Abstract
Background
Sustained pressure-overload induces pathologic cardiac hypertrophy and dysfunction. Oxidative stress linked to nitric oxide synthase (NOS) uncoupling may play an important role. We tested whether tetrahydrobiopterin (BH4) can re-couple NOS and reverse pre-established advanced hypertrophy, fibrosis, and dysfunction.
Methods and Findings
C57/Bl6 mice underwent transverse aortic constriction (TAC) for 4-wks increasing cardiac mass (+190%) and diastolic dimension (144%), lowering ejection fraction (−46%), and triggering NOS uncoupling and oxidative stress. Oral BH4 was then administered for five additional weeks of pressure-overload. Without reducing loading, BH4 reversed hypertrophy and fibrosis, re-coupled eNOS, lowered oxidant stress, and improved chamber and myocyte function, whereas untreated hearts worsened further. If BH4 was started at the onset of pressure-overload, it did not suppress hypertrophy after 1wk when NOS activity remained preserved even in untreated TAC hearts. However, BH4 stopped subsequent remodeling, when NOS activity was otherwise declining. A broad anti-oxidant Tempol also reduced oxidant stress, yet did not re-couple NOS nor reverse worsened hypertrophy/fibrosis from sustained TAC. Microarray analysis revealed very different gene expression profiles for both treatments. BH4 did not enhance net protein kinase G activity. Lastly, transgenic mice with enhanced BH4 synthesis confined to endothelial cells were unprotected against pressure-overload, indicating exogenous BH4 targeted myocytes and fibroblasts.
Conclusions
NOS re-coupling by exogenous BH4 ameliorates pre-existing advanced cardiac hypertrophy/fibrosis, and is more effective than a less targeted anti-oxidant approach (Tempol). These data highlight the importance of myocyte NOS uncoupling in hypertrophic heart disease, and support BH4 as potentially new approach to treat this disorder.
Keywords: nitric oxide, reactive oxygen species, heart failure, remodeling, therapeutics
INTRODUCTION
Sustained pressure-overload stimulates pathologic cardiac hypertrophy and dysfunction1, and reversing such maladaptations has emerged as an important therapeutic goal2. A prominent pathway is activation of reactive oxygen species (ROS) which contributes to chamber remodelling and contractile failure3. While treatment with ROS scavengers has not been very effective to date4,5, suppression of key ROS generators in the myocardium may prove more so. Myocardial ROS sources include xanthine and NADPH oxidases, mitochondrial electron transport, and nitric oxide synthase (NOS), and among these, some recent evidence suggests NOS may be particularly important to more advanced dilative disease. NOS behaves somewhat like Jekyll and Hyde, generating NO to provide anti-oxidant and anti-hypertrophic effects, yet contributing to cardiovascular pathobiology if it becomes functionally uncoupled6. This occurs if the normal flow of electrons from NADPH in the reductase domain to heme in the amino-terminus oxidase domain is disturbed, limiting NO synthesis and favouring superoxide generation by dissociation of the ferrous-dioxygen complex6,7. eNOS uncoupling has been documented in hypertension8, diabetes9, atherosclerosis10, and may have a prominent role in cardiac hypertrophic remodeling11.
A major cause for eNOS uncoupling is depletion and/or oxidation of tetrahydrobiopterin (BH4)12,13. BH4 is an obligate co-factor for the three aromatic amino acid hydroxylases14, and insufficiency of phenylalanine hydroxylase causes phenylketonuria, a genetic disorder characterized by progressive mental retardation. BH4 replacement therapy can reduce phenylalanine levels and appears useful for treating a substantial number of these patients15. BH4 is also required for normal NOS function (reviewed in16). It is synthesized de novo from guanosine triphosphate (GTP)17, with the rate limiting enzyme being GTP cyclohydrolase-1 (GTPCH). Models in which GTPCH-1 is genetically enhanced in endothelial cells show suppressed diabetic and atherosclerotic vasculopathy9,10. Effective BH4 levels also depend on redox state, since the oxidized form of BH4 (BH2) does not serve as a NOS cofactor. BH4 levels decline in pressure-overload hypertrophy in conjunction with NOS uncoupling11, but whether BH4 supplementation can treat already established advanced disease and if this indeed involves targeted eNOS re-coupling is unknown. Here, we demonstrate that exogenous BH4 can indeed re-couple NOS and reverse advanced hypertrophy/dilation more effectively than a less specific anti-oxidant strategy.
