compared to β-(1,4)-linked N-acetylglucosamine polymers (i.e. chitin).
Figure 4.

Eosinophils respond to β-glucan, but not to chitin. A, Isolated human eosinophils were incubated with medium alone or with serial dilutions of purified chitin particles or purified β-glucan particles (10~1000 μg/ml) for 18 h. EDN released into cell-free supernatants was measured by ELISA. Results show means±SEM from 4 independent experiments. * p<0.05 and ** p<0.01, compared to medium alone. The photomicrograph inset shows the morphology of eosinophils incubated with the larger β-glucan particles (original magnification, x400). B, Eosinophils were incubated with medium alone or purified β-glucan particles (500 μg/ml) for 18 h. Cell-free supernatants were collected and analyzed by a cytokine protein microarray, as described in the Materials and Methods. Four dots in the top row (left) and two dots in the bottom row (right) indicate positive controls provided by the manufacturer. Results are representative from two independent experiments.
In human eosinophils, a β2 integrin, CD11b, is involved in interactions with β-glucan and A. alternata
Having established that eosinophils respond to β-glucan, we investigated the molecular mechanisms involved in the eosinophils’ interaction with β-glucan and ultimately with A. alternata organisms. Initially, because eosinophils express a low affinity IgG receptor, FcγRII (CD32) (23), we suspected potential roles for the products of acquired immunity, such as antibodies. Perhaps the calf serum used in the eosinophil-A. alternata culture contains antibodies to β-glucan, and eosinophils may recognize β-glucan through these bound IgG antibodies. To test this hypothesis, eosinophils were preincubated with blocking anti-CD32 mAb and then incubated with β-glucan particles or with IgG immobilized onto the tissue culture plates, as a positive control. Eosinophils incubated with β-glucan particles showed marked degranulation; however, the degranulation induced by β-glucan was not affected by anti-CD32 mAb (Figure 5). Immobilized IgG induced a comparable level of eosinophil degranulation as that observed with β-glucan particles, and degranulation induced by IgG was abolished by anti-CD32 mAb. Furthermore, inactivation of complement by heat-inactivating the calf serum for 56 °C for 30 minutes or by using C3-depleted human sera did not affect eosinophil degranulation induced by β-glucan particles (data not shown). Thus, neither antibodies nor complement are likely to be involved in the eosinophils’ response to β-glucan particles.
Figure 5.
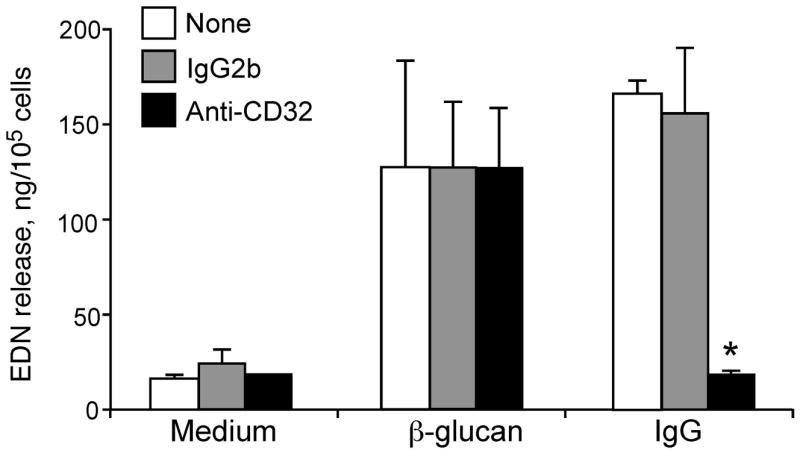
FcγRII (CD32) is not involved in the eosinophils’ response to purified β-glucan. Isolated human eosinophils were preincubated with medium alone (None), control mouse IgG2b, or anti-CD32 mAb (10 μg/ml) for 30 min at RT. Cells were then stimulated with medium alone, purified β-glucan particles (100 μg/ml), or human IgG (50 μg/ml) immobilized onto the tissue culture plates (IgG) for 18 h. Cell-free supernatants were collected and measured for EDN by ELISA. Results show means±SEM from 3 independent experiments. * p<0.05, compared to mouse IgG2b treatment stimulated with immobilized human IgG.
