Abstract
We previously observed that collagen IV regulates Caco-2 intestinal epithelial cell spreading and migration via Src-dependent p130Cas phosphorylation and stimulates focal adhesion kinase (FAK). However, the role of FAK and the related kinase, Pyk2, in Caco-2 spreading and migration is unclear. FAK- or Pyk2-specific siRNAs reduced protein levels by 90%. However, when detached cells were replated on collagen IV neither individual nor combined FAK and Pyk2 siRNAs affected the cell spreading rate. As combined FAK and Pyk2 siRNAs increased p130Cas protein levels, we cotransfected cells with 1 nm p130Cas siRNA to partially reduce p130Cas protein to control levels. Although p130Cas Tyr(P)249 phosphorylation was reduced by 60%, cell spreading was unaffected. Combined siRNA reduction of FAK, Pyk2 and p130Cas increased cell spreading by 20% compared to p130Cas siRNA alone, suggesting that FAK and Pyk2 negatively regulate spreading in addition to stimulating spreading via p130Cas. FAK-binding mutant SH3 domain-deleted rat p130Cas was not phosphorylated after adhesion and, unlike full-length p130Cas, did not restore spreading after human-specific p130Cas siRNA knockdown of endogenous p130Cas. Together, these data suggest that FAK positively regulates Caco-2 spreading on collagen IV via p130Cas phosphorylation, but also suggests that FAK may negatively regulate spreading through other mechanisms and the presence of additional FAK-independent pathways regulating p130Cas.
Keywords: cell spreading, focal adhesion kinase, intestinal epithelial cell, p130Cas, SH3 domain, type IV collagen
Introduction
Intestinal epithelial cells undergo a continual process of renewal in vivo, becoming progressively more differentiated as they move from the intestinal epithelial crypt up the villus before being released into the lumen (reviewed in Basson, 1998; Podolsky, 1999; Lussier et al., 2000). The differential expression of integrins in intestinal epithelial cells (reviewed in Lussier et al., 2000) and basement membrane extracellular matrix proteins (reviewed in Beaulieu, 1997) along the intestinal epithelial crypt-villus axis in vivo suggests a potential role for cell-matrix interactions in regulating intestinal epithelial cell differentiation and migration, but the signaling mechanisms which regulate this process are unclear. Our previous work in human Caco-2 intestinal epithelial cells, a cell line which progressively differentiates as it grows past confluence (Peterson and Mooseker, 1993; Peterson et al., 1993) and is thus a widely used human intestinal epithelial cell in vitro model system, suggests that Crk binding to Src-phosphorylated p130Cas is an important regulator of intestinal epithelial cell spreading and sheet migration on collagen IV (Sanders and Basson, 2005), one of the major components of the intestinal epithelial basement membrane in vivo. This work also suggests that Crk binding to paxillin, which plays an important role in regulating migration in some cell systems (Petit et al., 2000; Lamorte et al., 2003), is not as important for regulation of these processes in Caco-2 cells on collagen IV.
We have previously observed rapid and persistent autophosphorylation and Src-dependent phosphorylation of focal adhesion kinase (FAK) in Caco-2 cells on collagen IV (Sanders and Basson, 2000). However, the mechanisms by which FAK regulates cell spreading and migration and p130Cas function are not understood for intestinal epithelial cells. FAK overexpression, FAK-null fibroblast and small interfering RNAs (siRNA) FAK knockdown studies indicate a complex role for FAK in cell spreading and migration that varies in different cell types on different matrix proteins. FAK-null fibroblasts exhibit increased focal adhesion formation and impaired spreading and migration on fibronectin (Ilic et al., 1995), and overexpression of FAK, but not the p130Cas binding mutant FAK P712/715A in CHO cells increases chemotactic migration towards fibronectin (Cary et al., 1996, 1998). However, siRNA knockdown of FAK or p130Cas in HeLa cells did not noticeably inhibit the rate of cell spreading and migration on collagen I in the first 50 min after cell adhesion. Over longer time periods, FAK and p130Cas knockdown cells exhibited distinct morphologies, with FAK but not p130Cas knockdown cells exhibiting numerous aberrant protrusions and showing defects in collective cell migration through inhibition of N-cadherin cell junctions (Yano et al., 2004). In this system, the role of FAK in regulating p130Cas function was not described. Additionally, in vivo observations of three-dimensional focal adhesions indicate the presence of tyrosine-phosphorylated paxillin, but not autophosphorylated FAK, in these structures (Cukierman et al., 2001), and other studies indicate that FAK interacts with proteins that negatively regulate cell spreading (Taylor et al., 1998, 1999; Liu et al., 2002). In the present work, we utilized siRNAs to knock down protein levels of FAK and the related kinase Pyk2 in order to examine their role in regulation of p130Cas function and in Caco-2 cell spreading on collagen IV.
Results
Collagen IV adhesion stimulates Pyk2 phosphorylation in Caco-2 cells
FAK is rapidly phosphorylated following adhesion of Caco-2 cells to collagen IV (Sanders and Basson, 2000). As we did not observe an obvious effect of siRNA knockdown of FAK expression on Caco-2 cell spreading on collagen IV in preliminary experiments, we examined whether collagen IV stimulates phosphorylation of the related kinase Pyk2 in control siRNA and FAK siRNA transfected Caco-2 cells (Figure 1). While FAK siRNA transfection reduced FAK protein levels by 92±1% (n=3) and reduced the level of autophosphorylated Tyr(P)397 FAK by 83±1% (n=2), in FAK siRNA transfected cells, we only observed a partial reduction in p130Cas phosphorylation in cells adherent to collagen IV compared to control nontargeting siRNA 1 (NT1) transfected cells adherent to collagen IV. Additionally, we still observed a substantial increase in p130Cas Tyr(P)249 in the FAK siRNA transfected cells adherent to collagen IV compared to the control substrate poly-l-lysine (Figure 1). As observed in fibroblasts obtained from FAK knockout mice (Sieg et al., 1998), Pyk2 protein levels were higher in cells in which FAK protein levels were reduced by siRNA transfection (44.7±9.2% increase compared to control, n=6, p<0.01). Pyk2 Tyr(P)402 autophosphorylation was slightly stimulated following adhesion to collagen IV compared to the control substrate poly-l-lysine in NT1 transfected control cells. However, Pyk2 autophosphorylation was stimulated much more strongly in FAK siRNA transfected cells (Figure 1), which suggested that the inability of FAK siRNA to inhibit cell spreading might be due to an upregulation of Pyk2 activity in FAK siRNA transfected cells.