METHODS
General Experimental Model
76 male mice (C57BL/6, 8–9 wk, 22–24 g) underwent transverse aortic constriction (TAC) as previously described11,18. Animals were screened by echocardiography at 4-weeks for hypertrophy and an EF<70% (chamber dilation). From these animals, 10 were sacrificed for tissue analysis, and the remaining were randomized to receive BH4, Tempol, or vehicle treatment during 5 further weeks of TAC. At 9 wks, subsets of these animals were randomly selected and used for molecular, cellular, and enzymatic assays, histopathology, or in vivo function analysis. BH4 (200 mg/kg/d (Schircks Labs., Jona, Switzerland) or vehicle were mixed in soft diet, and Tempol (10 mg/g food; 1.67 g/kg/day)19 or vehicle premixed in solid food logs (Bio-Serv, Frenchtown, NJ). All animal protocols were approved by the Animal Care and Use Committee of Johns Hopkins University.
Endothelial GTPCH-Tg overexpression
GCH-Tg mice (n=24) and non-transgenic (NTG) control littermates (n=16)9 were subjected to 12-wks TAC. Age-matched sham controls were also generated. Serial echocardiography and final sacrifice tissue analysis were performed.
Cardiac Function and Geometry
In vivo cardiac geometry and function was serially assessed by transthoracic echocardiography (Acuson Sequoia C256, 13 MHz transducer; Siemens) in conscious mice. M-mode LV end-systolic dimensions (LVESD) and LV end-diastolic dimensions (LVEDD) were averaged from 3–5 beats, and data analyzed blinded to heart condition as described11. In a subset of mice, LV function was assessed by pressure-volume relations (SPR 839; Millar Instruments Inc.) in anesthetized animals as described11.
Histology
Myocardium was fixed in 10% formalin and stained using haematoxylin-eosin, PAS methenimine silver, or Masson-trichrome to determine myocyte cross-sectional diameter (mean of 40 cells from 3 slices in 4–5 different hearts) and interstitial fibrosis. Fibrosis was scored 0–3 by a pathologist blinded as to heart condition.
Whole cell myocyte shortening and calcium transients
Adult myocytes were isolated from left ventricles and cell shortening and calcium transient changes (Indo-1-AM) determined by fluorescence microscopy (Diaphot 200; Nikon, Inc) equipped with image/analysis (IonOptix, MyoCam, Milton, MA) as described20. Data were assessed in control and 9-wks TAC hearts with or without BH4 treatment.
eNOS Monomer/Dimer ratio and activity
Cold SDS-Page Western blot analysis was performed in self-made 7-4% SDS-Tris gels run overnight on ice, and then transferred for 3h to nitrocellulose membranes. Primary eNOS antibody (1:350, Santa Cruz, CA) was detected by enhanced chemiluminescence (Pierce, Rockford, IL). NOS activity was measured from myocardial homogenates (80 ig of protein) by C14 arginine to citrulline conversion (Stratagene, La Jolla, CA)11.
PKG activity
PKG-1 activity was assayed from whole heart protein lysates by enzyme linked immunosorbant assay (CycLex-PKG assay kit, MBL, Woburn, MA), and immunoblot for PKG-phosphorylated vasodilator-stimulated protein (VASP), using a monoclonal antibody to pS239 VASP (Alexis, Lausen, Switzerland) at 1:1000 dilution20.
Superoxide Determination
Myocardial superoxide was measured by dihydroethidine (DHE) fluorescent microtopography and lucigenin-enhanced chemiluminescence. Fresh frozen 8μm LV slices were incubated for 1hr at 37°C with DHE (2μM; Invitrogen) and fluorescence imaged as described11. For lucigenin analysis, fresh frozen myocardium was homogenized, centrifuged at 4000 RPM for 30 sec, lucigenin (5 μM) and NADPH (100μM) added to the supernatant, and chemiliminescence measured by scintillation counter (LS6000IC, Beckman Instruments) at 37°C. Data are reported as counts/min/mg protein after background subtraction.