Based on these observations, we suspected potential roles for innate receptors, which recognize the molecular patterns of microbes. Three molecules reportedly recognize β-glucan, including dectin-1, lactosylceramide, and a β2 integrin, CD11b (27). Dectin-1 was clearly detected by FACS on the surface of human DCs, but it was undetectable on the cell surface or the intracellular compartments of human eosinophils (Figure 6A). Another candidate receptor, lactosylceramide, was abundant on the surface of human neutrophils, but not on the surface of human eosinophils (Figure 6B). Furthermore, neither dectin-1 nor lactosylceramide were detectable on IL-5 primed-eosinophils (data not shown).
Figure 6.

Eosinophils do not express dectin-1 or lactosylceramide. A, Human monocyte-derived DCs or freshly isolated eosinophils were stained with anti-dectin-1 mAb, followed by PE-conjugated secondary antibody and were analyzed by flow cytometry. For intracellular staining, eosinophils were fixed and permeabilized, as described in Materials and Methods, before staining. B, Freshly isolated neutrophils or eosinophils were stained with anti-lactosylceramide mAb, followed by PE-conjugated secondary antibody and were analyzed by flow cytometry. Figures are representative histograms from three independent experiments.
Because both the I-domain and lectin-domain of CD11b are involved in glucan recognition by phagocytic cells (28–30), we examined the expression of CD11b and its specific domains by human eosinophils and neutrophils. Two anti CD11b Ab, namely anti-I-domain mAb (clone 2LPM19c) and lectin-function blocking mAb (clone M1/70) clearly detected CD11b expressed on the eosinophils’ surface (Figure 7A). Similar levels of CD11b expression were also observed on neutrophils. To examine the functional relevance of these CD11b molecules, neutrophils or eosinophils were preincubated with anti-CD11b antibodies and exposed to β-glucan particles. Neutrophil MPO release induced by β-glucan was inhibited by both the anti-I-domain mAb (clone 2LPM19c) and, as expected, by the lectin-function blocking mAb (clone M1/70) (Figure 7B), suggesting that both the I-domain and lectin-domain are involved in the neutrophils’ response to β-glucan. The anti-I-domain mAb (clone 2LPM19c) also abolished eosinophil degranulation induced by β-glucan particles (Figure 7B). In contrast, the lectin-function blocking mAb (clone M1/70) showed no effects on β-glucan-induced eosinophil degranulation, suggesting that the I-domain, but not the lectin-domain, of CD11b is involved in the eosinophils’ response to β-glucan.
Figure 7.
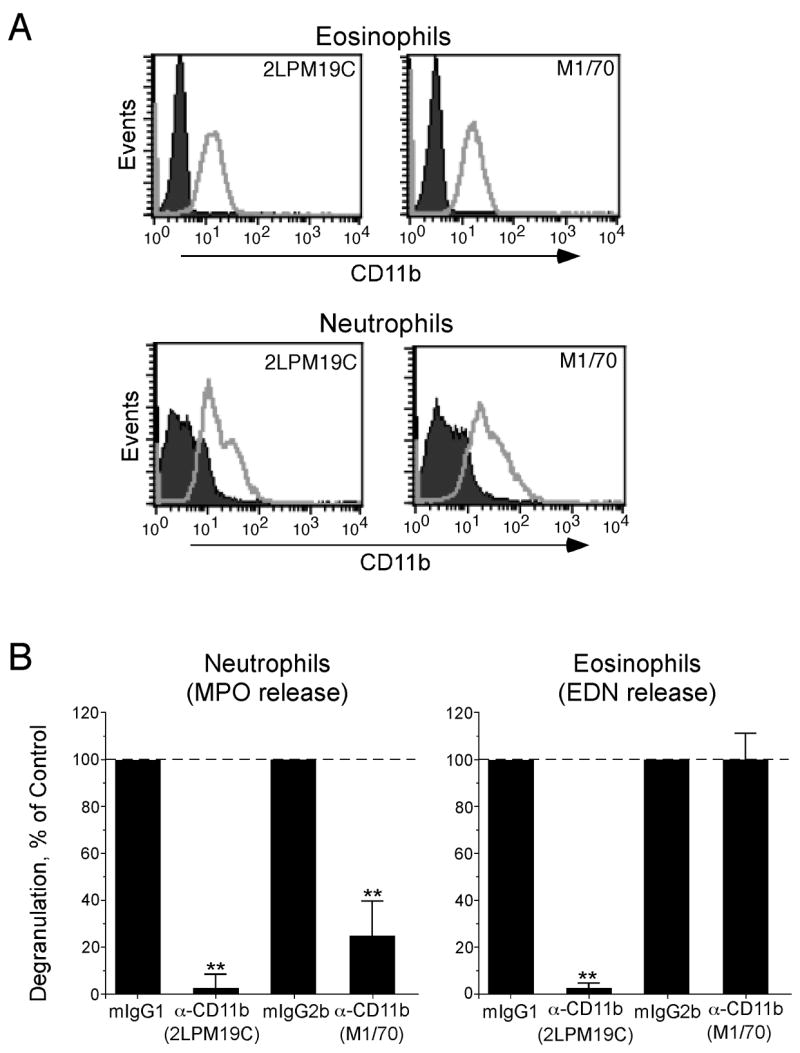