Figure 1.
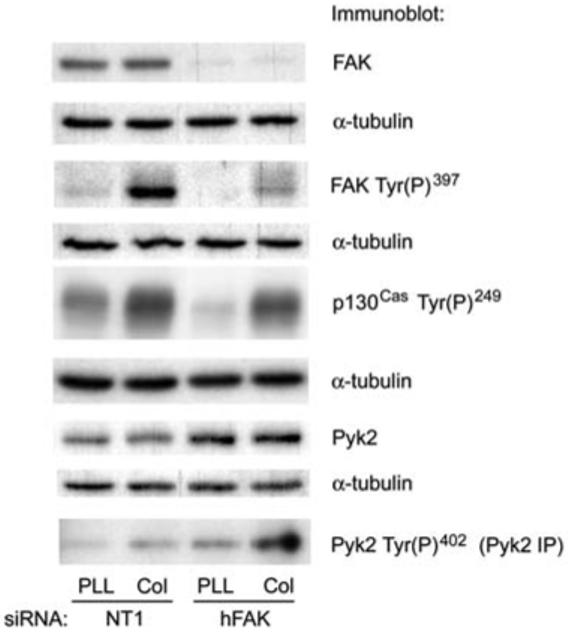
Effect of FAK siRNA on p130Cas phosphorylation and Pyk2 activation initiated by collagen IV.
Caco-2 cells were transfected with 100 nm NT1 control siRNA or siRNA targeting human FAK as described in the materials and methods section. Lysates of cells adherent to the control substrate poly-l-lysine or collagen IV for 20 min were immunoblotted with antibodies to FAK or Pyk2 or antibodies to the autophosphorylation site of each protein and to p130Cas Tyr(P)249.In Caco-2 cells, FAK appears as a single band at approximately 120 kDa, Pyk2 appears as a single band at approximately 110 kDa, and phosphorylated p130Cas appears as a broad band from 125 to 130 kDa. Immunoblots shown are representative of at least two independent experiments with similar results.
Caco-2 cell spreading rate is not affected by siRNA knockdown of FAK and Pyk2
We next examined cell spreading in Caco-2 cells transfected with FAK and Pyk2 siRNAs. Formation of lamellipodial-type extensions was not affected by transfection with FAK and Pyk2 siRNAs alone or in combination (Figure 2A), in contrast to p130Cas or combined Crk and CrkL siRNA transfection which strongly inhibited these processes (Sanders and Basson, 2005). Pyk2 siRNA transfection strongly reduced Pyk2 protein levels by 91±3% (n=3), and both siRNAs reduced protein levels similarly by themselves and in combination with the other siRNA (Figure 2B). Surprisingly, however, when cells were detached and replated on collagen IV, we did not observe a significant effect of either the FAK and Pyk2 siRNAs on the rate of cell spreading (as indicated by measurements of cell size) on collagen IV (Figure 2C). While the combination of FAK and Pyk2 siRNAs slightly inhibited cell spreading, this inhibition did not achieve statistical significance and was much less than the inhibition we previously observed in Caco-2 cells transfected with p130Cas siRNA or the combination of Crk and CrkL siRNAs (Sanders and Basson, 2005).
Figure 2.
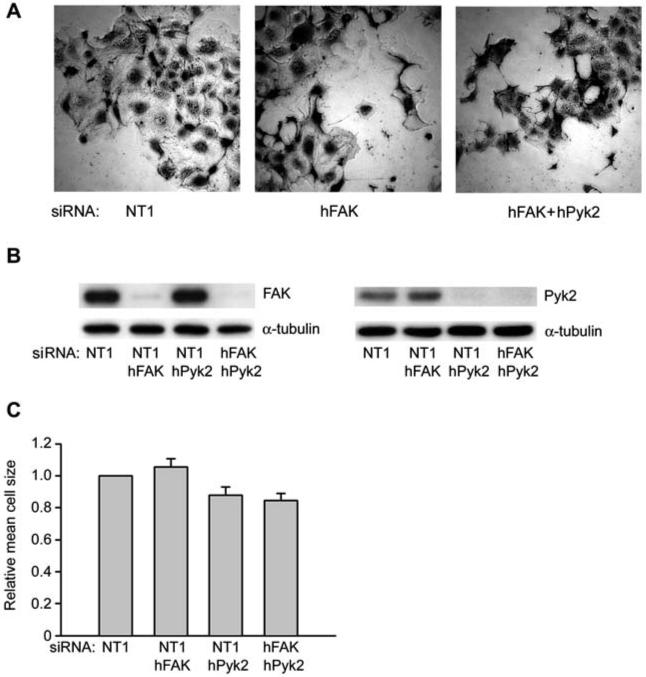
Effect of FAK, Pyk2 or combined FAK and Pyk2 siRNAs on Caco-2 cell spreading on collagen IV.
(A) FAK siRNA and combined FAK and Pyk2 siRNA transfected Caco-2 cells before they were trypsinized and allowed to spread on collagen IV. (B) One of three or more similar immunoblots for FAK or Pyk2 expression is shown from lysates of Caco-2 cells allowed to adhere to collagen IV for 30 min in parallel with cell spreading studies. (C) siRNA transfected cells pictured in panel (A) were trypsinized and allowed to spread on collagen IV for 60-75 min after initial 15 min adhesion as described in the materials and methods section. Cells were plated at low density (ca. 5000 cells per well of a six-well dish) to minimize cell-cell contacts that might interfere with cell spreading. Results are based on three or more independent experiments. Cell size was determined from measurements of at least 200 cells for each condition in each experiment.