Microarray Analysis
Microarrays for 9wks TAC with and without delayed BH4 and Tempol treatment were performed using the Mouse Genome 430 2.0 array chip (Affymetrix). Details are provided in supplemental methods.
PCR Analysis
Quantitative PCR was performed with an Applied Biosystems Prism 7900HT Sequence Detection System using TaqMan® universal PCR master mix according to the manufacturer’s specifications (Applied Biosystems Inc.). Mann Whitney U-test was used to compare the different groups (SigmaStat®). Details are provided in supplemental methods.
Myocardial BH4/BH2 analysis
Myocardial BH4 and BH2 levels were determined by direct HPLC analysis of frozen tissue homogenates. Details are provided in supplemental methods.
Statistical analysis
All data are expressed as mean ± SEM. Group data were compared using 1 and 2-way ANOVA. Non-parametric data were analyzed using Kruskall-Wallis and Mann-Whitney U test. Reported p values were Bonferoni or Tukey Test adjusted for multiple comparisons (3–5 comparisons depending on the data analyzed). The minimum sample size was n = 4 for any group, and other specific details are provided in the text.
RESULTS
BH4 reverses chronic hypertrophic remodelling and fibrosis
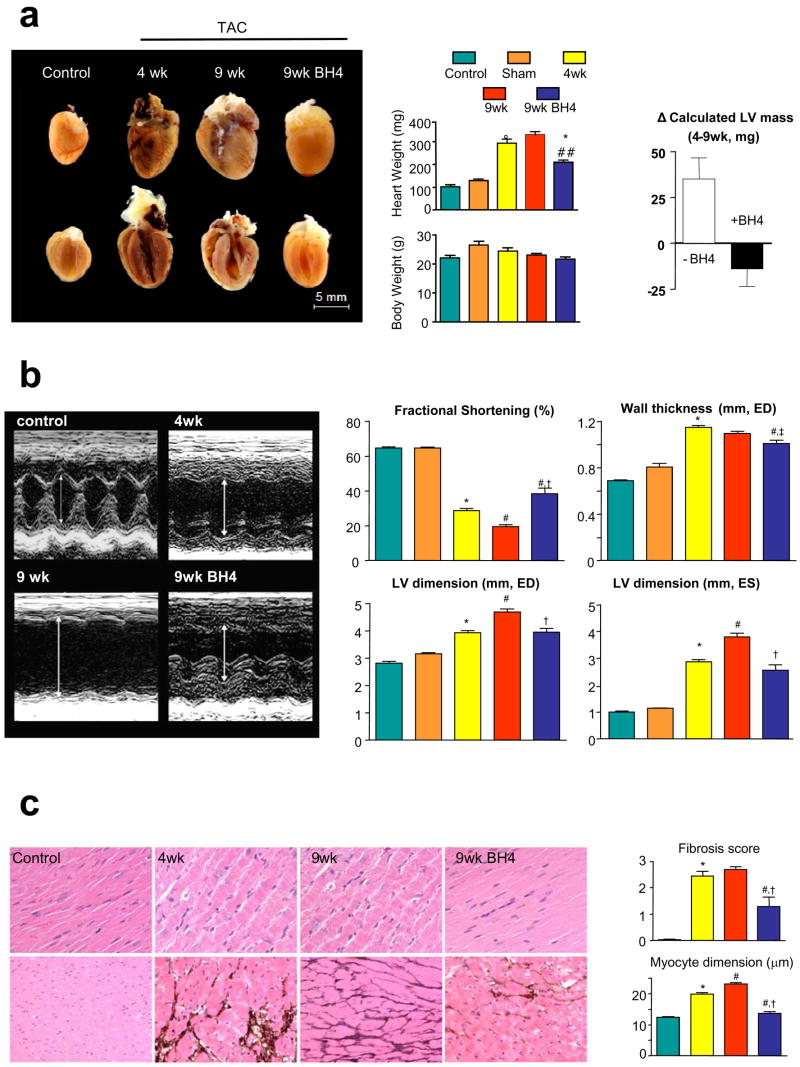
Four weeks of TAC induced substantial remodelling, increasing cardiac mass by 190%, chamber end-diastolic dimension by 140%, and lowering fractional shortening by 44% (Fig 1a,b). Ejection fraction (EF) declined from 87.4±0.5 to 45.7±1.6% (p<0.001). Hypertrophy reversed and heart function improved in mice that subsequently received BH4 for 5-wks of continued TAC (Fig. 1a,b). Chamber dilation was arrested at levels present at the onset of treatment. By contrast, all these features worsened in vehicle-treated mice. Myocyte enlargement and interstitial fibrosis was present at 4-wks TAC and also reversed by BH4 treatment (Fig 1c), whereas both remained elevated or worsened in un-treated 9-wk TAC mice.