A β2 integrin, CD11b, expressed by eosinophils is involved in the cells’ response to β-glucan. A, Freshly isolated human eosinophils or neutrophils were stained with anti-CD11b mAbs (clone 2LPM19c and clone M1/70). Cells were stained with PE-conjugated secondary antibody and analyzed by flow cytometry. Representative histograms from two independent experiments are shown. B, Neutrophils or eosinophils were pre-incubated with medium alone, control mouse IgGs (mIgG1 or mIgG2b), or anti-CD11b mAbs (clone 2LPM19c or clone M1/70) (10 μg/ml) for 30 minutes at RT and then incubated with medium alone or purified β-glucan particles (100 μg/ml) for 18 h. After incubation, cell-free supernatants were collected and analyzed for MPO (neutrophils) and EDN (eosinophils). Data were normalized to the values with appropriate control mouse IgGs plus β-glucan particles as 100%. Spontaneous levels of MPO and EDN release without β-glucan particles were an average of 38% and 31%, respectively, of the values with control mouse IgGs plus β-glucan particles, and these values were subtracted before normalization. Results show means±SEM from 3 independent experiments. **; p<0.01 compared to values with control IgGs.
Finally, we examined whether these same CD11b molecules are involved in the eosinophils’ interaction with A. alternata organisms. Immunohistochemical analysis using anti-β-glucan antibody clearly showed that β-glucan is expressed on the surface of A. alternata hyphae (Figure 8A). Furthermore, eosinophil degranulation induced by A. alternata was completely inhibited by preincubation of eosinophils with anti-I-domain mAb (clone 2LPM19c) (Figure 8B). Thus, CD11b, in particular its I-domain, likely plays a pivotal role in how eosinophils recognize and/or interact with β-glucan as well as A. alternata organisms.
Figure 8.

A. alternata organisms express β-glucan on their hyphal surface, and CD11b is involved in the eosinophils’ responses to whole A. alternata organisms. A, A. alternata fungal spores were allowed to germinate for 18 h, and then hyphae were stained with control mouse IgM or anti-β-glucan followed by Texas-Red-conjugated secondary Ab. Left panels show bright field photomicrographs; right panels show fluorescence photomicrographs. Original magnification, 400x. Figures are representative from three independent experiments. B, A. alternata spores germinated for 18 h; isolated eosinophils were pre-incubated with medium alone, control mouse IgG1, or anti-CD11b Ab (clone 2LPM19c) (10 μg/ml) for 30 minutes at RT, and then cultured with medium alone or A. alternata organisms for 18 h. After incubation, cell-free supernatants were analyzed for EDN. Results show means±SEM from 3 independent experiments. * p<0.05, compared to control mouse IgG1 plus A. alternata.