siRNA inhibition of FAK and Pyk2 only partially inhibits p130Cas phosphorylation
As our previous work in Caco-2 cells (Sanders and Basson, 2005) and studies in several other cell types (Yano et al., 2000; Huang et al., 2002; Goldberg et al., 2003) indicate an important role for p130Cas in lamellipodial extension and cell spreading, we examined p130Cas expression and phosphorylation in FAK and Pyk2 siRNA transfected cells. Interestingly, we observed a significant increase (Figure 3A) in the p130Cas protein level in FAK siRNA transfected cells compared to control siRNA transfected cells (70±20% increase compared to control, n=4, p<0.05). In Caco-2 cells transfected with both FAK and Pyk2 siRNAs, we observed a 121±21% increase in the p130Cas protein level above that in control transfected cells (n=6, p<0.05 compared to NT1 control). In Figure 3, p130Cas appears as a doublet. In the original paper describing the cloning of p130Cas (Sakai et al., 1994), two different cDNAs are described. One cDNA has an open reading frame of 874 amino acids, while the other has an open reading frame of 968 amino acids. Thus, the doublets observed in this and subsequent immunoblots for p130Cas most likely result from these alternatively spliced forms of p130Cas. We obtained similar results using two different sets of FAK and Pyk2 siRNAs, indicating that the increased expression did not result from a non-specific off-target effect of the siRNA. However, expression of the focal adhesion proteins, paxillin and Crk, was not affected in FAK and Pyk2 siRNA transfected cells (data not shown). As can be seen in Figure 1, phosphorylation of p130Cas Tyr249, which studies on fibroblasts indicate as one of the most important substrate-domain phosphorylation sites for coupling to Crk (Shin et al., 2004), were partially reduced in FAK siRNA transfected cells (46.7±3.6% inhibition, n=3, p<0.01). However, perhaps due to increased p130Cas protein expression, we observed only a moderate reduction in p130Cas Tyr(P)249 in cells transfected with both FAK and Pyk2 siRNAs (Figure 3A; 25.4±9.2% inhibition compared to NT1 control, n=3, p=0.11). Additionally, we observed a comparable increase in p130Cas Tyr(P)249 in combined FAK and Pyk2 siRNA transfected cells adherent to collagen IV compared to cells adherent to the control substrate poly-l-lysine, as observed for FAK siRNA transfected cells in Figure 1 (data not shown).
Figure 3.
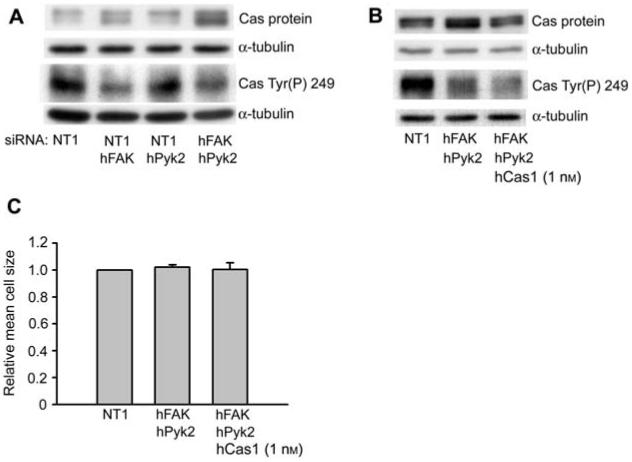
Effect of FAK and Pyk2 siRNAs on p130Cas protein levels and phosphorylation.
(A) Lysates from cell spreading studies described in Figure 2 were immunoblotted for p130Cas protein and p130Cas Tyr(P)249. One of three or more similar immunoblots is shown. In Caco-2 cells, p130Cas appears as a doublet with bands at approximately 120 and 125 kDa. (B) Lysates from cells transfected with 200 nm NT1 control siRNA, combined 100 nm FAK and 100 nm Pyk2 siRNAs, or with 1 nm p130Cas siRNAs in addition to combined FAK and Pyk2 siRNAs were immunoblotted for p130Cas protein and p130Cas Tyr(P)249 after adhesion to collagen IV for 30 min. Lysates were prepared in parallel with spreading studies as for Figure 2. Representative immunoblots from one of three independent experiments are shown. In this representative immunoblot the lanes were rearranged from their original order for clarity of presentation. (C) siRNA transfected cells were allowed to spread on collagen IV for 60-75 min after initial 15 min adhesion as described in the materials and methods section. Results are based on three or more independent experiments. Cell size was determined from measurements of at least 200 cells for each condition in each experiment.
When FAK and Pyk2 siRNA transfected cells were cotransfected with a low concentration of p130Cas siRNA (1 nm) to partially reduce p130Cas protein to similar levels as NT1 control transfected cells (1.06±0.07 p130Cas expression relative to NT1 control siRNA transfected cells, n=3), we observed a 59.6±4.9% (n=3, p<0.01 compared to control) reduction in p130Cas Tyr(P)249 phosphorylation (Figure 3B). However, cell spreading did not significantly differ between Caco-2 cells cotransfected with the combination of FAK, Pyk2, and 1 nm p130Cas siRNA, those transfected only with FAK and Pyk2 siRNA, and control transfected cells (Figure 3C). This indicates that the failure of FAK and Pyk2 siRNAs to affect cell spreading was not caused by increased p130Cas expression.
FAK and Pyk2 siRNA transfection stimulates cell spreading in p130Cas siRNA transfected cells
One possible explanation for the failure of FAK and Pyk2 siRNA transfection to affect cell spreading, despite its partial inhibition of p130Cas phosphorylation, is that FAK and Pyk2 may negatively regulate the rate of cell spreading through other signaling pathways that are involved in this process. If this were true, it might be predicted that the combination of FAK, Pyk2 and p130Cas siRNA treatment would result in increased cell spreading compared to p130Cas siRNA treatment alone. We examined this by comparing cell spreading of FAK, Pyk2 and 100 nm p130Cas siRNA transfected cells and cells transfected only with 100 nm p130Cas siRNA. p130Cas siRNA (100 nm) was equally effective in reducing p130Cas expression alone (89.7±1.0% reduction compared to NT1 control) and in combination with the FAK and Pyk2 siRNAs (87.0±2.6% reduction compared to NT1 control; Figure 4). As we observed previously, siRNA reduction of p130Cas expression strongly inhibited cell spreading by 41±2% (n=5). However, consistent with negative regulatory cell spreading pathways initiated by FAK and Pyk2, the combination of FAK and Pyk2 siRNAs in the presence of 100 nm p130Cas siRNA stimulated cell spreading by 25% compared to 100 nm p130Cas siRNA treatment alone (p<0.05 compared to 100 nm p130Cas siRNA only knockdown cells; Figure 4). We obtained similar results with either of the two FAK and Pyk2 siRNA combinations, indicating this result was not due to non-specific off-target effects of the siRNA. This suggests that FAK and Pyk2 may negatively regulate the rate of cell spreading in Caco-2 cells on collagen IV through pathways independent of p130Cas, in addition to their potential positive regulation of cell spreading via p130Cas phosphorylation.