Figure 1.
a) BH4 treatment reverses advanced hypertrophy due to sustained pressure-overload. TAC stimulated increases in heart weight at 4-weeks were reversed by the subsequent addition of oral BH4, whereas untreated hearts continued to enlarge. The panel to the right shows paired changes in LV mass between weeks 4–9 (treatment period) between untreated and BH4 treated hearts. These were significantly different (p<0.01). b) Example M-mode echocardiograms showing increased dilation, wall thickening, and reduced fractional shortening after 4-week TAC. This improved in mice treated with BH4. (*: p<0.001 vs control; †,‡ p<0.001, p<0.05 vs 9 wks TAC; #: p<0.001 vs 4-wks TAC). c) BH4 treatment reverses myocyte enlargement and fibrosis after 4-wks TAC. Panels show H/E staining (top) and PAS-m silver-staining (lower). Summary data are provided to the right. Color coding for all panels in this figure follow the legend at the top. (* p<0.001 vs control; † p<0.001 vs 9 wks TAC; # p<0.01 vs 4-wks TAC).
BH4 prevents progressive deterioration of myocardial function
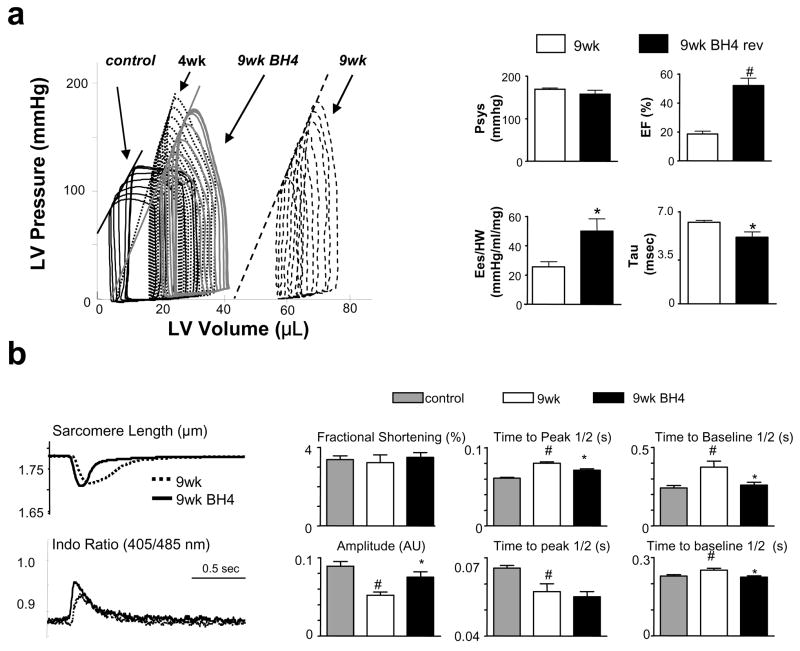
Pressure-volume (PV) relations were obtained to better assess LV function (Fig 2a, Table 1). Rest conditions are reflected by the most rightward PV loop for each set. At 4-wks TAC, hearts were dilated and had increased end-systolic elastance (arrow) typical of hypertrophy. After 9-wks TAC, they became markedly dilated and had depressed function (e.g. reduced end-systolic elastance; Table 1). These changes were prevented by BH4, with end-systolic pressure-volume relations maintaining their position at 4-wks TAC (summary data to the right and Table 1). Importantly, BH4 did not alter ventricular afterload assessed by peak systolic pressure (top left, Fig 2a) or total resistive load (p=0.3, data not shown).