Discussion
The key observation in this study is the immunological response of human eosinophils against the common environmental non-pathogenic fungus, Alternaria alternata. Eosinophils may possess anti-helminth immune effector functions (2, 31–33). Indeed, the cytotoxic granule proteins of eosinophils (e.g. MBP) bind strongly to the parasite targets and directly damage schistosomules of Schistosoma mansoni (34) and other parasites (35). Interestingly, MBP and ECP also exhibit robust antimicrobial activities against certain bacteria, such as Staphylococcus aureus and Escherichia Coli (36). Furthermore, EDN and ECP possess anti-viral activities against respiratory syncytial virus (37). While the immunological activities of eosinophil granule proteins have been relatively well characterized, little has been described regarding the immunological functions of eosinophils. We found that eosinophils respond vigorously to the common environmental fungus, A. alternata. Eosinophils bound to whole A. alternata organisms, in particular their hyphae (Figure 1), released cytotoxic granule proteins (Figures 1 and 2), and damaged the fungus (Figure 3). While the biological significance of these observations needs to be verified in animal models and in humans, these findings strongly suggest that eosinophils have the capacity to recognize and exert immunological responses to certain fungi. Thus, the innate immune functions of eosinophils may be more diverse than previously recognized.
β-glucan is one of the major cell wall components of fungus and is detected by several pattern recognition receptors, such as dectin-1 and lactosylceramide, as well as by CD11b (38). β-glucan is involved in anti-fungal immune responses in vivo (39, 40). Previously, we found that the soluble protease-like molecules secreted by A. alternata activate eosinophils likely through the protease-activated receptor-2 (41). We now report that eosinophils directly recognize purified β-glucan and release granule proteins (Figure 4A); and these eosinophils actively produce and release several chemokines (Figure 4B). Thus, there seems to be at least two fundamental, but non-exclusive, mechanisms involved in the eosinophils’ responses to fungal organisms, including the recognition of secreted and soluble proteases and of β-glucan expressed on the cell wall. Chitin is also a major cell wall component of fungi. A recent report showed that chitin activates macrophages; this activation is essential to recruit IL-4-producing eosinophils and basophils into the lungs in vivo in mice (14). In contrast, purified chitin did not activate eosinophils, even at high concentrations in our study (Figure 4). Furthermore, an authentic receptor for chitin, namely the mannose receptor, was undetectable on eosinophils (data not shown). Therefore, different innate immune cells, such as macrophages/DCs, neutrophils, and eosinophils, may recognize different fungal cell wall components (and maybe different genera of fungi) and may produce distinct immunological functions, including the recruitment of inflammatory cells, production of inflammatory mediators, and elimination of the organisms.
Eosinophils likely use a versatile β2 integrin, CD11b, to recognize and/or to interact with β-glucan and A. alternata hyphae, while they lack expression of dectin-1 and lactosylceramide (Figure 6). Furthermore, unlike neutrophils, the eosinophil shows limited expression of TLR-2 (42), which can recognize yeast zymosan (43). Indeed, anti-CD11b blocking mAb (clone 2LPM19c) completely inhibited eosinophil activation induced by purified β-glucan and whole A. alternata organisms (Figures 7B and 8B), suggesting a pivotal role for CD11b in the eosinophils’ response to filamentous fungi. We initially suspected that serum components, which directly bind to β-glucan, might be involved in how eosinophils recognize fungi. For example C3bi, one of the major serum proteins in the complement system, opsonizes target pathogens, which then can be recognized by the I-domain of eosinophil CD11b (44, 45). Serum could also contain anti-β-glucan antibody, which binds to β-glucan on A. alternata, and the bound antibody is subsequently recognized by the Fc receptor of eosinophils, leading to eosinophil activation in a β2 integrin-dependent manner (46). However, depletion of C3bi or heat activation of serum in culture medium did not affect the eosinophils’ response to A. alternata organisms nor did FcγRII blocking antibody (Figure 5). Thus, the β-glucan molecules on the A. alternata cell wall are likely recognized directly by eosinophil CD11b, although the potential involvement of unknown serum-derived intermediate molecules cannot be excluded.