Figure 4.
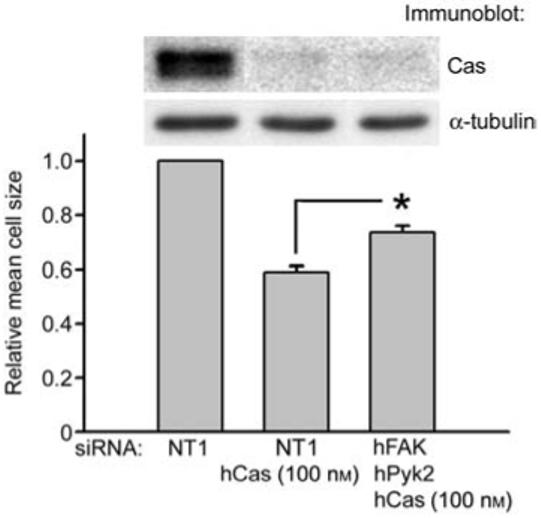
Effect of FAK and Pyk2 siRNAs on Caco-2 cell spreading in p130Cas siRNA knockdown cells.
Caco-2 cells were transfected with 300 nm NT1 control, 200 nm NT1 control and 100 nm p130Cas siRNAs or 100 nm each FAK, Pyk2 and p130Cas siRNAs. Lysates were prepared from cells adherent to collagen IV for 30 min and cell spreading studies on collagen IV were performed in parallel as in Figures 2 and 3. Cell size was determined from measurements of at least 200 cells for each condition in each of five independent experiments using two different combinations of FAK and Pyk2 siRNAs (three experiments using hFAK3 and hPyk2-1 siRNAs, two experiments using hFAK2 and hPyk2-2 siRNAs). In the representative immunoblot, lanes were rearranged from their original order for clarity of presentation. Note that in Figure 4 p130Cas siRNA was used at 100 nm, whereas in Figure 3B and C it was used at a reduced concentration of 1 nm to only partially inhibit the expression of p130Cas.
The SH3 domain of p130Cas is required to rescue inhibition of cell spreading by p130Cas siRNA
p130Cas interacts with FAK primarily via binding of its SH3 domain to the FAK proline-rich region, spanning amino acids 712 to 718 (Polte and Hanks, 1995; Harte et al., 1996), and this region is necessary for association of p130Cas with focal adhesions in Cos-7 cells and Src-transformed NIH3T3 cells (Nakamoto et al., 1997). We sought to confirm a role for FAK signaling through p130Cas in regulation of cell spreading by knocking out endogenous expression of p130Cas and re-expressing either wild type or SH3 domain-deleted rat p130Cas. The human p130Cas siRNA used in these studies does not affect rat p130Cas, as it differs at 6 of 19 nucleotides from the corresponding sequence in rat p130Cas (Sanders and Basson, 2005). While the expression levels of full-length p130Cas and the SH3 domain-deleted rat p130Cas were similar, we observed greatly reduced Tyr(P)249 phosphorylation of SH3 domain-deleted rat p130Cas (lane 4 in Figure 5A) compared to full-length rat p130Cas (lane 3 in Figure 5A). The phosphorylation of SH3 domain-deleted p130Cas did not appear to differ from the background signal in p130Cas siRNA knockdown cells (lane 2 in Figure 5A). However, consistent with the data shown in Figures 3 and 4 suggesting alternate pathways for phosphorylation of p130Cas, expression of the p130Cas binding mutant FAK P712/715A only had a slight effect (21.4±3.9% reduced phosphorylation compared to pKH3 vector control, n=3, p<0.05) on p130Cas Tyr(P)249 (Figure 5B). Although re-expression of full-length rat p130Cas restored cell spreading to control levels, SH3 domain-deleted p130Cas re-expression had no effect on cell spreading on collagen IV (Figure 5C,D). Transfection with the p130Cas binding mutant FAK P712/715A only slightly, but significantly, inhibited cell spreading on collagen IV (13.4±4.5% inhibition, n=5, p<0.05), while overexpression of wild type FAK did not affect cell spreading (Figure 5E). This partial inhibition of cell spreading by the p130Cas binding mutant FAK P712/715A is also consistent with the presence of additional signaling pathways that can initiate p130Cas phosphorylation and cell spreading in the absence of FAK and Pyk2.
Figure 5.
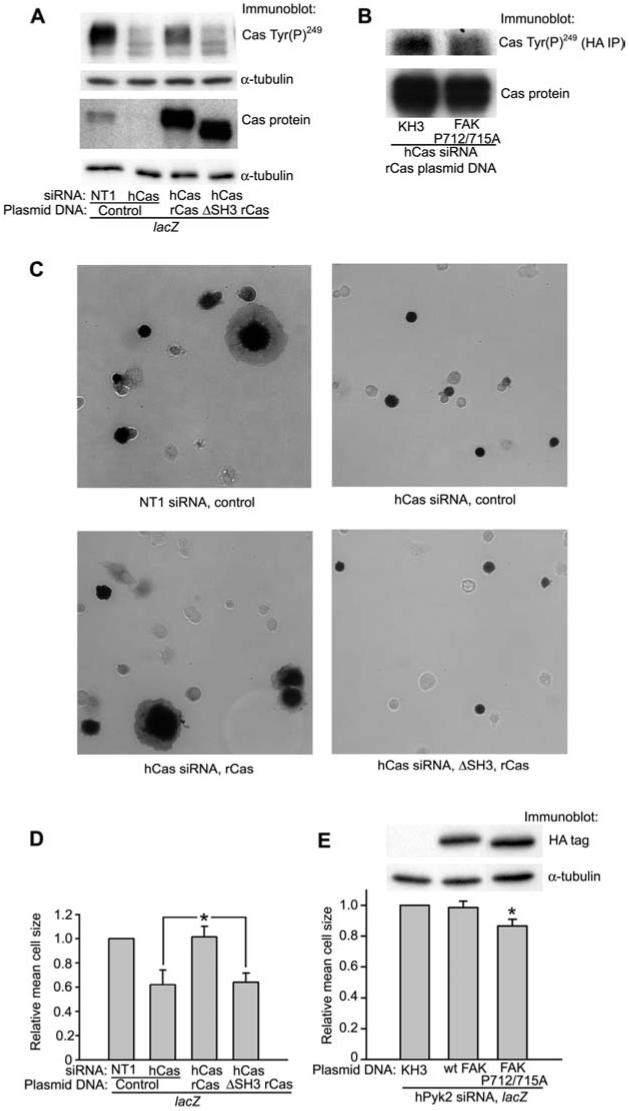
The SH3 domain of p130Cas is required for p130Cas phosphorylation and Caco-2 cell spreading.