Figure 2.
a) BH4 treatment improves intact heart function. Pressure-volume loops are measured before and during transient inferior vena cava occlusion, with the most rightward loop reflecting rest conditions. 4-wks TAC induced moderate dilation, reduced EF, and increased chamber end-systolic elastance (slope of the end-systolic pressure-volume relation, Ees, arrow) consistent with hypertrophy but contractile compensation. At 9 wks TAC, the loops shifted rightward (dilation) and Ees declined, whereas BH4 treated hearts had less dilation, improved EF, Ees (normlalized to heart mass), and relaxation time constant (tau). Peak systolic pressure (Psys) was similar among groups. (*: p<0.05 vs. 9wks vehicle) b) BH4 improves sarcomere shortening kinetics and calcium transients. Left panel shows example tracings, and summary data are provided to the right. (#: p<0.05 between control and TAC 9wks ; *: p<0.05 between TAC 9wks and TAC 9wks+BH4).
Table 1.
Invasive hemodynamic analysis of TAC and TAC +delayed-BH4 treatment based on pressure-volume analysis.
| Control | TAC 4wk | TAC 9wk + Vehicle (P) | TAC 9wk + BH4 | 4-group | 9wkTAC-P vs 9wkTAC-BH4 | 4wkTAC-P vs 9wkTAC-BH4 | |
|---|---|---|---|---|---|---|---|
| n=5 | n=6 | n=6 | n=5 | p-value | p-value | p-value | |
| Hemodynamics | |||||||
| Heart Rate (beats/min) | 523 ± 14 | 520 ± 13 | 540 ± 15 | 490 ± 20 | 0.17 | ---- | ---- |
| Heart Mass (mg) | 117.6 ± 3.7 | 232.8 ± 6.8 | 303.7 ± 32.8 | 204.6 ± 14.2 | <0.001 | 0.01 | 0.20 |
| LV Peak Pressure (mmHg) | 107 ± 2.2 | 179.9 ± 3.2 | 168.5 ± 3.4 | 157 ±6.1 | <0.001 | 0.14 | 0.018 |
| LV end-diastolic Pressure (mmHg) | 5.4 ± 0.6 | 7.1 ± 1.4 | 6.1 ± 0.8 | 8.0 ± 0.4 | 0.1 | ---- | ---- |
| LV end-systolic Volume (Pl) | 10.2 ± 1.0 | 23.3 ± 3.3 | 55.9 ± 7.5 | 16.9 ± 4.5 | 0.001 | 0.006 | 0.36 |
| LV end-diastolic Volume (Pl) | 29.0 ± 2.0 | 38.8 ± 3.4 | 68.4 ± 7.3 | 33.5 ± 6.0 | 0.006 | 0.01 | 0.59 |
| Stroke Volume | 18.9 ± 1.4 | 15.5 ± 0.6 | 12.5 ± 0.7 | 16.6 ± 2.6 | 0.056 | 0.20 | 0.71 |
| Cardiac Output | 9.9 ± 0.7 | 8.0 ± 0.3 | 6.7 ± 0.3 | 8.2 ± 1.5 | 0.047 | 0.36 | 0.86 |
| Ejection Fraction (%) | 65.1 ± 2.1 | 41.3 ± 3.6 | 18.7 ± 2.2 | 52.0 ± 5.3 | <0.001 | 0.006 | 0.10 |
| Systolic Function | |||||||
| dP/dt max | 13368 ± 370 | 12602 ± 620 | 9836 ± 421 | 10210 ± 618 | 0.001 | 0.36 | 0.006 |