Interestingly, there were clear differences in the various anti-CD11b mAbs used to block eosinophil and neutrophil responses to γ-glucan. An anti-I-domain antibody (clone 2LPM19c) prevented the activation of both eosinophils and neutrophils stimulated with purified γ-glucan. In contrast, an anti-CD11b mAb (clone M1/70), which blocks the lectin-domain function of CD11b, and another lectin-domain blocking mAb (clone 5C6) (data not shown) failed to block eosinophil activation by γ-glucan, but the clone M1/70 did inhibit neutrophil activation by more than 70% (Figure 7B). Both clones, 2LPM19C and M1/70, bound similarly to eosinophils and neutrophils (Figure 7A). Then, how can we explain the different inhibitory effects of anti-CD11b blocking mAbs, in particular the differential effects of the antibodies, which block lectin-domain function on eosinophils and neutrophils? In eosinophils and neutrophils CD11b may have different carbohydrate modifications or different surface interacting molecules or both. For example, CD11b may be physically associated with a low affinity IgG receptor, FcγRIII (CD16), which is expressed abundantly on neutrophils, but shows limited expression on eosinophils (47, 48). In contrast, we found recently that a GPI-anchored protein, CD66b, is constitutively associated with CD11b and plays a critical role in eosinophil activation (49). Therefore, while CD11b is expressed by both eosinophils and neutrophils, it may be involved differently in the effector functions of these cell types. In general, the lectin-domain of CD11b is considered essential for glucan recognition by phagocytic cells (28–30). Therefore, a major question still remains: how does the CD11b on eosinophils recognize and/or interact with γ-glucan without the involvement of a lectin-domain?
Fungi, including A. alternata, are multicellular organisms and express various biological molecules during different stages of their lives. Therefore, the mechanisms involved in the immunological and inflammatory responses against fungi are likely complex. Furthermore, fungi are genetically highly diverse; thus the immunological responses to them may also be diverse. For example, Candida spores are small and phagocytosable (50); in contrast, A. alternata spores are much larger (~50 μm) than the size of leukocytes. This study shows for the first time that eosinophils demonstrate anti-fungal immune responses with intact A. alternata. The potential clinical implications of this observation and the further elucidation of the pathophysiology of human diseases are likely substantial. Apparently, the innate immunity exerted by human eosinophils may reach beyond parasitic helminths and may extend to other non-phagocytosable organisms such as filamentous fungi. Furthermore, recent studies suggest that the fundamental pathogenesis of asthma and allergic responses may be dysregulated immune responses to chitin-encased insects and fungi (15, 16), which were evolutionally developed to protect against chitin-encased parasites. The immunological responses of eosinophils to both parasitic helminths and fungi fit perfectly with this model and lead us to speculate that eosinophils may play an important role in such dysregulated immune responses. Several epidemiological studies also implicate exposure or sensitization to Alternaria in the development and exacerbation of allergic airway diseases (17–19). Thus, dysregulated immune responses of eosinophils to A. alternata, other filamentous fungi, and potentially other chitin-encased insects, such as mites and cockroaches, may play a pivotal role in chronic inflammation and the pathology of the airways in human disease, such as asthma.
Acknowledgments
The authors thank Diane L. Squillace, James L. Checkel and Gail M. Kephart for technical assistance, Cheryl R. Adolphson for editorial assistance and LuRaye S. Eischens for secretarial help.