(A) Cells transfected with either NT1 control or p130Cas siRNA were transfected with either control plasmid or rat p130Cas expression plasmids as described in the Materials and methods section. Cells were cotransfected with a lacZ expression plasmid to indicate transfected cells. (B) Cells were transfected with the p130Cas binding mutant, FAK P712/715A, and the full-length rat p130Cas expression plasmid in a 4:1 ratio after reduction of endogenous p130Cas with siRNA. Cell lysates were immunoprecipitated with anti-HA antibody and immunoblotted for p130Cas Tyr(P)249. Expression of the FAK P712/715A mutant is shown in panel (E). (C, D) In parallel with the experiment shown in panel (A), cells not used for lysis were allowed to spread for 75 min on collagen IV as described, and then cells were stained for β-galactosidase expression to indicate transfected cells. For the quantification in (D), the size of at least 150 lacZ positive cells (dark cells in the representative pictures in panel C) for each condition was measured. (E) Cells transfected with Pyk2 siRNA were transfected with either control or HA-tagged FAK expression plasmids along with a lacZ expression plasmid to indicate transfected cells. Lysates were prepared and spreading studies performed as in (C) and (D). Cell size was determined from three independent experiments for wild type HA-FAK and five independent experiments for HA-FAK P712/715A. In the representative immunoblot, lanes were rearranged from their original order for clarity of presentation. In each experiment, the size of at least 150 lacZ-positive cells for each condition was measured.
Discussion
The α1β1 and α2β1 integrins, which are the major collagen IV binding integrins in Caco-2 cells (Sanders and Basson, 2004), are differentially expressed in intestinal epithelial cells along the intestinal epithelial crypt-villus axis in vivo, with expression highest in the undifferentiated cells of the crypt region (Beaulieu, 1992). While this differential expression suggests an important role for collagen IV in regulating intestinal epithelial cell behavior in vivo, the mechanisms for this are unclear. In the widely studied Caco-2 intestinal epithelial cell model system, both α1β1 and α2β1 integrins are capable of initiating FAK Tyr(P)397 autophosphorylation, Src-dependent FAK Tyr(P)576 and Tyr(P)925 phosphorylation and cell spreading on collagen IV (Sanders and Basson, 2004). While our previous work indicates that siRNA knockdown of p130Cas and combined siRNA knockdown of the p130Cas binding proteins, Crk and CrkL, strongly inhibits cell spreading, sheet migration and lamellipodial formation, the role of FAK in regulation of p130Cas function in intestinal epithelial cells is unclear. As recent studies have indicated a complex role for FAK in regulation of cell migration depending on the cell type and matrix protein studied (Ilic et al., 1995; Cary et al., 1996, 1998; Cukierman et al., 2001; Yano et al., 2004), and in various cell systems FAK associates with numerous proteins in addition to p130Cas (reviewed in Parsons, 2003; Cohen and Guan, 2005; Mitra et al., 2005), we used siRNA knockdown of FAK and the related kinase Pyk2 to examine their role in regulation of Caco-2 cell p130Cas phosphorylation and cell spreading on collagen IV. Our data clearly indicate an important role for FAK in regulation of p130Cas phosphorylation, but also indicate that FAK regulates cell spreading by additional signaling pathways in Caco-2 cells.
FAK and Pyk2 regulation of p130Cas
The p130Cas SH3 domain mediates its association with FAK (Polte and Hanks, 1995). p130Cas association with Pyk2 (Astier et al., 1997; Keogh et al., 2002), which can partially compensate for FAK function in FAK knockout fibroblasts (Sieg et al., 1998), is also mediated by the p130Cas SH3 domain (Lakkakorpi et al., 1999). Our data indicate that p130Cas Tyr(P)249 phosphorylation and its ability to regulate cell spreading is strongly dependent on its SH3 domain, which has been demonstrated to mediate its interaction with FAK and Pyk2 and is required for FAK-dependent cell migration in transfected CHO cells (Cary et al., 1998). However, we did not observe a complete reduction in p130Cas phosphorylation in FAK and combined FAK and Pyk2 knockdown cells (Figures 2 and 3). Additionally, while overexpression of the p130Cas binding mutant FAK P712/715A inhibited cell spreading on collagen IV, this effect was much less than that which we have previously observed in p130Cas siRNA knockdown cells (Sanders and Basson, 2005) and only resulted in a slight reduction in p130Cas Tyr(P)249 phosphorylation (Figure 5). While the siRNA knockdowns of both FAK and Pyk2 reduced expression of each protein by more than 90% (Figures 1 and 2), it remains possible that this reduced expression level of FAK and Pyk2 is sufficient to explain the incomplete inhibition of p130Cas phosphorylation. However, as p130Cas phosphorylation requires the p130Cas SH3 domain (Figure 5A), these observations suggest that p130Cas may associate with additional proteins via its SH3 domain and that this association may allow it to be partially phosphorylated and function in cell spreading and migration. Phosphatidylinositol 3-kinase dependent phosphorylation of p130Cas independent of FAK is observed in GD25 cells (Armulik et al., 2004), though it was not determined whether this phosphorylation requires the p130Cas SH3 domain. p130Cas also associates via its SH3 domain with the guanine-nucleotide exchange factor C3G (Kirsch et al., 1998). The potential role of these signaling pathways in regulation of p130Cas function in Caco-2 cells on collagen IV remains to be determined.