| Ees(norm) | 37.9 ± 5.9 | 70.20 ± 13.40 | 25.6 ± 3.2 | 50.2 ± 8.2 | 0.018 | 0.028 | 0.27 |
| MSW | 79.5 ± 4.1 | 120.75 ± 12.6 | 79.2 ± 8.4 | 113.9 ± 13.7 | 0.01 | 0.028 | 1 |
| Diastolic Function | |||||||
| dP/dt min | −10728 ± 236 | −10508 ± 500 | −8550 ± 189 | −9908 ± 813 | 0.008 | 0.10 | 0.46 |
| Tau(norm) | 3.6 ± 0.1 | 4.4 ± 0.2 | 5.6 ± 0.1 | 4.1 ± 0.2 | <0.001 | 0.006 | 0.27 |
| PFR/EDV | 37.1 ± 5.6 | 24.4 ± 1.4 | 14.5 ± 2.2 | 31.4 ± 4.0 | 0.003 | 0.018 | 0.10 |
LVP: Left ventricular pressure; LVV: Left ventricular volume; dP/dt max: peak rate of pressure rise; Ees(norm) – LV end-systolic elastance (stiffness) normalized to heart mass; MSW: slope of stroke work-end diastolic volume relationship; contractility index; dP/dt min: peak rate of pressure decline; Tau(norm): time constant or relaxation normalized to heart rate; PFR/EDV: Peak filling rate normalized to end-diastolic volume. Four-way group analysis performed by Kruskal-Wallis test; and post hoc comparisons between two groups by Mann-Whitney U test.
To further assess the effect of BH4 on myocardial function, myocytes were isolated from treated and non-treated 9-wk TAC hearts (Fig 2b). The rate of sarcomere shortening and re-lengthening improved with BH4 treatment, and were associated with higher peak calcium transients and a faster transient decay, consistent with improved calcium cycling.
BH4 re-couples eNOS
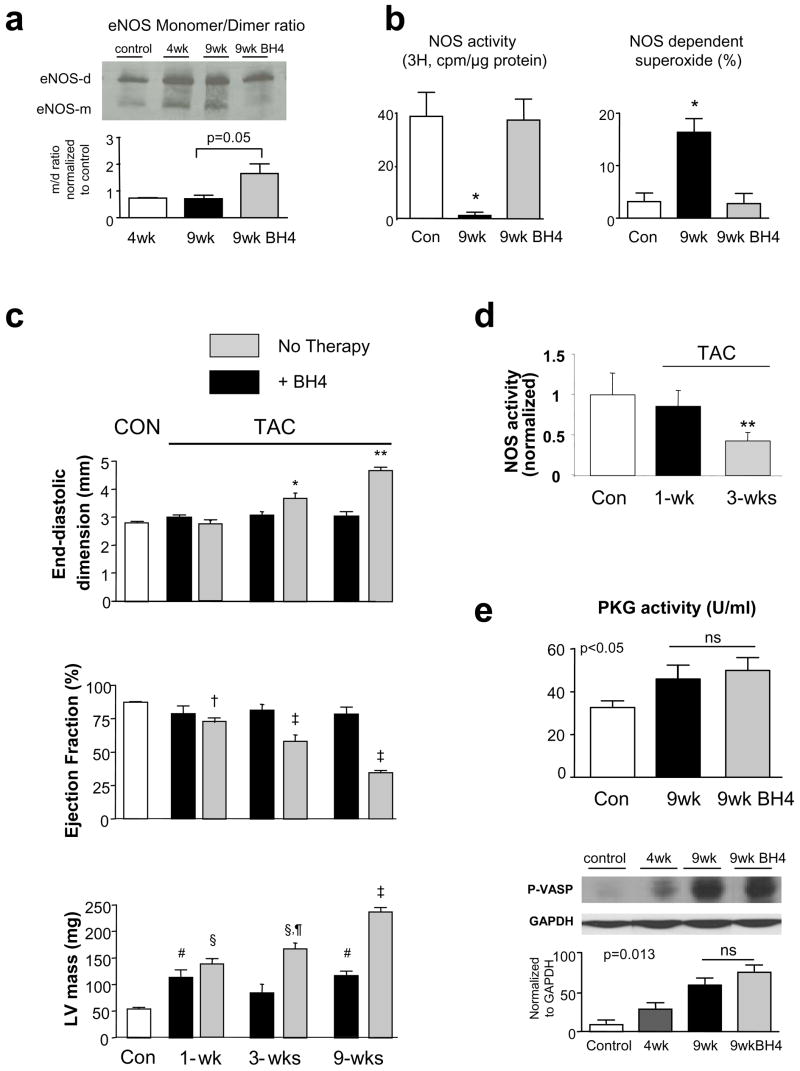
As previously reported, 3–4 wks TAC results in NOS uncoupling indexed by eNOS homodimer instability, reduced Ca2+-dependent NOS activity, and increased NOS-derived ROS11. Here we show data for homodimer instability (higher monomer/dimer ratio in cold-SDS non-reducing gels; Fig 3a). Untreated 9-wk TAC had persistence of this instability, with a marked decline in NOS activity and increased NOS-dependent ROS generation (Fig 3b). These behaviours were restored to normal with BH4 treatment. Total eNOS (monomer + dimer) was unchanged.
Figure 3.