Abbreviations
- CS
Calf Serum
- DCs
Dendritic cells
- ECP
eosinophil cationic protein
- EDN
eosinophil-derived neurotoxin
- MBP
eosinophil major basic protein
- MPO
myeloperoxidase
- PI
Propidium iodide
References
- 1.Kita H, Adolphson CR, Gleich GJ. Biology of eosinophils. In: Middleton E Jr, Reed CE, Ellis EF, Adkinson NF Jr, Yunginger JW, Busse WW, editors. Allergy: Principles and practice. 6. St. Louis: Mosby-Year Book, Inc; 2003. pp. 305–332. [Google Scholar]
- 2.Behm CA, Ovington KS. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol Today. 2000;16:202–209. doi: 10.1016/s0169-4758(99)01620-8. [DOI] [PubMed] [Google Scholar]
- 3.Lee CH, Chuang HY, Shih CC, Jee SH, Wang LF, Chiu HC, Chang CH, Wu CS, Yu HS. Correlation of serum total IgE, eosinophil granule cationic proteins, sensitized allergens and family aggregation in atopic dermatitis patients with or without rhinitis. J Dermatol. 2004;31:784–793. doi: 10.1111/j.1346-8138.2004.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 4.Ponikau JU, Sherris DA, Kephart GM, Kern EB, Congdon DJ, Adolphson CR, Springett MJ, Gleich GJ, Kita H. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005;116:362–369. doi: 10.1016/j.jaci.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Walsh GM. Eosinophil granule proteins and their role in disease. Curr Opin Hematol. 2001;8:28–33. doi: 10.1097/00062752-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Chung HL, Hwang JB, Kwon YD, Park MH, Shin WJ, Park JB. Deposition of eosinophil-granule major basic protein and expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in the mucosa of the small intestine in infants with cow’s milk-sensitive enteropathy. J Allergy Clin Immunol. 1999;103:1195–1201. doi: 10.1016/s0091-6749(99)70199-5. [DOI] [PubMed] [Google Scholar]
- 7.Kapp A. The role of eosinophils in the pathogenesis of atopic dermatitis--eosinophil granule proteins as markers of disease activity. Allergy. 1993;48:1–5. doi: 10.1111/j.1398-9995.1993.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 8.Leiferman KM, Ackerman SJ, Sampson HA, Haugen HS, Venencie PY, Gleich GJ. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N Engl J Med. 1985;313:282–285. doi: 10.1056/NEJM198508013130502. [DOI] [PubMed] [Google Scholar]
- 9.Peters MS, Schroeter AL, Kephart GM, Gleich GJ. Localization of eosinophil granule major basic protein in chronic urticaria. J Invest Dermatol. 1983;81:39–43. doi: 10.1111/1523-1747.ep12538380. [DOI] [PubMed] [Google Scholar]
- 10.Greenberger PA. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2002;110:685–692. doi: 10.1067/mai.2002.130179. [DOI] [PubMed] [Google Scholar]
- 11.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 12.Ponikau JU, Sherris DA, Kephart GM, Adolphson C, Kita H. The role of ubiquitous airborne fungi in chronic rhinosinusitis. Clin Rev Allergy Immunol. 2006;30:187–194. doi: 10.1385/CRIAI:30:3:187. [DOI] [PubMed] [Google Scholar]
- 13.Wagner JM, Franco M, Kephart GM, Gleich GJ. Localization of eosinophil granule major basic protein in paracoccidioidomycosis lesions. Am J Trop Med Hyg. 1998;59:66–72. doi: 10.4269/ajtmh.1998.59.66. [DOI] [PubMed] [Google Scholar]
- 14.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wills-Karp M, Karp CL. Chitin checking--novel insights into asthma. N Engl J Med. 2004;351:1455–1457. doi: 10.1056/NEJMcibr041294. [DOI] [PubMed] [Google Scholar]
- 16.Dickey BF. Exoskeletons and exhalation. N Engl J Med. 2007;357:2082–2084. doi: 10.1056/NEJMe0706634. [DOI] [PubMed] [Google Scholar]
- 17.Salo PM, Arbes SJ, Jr, Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118:892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantani A, Ciaschi V. Epidemiology of alternaria alternata allergy: a prospective study in 6840 Italian asthmatic children. Eur Rev Med Pharmacol Sci. 2004;8:289–294. [PubMed] [Google Scholar]