Additional functions of FAK
Our data indicate that FAK is necessary for complete p130Cas Tyr(P)249 phosphorylation. The findings that combined FAK and Pyk2 siRNA knockdown has no overall net effect on cell spreading even when p130Cas phosphorylation is reduced by 60% (Figure 3), that FAK and Pyk2 siRNA knockdown in combination with p130Cas siRNA knockdown actually increases cell spreading compared to p130Cas knockdown alone (Figure 4) and that overexpression of FAK has no effect on cell spreading (Figure 5) suggests that FAK may actually initiate signaling pathways that negatively regulate cell spreading in addition to its requirement for full p130Cas phosphorylation. The apparent ability of FAK to exert contrasting effects on cell spreading may relate to its central role as both a signaling kinase and as a scaffolding protein in the regulation of focal adhesion turnover and lamellipodium formation (reviewed in Mitra et al., 2005). While our previous data indicate that p130Cas is an essential regulator of lamellipodium formation (Sanders and Basson, 2005), cell spreading and the initial extension of the migrating front in the healing of wounded epithelial cell monolayers is a dynamic process requiring the continuous assembly and disassembly of focal adhesion structures at the migrating front in addition to the extension of actin filaments in formation of lamellipodia (reviewed in Basson, 2001; Dignass, 2001; Wang, 2005). One possible signaling pathway which may explain the contrasting effects of FAK on cell spreading could be the Rho GTPase-activating protein, Graf1, which associates with FAK via the second SH3 domain binding site in the FAK C-terminal region (Hildebrand et al., 1996) and inhibits cell spreading on fibronectin when overexpressed in NIH3T3 cells (Taylor et al., 1999). In preliminary experiments, we have observed that RhoA siRNA knockdown strongly decreases cell spreading in Caco-2 cells (data not shown), which would be consistent with a potential role for FAK inhibition of RhoA in regulation of cell spreading. The ADP ribosylation factor-GTPase activating protein, ASAP1, also associates with FAK via the second SH3 domain and inhibits cell spreading on fibronectin when overexpressed in REF52 cells (Liu et al., 2002). However, the identification and characterization of these additional FAK-dependent signaling pathways in Caco-2 cells is beyond the scope of the present investigation.
In conclusion, we have observed an important role for FAK in regulation of p130Cas phosphorylation in human intestinal epithelial Caco-2 cells. Additionally, our data indicate that interaction of FAK and p130Cas participates in regulation of cell spreading of Caco-2 cells on collagen IV. However, our data also suggest that FAK may regulate cell spreading through additional signaling pathways that remain to be determined. Additionally, our data indicate that there may be additional FAK-independent pathways for collagen IV initiation of p130Cas phosphorylation. Taken together with our previous observations (Yu et al., 2000; Sanders and Basson, 2004, 2005), the results described in this manuscript suggest that FAK and Src-dependent phosphorylation of p130Cas initiated by intestinal epithelial basement membrane matrix proteins, such as type IV collagen, may regulate intestinal epithelial cell migration.
Materials and methods
Materials
Dulbecco’s modified Eagle medium, Oligofectamine, Lipofectamine and Plus Reagent and β-galactosidase detection kit were obtained from Invitrogen (La Jolla, CA, USA). Human transferrin was obtained from Roche Applied Science (Indianapolis, IN, USA). Trypsin, soybean trypsin inhibitor, collagen IV, poly-l-lysine (PLL; Mr 70,000-150,000) and horseradish peroxidase conjugated rabbit anti-mouse IgG were obtained from Sigma (St. Louis, MO, USA). Crk, Paxillin, Pyk2 and p130Cas monoclonal antibodies were obtained from Transduction Laboratories (Lexington, KY, USA). Monoclonal antibodies to α-tubulin, FAK (4.47) and HA tag (12CA5) were obtained from Calbiochem (San Diego, CA, USA), Upstate Biotechnology (Charlottesville, VA, USA) and Roche Applied Science, respectively. Phosphospecific polyclonal antibodies to FAK Tyr(P)397 and Pyk2 Tyr(P)402 were obtained from Biosource International (Camirillo, CA, USA). Phosphospecific polyclonal antibody to p130Cas Tyr(P)249 was obtained from Cell Signaling Technology (Beverly, MA, USA). Protein A sepharose was obtained from GE Healthcare (Piscataway, NJ, USA). siRNAs to human p130Cas, FAK, Pyk2 and control non-targeting siRNA 1 (NT1 siRNA) were purchased from Dharmacon (Lafayette, CO, USA). siRNA sequences were selected using Dharmacon Smartdesign® and corresponded to the following sequences: human p130Cas, 5′-GGT CGA CAG TGG TGT GTA T-3′; human FAK, 5′-TTT GGC GGT TGC AAT TAA A-3′, and 5′-ACC TCG CAG TCA TTT ATC A-3′; human Pyk2, 5′-GGA TCA TCA TGG AAT TGT A-3′, and 5′-ATT CAA GGA TGG AAC ATT A-3′. pSSRα, HA-p130Cas and HA-ΔSH3-p130Cas expression vectors were generously provided by Drs. Tetsuya Nakamoto and Hisamaru Hirai (University of Tokyo, Tokyo, Japan). pKH3, HA-FAK and HA-FAK P712/715A expression vectors were generously provided by Dr. Jun-Lin Guan (Cornell University, Ithaca, NY, USA).
Cell culture
The Caco-2 cell line used for this work was a subclone (Caco-2BBE) of the original Caco-2 cell line that was selected for its ability to differentiate in culture, as indicated by formation of an apical brush border and brush border enzyme expression, and has been previously described (Peterson and Mooseker, 1993; Peterson et al., 1993). Passage 45-67 Caco-2 cells were maintained at 37°C with 8% CO2 in Dulbecco’s modified Eagle medium with 4.5 g/l d-glucose, 4 mm glutamine, 1 mm sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 μg/ml transferrin, 0.1 mm MEM non-essential amino acids solution, 10 mm HEPES pH 7.4, 3.7 g/l NaHCO3 and supplemented with 10% fetal bovine serum.
Coating of cell culture dishes
Cell culture dishes were coated with a saturating concentration (Madri et al., 1988) of collagen IV (12.5 μg/ml) in precoating buffer (15 mm Na2CO3, 35 mm NaHCO3, pH 9.4). Collagen IV-coated tissue culture dishes were overlaid with 1% heat-inactivated (80°C, 30 min) bovine serum albumin (BSA) in PBS for 45 min at 37°C prior to spreading studies.
Cell lysis
Cells were lysed on ice in modified radioimmunoprecipitation buffer (50 mm Tris-HCl pH 7.4, 150 mm NaCl, 1% Triton X-100, 10% glycerol, 1% deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS), 1 mm ethylenediaminetetraacetic acid, 1 mm ethylene glycol-bis[β-aminoethyl ether]-N,N,N’N’-tetraacetic acid, 50 mm phenylmethylsulfonyl fluoride, 1 mm Na3VO4, 1 mm NaF, 10 mm sodium pyrorophosphate, 2 μg/ml aprotinin, 2 μg/ml leupeptin. Lysates were centrifuged at 15,000 g for 10 min at 4°C and supernatants were stored at -80°C. Protein concentrations were determined by the BCA® method (Pierce Chemical, Rockford, IL, USA). Gel loading buffer was then added and samples were boiled and resolved on SDS-polyacrylamide gels. Blots were detected by the ECL® method (GE Healthcare) after transfer of gels to Immobilon P membranes (Millipore, Bedford, MA, USA). Densitometry on autoradiographs of immunoblots was performed using a Kodak 440CF Image Station (Kodak, Rochester, NY, USA) within the linear range of exposure and assay. As indicated in the Figure legends, in some representative immunoblots, lanes were rearranged from their original order for clarity of presentation.
Transfections
Caco-2 cells were plated on collagen I-coated petri dishes at 15-20% confluency the day before siRNA transfection. siRNAs were combined with Plus Reagent in Optimem for plasmid DNA transfection, as described previously (Sanders and Basson, 2000). Oligofectamine in Optimem was used for transfection, according to the manufacturer’s protocol. After overnight transfection, transfection medium was replaced with normal medium overnight, and then normal medium was replaced with 0.3% serum medium 18-24 h prior to spreading experiments. For experiments in which cells were transfected with rat p130Cas plasmid DNA or FAK plasmid DNA, cells transfected overnight with siRNA were transfected with plasmid DNA for 6 h, as described previously (Sanders and Basson, 2000), except that the final Lipofectamine concentration was 5 μg/ml. Transfection medium was then replaced with normal medium overnight before replacement with 0.3% serum medium 18-24 h prior to spreading experiments. For each p100 dish, cells were cotransfected with 4 μg of vector control, rat p130Cas plasmid DNA or FAK plasmid DNA along with 1 μg of β-galactosidase expression vector to indicate cells transfected with plasmid DNA.
Cell spreading assays
Transfected cells were harvested using trypsin-ethylenediaminetetraacetic acid, and trypsinization was stopped using soybean trypsin inhibitor. Cells were then rinsed twice with serum-free medium with 0.1% BSA and allowed to initially adhere for 15 min at 37°C to collagen IV-coated dishes blocked with BSA. Non-adherent cells were then rinsed off with serum free medium and cells were allowed to continue spreading in serum-free medium at 37°C for 60 to 75 min. Cells were plated at low density (ca. 5000 cells per well of a six-well dish) to minimize cell-cell contacts that might interfere with cell spreading. Cells were then fixed with 10% formalin solution and stained with Harris Modified Hematoxylin (Sigma). Measurements of cell size were based on at least 200 cells for each condition in each experiment. For experiments in which cells were transfected with rat p130Cas plasmid DNA, cells were fixed, and β-galactosidase expression was detected using a staining kit from Invitrogen. Measurements of cell size were based on at least 150 lacZ-positive cells for each condition in each experiment.
Statistical analysis
Where indicated, results were compared using the Student t-test and considered statistically significant at a p-value of <0.05. All experiments were carried out independently at least three times unless otherwise indicated.
Acknowledgments
The authors would like to thank the following for technical assistance and generous gifts of expression vectors: the laboratory of Dr. Paul Skoff (Department of Anatomy, Wayne State University Medical School) for assistance with microscopy, Drs. Tetsuya Nakamoto and Hisamaru Hirai (University of Tokyo, Japan) for providing pSSRα, HA-p130Cas and HA-ΔSH3-p130Cas expression vectors and Dr. Jun-Lin Guan (University of Michigan, Ann Arbor, MI, USA) for providing pKH3, HA-FAK and HA-FAK P712/715A expression vectors. This research was supported in part by NIH grant RO1DK067257 and a Department of Veterans Affairs Merit Award (M.D.B.).