- 19.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Ide M, Weiler D, Kita H, Gleich GJ. Ammonium chloride exposure inhibits cytokine-mediated eosinophil survival. J Immunol Methods. 1994;168:187–196. doi: 10.1016/0022-1759(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 21.Filley WV, Ackerman SJ, Gleich GJ. An immunofluorescent method for specific staining of eosinophil granule major basic protein. J Immunol Methods. 1981;47:227–238. doi: 10.1016/0022-1759(81)90123-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee DG, Kim DH, Park Y, Kim HK, Kim HN, Shin YK, Choi CH, Hahm KS. Fungicidal effect of antimicrobial peptide, PMAP-23, isolated from porcine myeloid against Candida albicans. Biochem Biophys Res Commun. 2001;282:570–574. doi: 10.1006/bbrc.2001.4602. [DOI] [PubMed] [Google Scholar]
- 23.Kim JT, Schimming AW, Kita H. Ligation of Fc gamma RII (CD32) pivotally regulates survival of human eosinophils. J Immunol. 1999;162:4253–4259. [PubMed] [Google Scholar]
- 24.Bowman SM, Free SJ. The structure and synthesis of the fungal cell wall. Bioessays. 2006;28:799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 26.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 27.Brown GD. Dectin-1: a signaling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 28.Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol. 1999;162:2281–2290. [PubMed] [Google Scholar]
- 29.Vetvicka V, Thornton BP, Ross GD. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 31.Culley FJ, Brown A, Girod N, Pritchard DI, Williams TJ. Innate and cognate mechanisms of pulmonary eosinophilia in helminth infection. Eur J Immunol. 2002;32:1376–1385. doi: 10.1002/1521-4141(200205)32:5<1376::AID-IMMU1376>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 33.Dixon H, Blanchard C, Deschoolmeester ML, Yuill NC, Christie JW, Rothenberg ME, Else KJ. The role of Th2 cytokines, chemokines and parasite products in eosinophil recruitment to the gastrointestinal mucosa during helminth infection. Eur J Immunol. 2006;36:1753–1763. doi: 10.1002/eji.200535492. [DOI] [PubMed] [Google Scholar]
- 34.Butterworth AE, Wassom DL, Gleich GJ, Loegering DA, David JR. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979;122:221–229. [PubMed] [Google Scholar]
- 35.Hamann KJ, Gleich GJ, Checkel JL, Loegering DA, McCall JW, Barker RL. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J Immunol. 1990;144:3166–3173. [PubMed] [Google Scholar]
- 36.Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989;142:4428–4434. [PubMed] [Google Scholar]
- 37.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- 38.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 39.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 41.Inoue Y, Matsuwaki Y, Shin SH, Ponikau JU, Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol. 2005;175:5439–5447. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- 42.Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson M, Weineisen M, Andersson T, Truedsson L, Sjobring U. Critical role for complement receptor 3 (CD11b/CD18), but not for Fc receptors, in killing of Streptococcus pyogenes by neutrophils in human immune serum. Eur J Immunol. 2005;35:1472–1481. doi: 10.1002/eji.200424850. [DOI] [PubMed] [Google Scholar]
- 45.Carlson M, Peterson C, Venge P. The influence of IL-3, IL-5, and GM-CSF on normal human eosinophil and neutrophil C3b-induced degranulation. Allergy. 1993;48:437–442. [PubMed] [Google Scholar]
- 46.Capron M, Kazatchkine MD, Fischer E, Joseph M, Butterworth AE, Kusnierz JP, Prin L, Papin JP, Capron A. Functional role of the alpha-chain of complement receptor type 3 in human eosinophil-dependent antibody-mediated cytotoxicity against schistosomes. J Immunol. 1987;139:2059–2065. [PubMed] [Google Scholar]
- 47.Galon J, Gauchat JF, Mazieres N, Spagnoli R, Storkus W, Lotze M, Bonnefoy JY, Fridman WH, Sautes C. Soluble Fcgamma receptor type III (FcgammaRIII, CD16) triggers cell activation through interaction with complement receptors. J Immunol. 1996;157:1184–1192. [PubMed] [Google Scholar]
- 48.Valerius T, Repp R, Kalden JR, Platzer E. Effects of IFN on human eosinophils in comparison with other cytokines. A novel class of eosinophil activators with delayed onset of action. J Immunol. 1990;145:2950–2958. [PubMed] [Google Scholar]
- 49.Yoon J, Terada A, Kita H. CD66b Regulates Adhesion and Activation of Human Eosinophils. J Immunol. 2007;179:8454–8462. doi: 10.4049/jimmunol.179.12.8454. [DOI] [PubMed] [Google Scholar]
- 50.d’Ostiani CF, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191:1661–1674. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]