References
- Armulik A, Velling T, Johansson S. The integrin β1 subunit transmembrane domain regulates phosphatidylinositol 3-kinase-dependent tyrosine phosphorylation of Crk-associated substrate. Mol. Biol. Cell. 2004;15:2558–2567. doi: 10.1091/mbc.E03-09-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier A, Avraham H, Manie SN, Groopman J, Canty T, Avraham S, Freedman AS. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after β1-integrin stimulation in B cells and binds to p130cas. J. Biol. Chem. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- Basson MD. Role of integrins in enterocyte migration. Clin. Exp. Pharmacol. Physiol. 1998;25:280–285. doi: 10.1111/j.1440-1681.1998.t01-10-.x. [DOI] [PubMed] [Google Scholar]
- Basson MD. In vitro evidence for matrix regulation of intestinal epithelial biology during mucosal healing. Life Sci. 2001;69:3005–3018. doi: 10.1016/s0024-3205(01)01408-4. [DOI] [PubMed] [Google Scholar]
- Beaulieu JF. Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J. Cell Sci. 1992;102:427–436. doi: 10.1242/jcs.102.3.427. [DOI] [PubMed] [Google Scholar]
- Beaulieu JF. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 1997;31:1–78. doi: 10.1016/s0079-6336(97)80001-0. [DOI] [PubMed] [Google Scholar]
- Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr. Cancer Drug Targets. 2005;5:629–643. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm. Bowel Dis. 2001;7:68–77. doi: 10.1097/00054725-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Alexander DB, Pellicena P, Zhang ZY, Tsuda H, Miller WT. Src phosphorylates Cas on tyrosine 253 to promote migration of transformed cells. J. Biol. Chem. 2003;278:46533–46540. doi: 10.1074/jbc.M307526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J. Biol. Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Taylor JM, Parsons JT. An SH3 domain-containing GTPase-activating protein for Rho and Cdc42 associates with focal adhesion kinase. Mol. Cell Biol. 1996;16:3169–3178. doi: 10.1128/mcb.16.6.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Hamasaki H, Nakamoto T, Honda H, Hirai H, Saito M, Takato T, Sakai R. Differential regulation of cell migration, actin stress fiber organization, and cell transformation by functional domains of Crk-associated substrate. J. Biol. Chem. 2002;277:27265–27272. doi: 10.1074/jbc.M203063200. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakat-suji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Keogh RJ, Houliston RA, Wheeler-Jones CP. Human endothelial Pyk2 is expressed in two isoforms and associates with paxillin and p130Cas. Biochem. Biophys. Res. Commun. 2002;290:1470–1477. doi: 10.1006/bbrc.2002.6350. [DOI] [PubMed] [Google Scholar]
- Kirsch KH, Georgescu MM, Hanafusa H. Direct binding of p130Cas to the guanine nucleotide exchange factor C3G. J. Biol. Chem. 1998;273:25673–25679. doi: 10.1074/jbc.273.40.25673. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi PT, Nakamura I, Nagy RM, Parsons JT, Rodan GA, Duong LT. Stable association of PYK2 and p130(Cas) in osteoclasts and their co-localization in the sealing zone. J. Biol. Chem. 1999;274:4900–4907. doi: 10.1074/jbc.274.8.4900. [DOI] [PubMed] [Google Scholar]
- Lamorte L, Rodrigues S, Sangwan V, Turner CE, Park M. Crk associates with a multimolecular Paxillin/GIT2/β-PIX complex and promotes Rac-dependent relocalization of Paxillin to focal contacts. Mol. Biol. Cell. 2003;14:2818–2831. doi: 10.1091/mbc.E02-08-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Loijens JC, Martin KH, Karginov AV, Parsons JT. The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol. Biol. Cell. 2002;13:2147–2156. doi: 10.1091/mbc.E02-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier C, Basora N, Bouatrouss Y, Beaulieu JF. Integrins as mediators of epithelial cell-matrix interactions in the human small intestinal mucosa. Microsc. Res. Tech. 2000;51:169–178. doi: 10.1002/1097-0029(20001015)51:2<169::AID-JEMT8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Madri JA, Pratt BM, Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am. J. Pathol. 1988;132:18–27. [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Nakamoto T, Sakai R, Honda H, Ogawa S, Ueno H, Suzuki T, Aizawa S, Yazaki Y, Hirai H. Requirements for localization of p130cas to focal adhesions. Mol. Cell. Biol. 1997;17:3884–3897. doi: 10.1128/mcb.17.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J. Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. I. Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J. Cell Sci. 1993;105:445–460. doi: 10.1242/jcs.105.2.445. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Bement WM, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. II. Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J. Cell Sci. 1993;105:461–472. doi: 10.1242/jcs.105.2.461. [DOI] [PubMed] [Google Scholar]
- Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Valles AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J. Cell Biol. 2000;148:957–970. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am. J. Physiol. 1999;277:G495–499. doi: 10.1152/ajpgi.1999.277.3.G495. [DOI] [PubMed] [Google Scholar]
- Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc. Natl. Acad. Sci. USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MA, Basson MD. Collagen IV-dependent ERK activation in human Caco-2 intestinal epithelial cells requires focal adhesion kinase. J. Biol. Chem. 2000;275:38040–38047. doi: 10.1074/jbc.M003871200. [DOI] [PubMed] [Google Scholar]
- Sanders MA, Basson MD. Collagen IV regulates Caco-2 migration and ERK activation via α1β1- and α2β1-integrin-dependent Src kinase activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G547–557. doi: 10.1152/ajpgi.00262.2003. [DOI] [PubMed] [Google Scholar]
- Sanders MA, Basson MD. p130cas but not paxillin is essential for Caco-2 intestinal epithelial cell spreading and migration on collagen IV. J. Biol. Chem. 2005;280:23516–23522. doi: 10.1074/jbc.M413165200. [DOI] [PubMed] [Google Scholar]
- Shin NY, Dise RS, Schneider-Mergener J, Ritchie MD, Kil-kenny DM, Hanks SK. Subsets of the major tyrosine phosphorylation sites in Crk-associated substrate (CAS) are sufficient to promote cell migration. J. Biol. Chem. 2004;279:38331–38337. doi: 10.1074/jbc.M404675200. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK-cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Hildebrand JD, Mack CP, Cox ME, Parsons JT. Characterization of graf, the GTPase-activating protein for rho associated with focal adhesion kinase. Phosphorylation and possible regulation by mitogen-activated protein kinase. J. Biol. Chem. 1998;273:8063–8070. doi: 10.1074/jbc.273.14.8063. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Macklem MM, Parsons JT. Cytoskeletal changes induced by GRAF, the GTPase regulator associated with focal adhesion kinase, are mediated by Rho. J. Cell Sci. 1999;112:231–242. doi: 10.1242/jcs.112.2.231. [DOI] [PubMed] [Google Scholar]
- Wang JY. Polyamines regulate expression of E-cadherin and play an important role in control of intestinal epithelial barrier function. Inflammopharmacology. 2005;13:91–101. doi: 10.1163/156856005774423890. [DOI] [PubMed] [Google Scholar]
- Yano H, Uchida H, Iwasaki T, Mukai M, Akedo H, Nakamura K, Hashimoto S, Sabe H. Paxillin α and Crk-associated substrate exert opposing effects on cell migration and contact inhibition of growth through tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA. 2000;97:9076–9081. doi: 10.1073/pnas.97.16.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J. Cell Biol. 2004;166:283–295. doi: 10.1083/jcb.200312013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CF, Sanders MA, Basson MD. Human caco-2 motility redistributes FAK and paxillin and activates p38 MAPK in a matrix-dependent manner. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G952–966. doi: 10.1152/ajpgi.2000.278.6.G952. [DOI] [PubMed] [Google Scholar]