a) Right: eNOS dimer/monomer gel electrophoresis. Control (Con) tissue has principally dimers in the gel, whereas monomers are more apparent at 4 and 9 wks TAC. BH4 restored the control appearance. Relative dimer/monomer density normalized to control is shown in summary. Middle: NOS Ca2+-dependent arginine-citrulline conversion (NOS activity) is reduced after 9 wks TAC and restored to normal by BH4. Right: NOS-dependent superoxide determined as the relative lucigenin chemiluminescent signal reduced after blocking NOS (L-NAME, 100 μM) (*: p<0.01 vs control and 9 wks treated). b) Effect of BH4 treatment from the onset of TAC over 9 week period. Hypertrophy generated after 1 week is unaltered, but thereafter, chamber dilation and hypertrophy progression is blocked by BH4 treatment. Statistics from Tukey Test based on 2-way ANOVA: * p<0.001 vs Con, 1wk, 9wk-TAC, p=0.021 vs untreated 3wk-TAC; ** p<0.001 vs other groups and 9wk untreated; †-p<0.05 vs Con; p<0.005 vs 3, 9wk-TAC; ‡-p<0.005 vs all other groups, p<0.001 vs untreated; #p<0.02 vs Con; § - p<0.001 vs Con, 9wk-TAC; ¶- p<0.001 vs untreated. c) NOS activity measured in non-treated TAC hearts at 1 and 3 wks, data shown normalized to normal control. d) PKG activity increases with 9 wks TAC similarly with or without BH4 treatment. Results for in vitro assay (upper) and p-VASP immunoblot (lower) are shown.
In 6 additional animals, BH4 treatment was initiated at the onset of TAC and continued for 9-wks. After 1-wk TAC, hearts developed non-dilated hypertrophy which was not suppressed by BH4; however, the progressive rise in LV mass, chamber dilation, and decline in EF observed thereafter in controls was prevented by BH4 treatment (Fig 3c; p<0.001 for treatment, time, and treatment×time interaction for each parameter based on 2 way-ANOVA). This result was consistent with the time course of reduced NOS activity. After 1-wk TAC, in vitro NOS activity remained at control levels, whereas it declined by ~50% after 3-wks (Fig 3d), consistent with our earlier report11, and even more by 9 wks (Fig 3b). Thus, BH4 became effective once NOS activity otherwise started to decline.
Effect of BH4 on PKG activity
Improved eNOS activity could potentially suppress hypertrophy by stimulating downstream cGMP-dependent protein kinase (PKG)18,21. PKG activity rose with 9-wks TAC as previously reported with 3-wk TAC18, but was not further enhanced by BH4 treatment (Fig 3e). This was demonstrated by both in vitro activity and pVASP assay (upper and lower panels, respectively).
Antioxidant effect of BH4 and comparison to Tempol
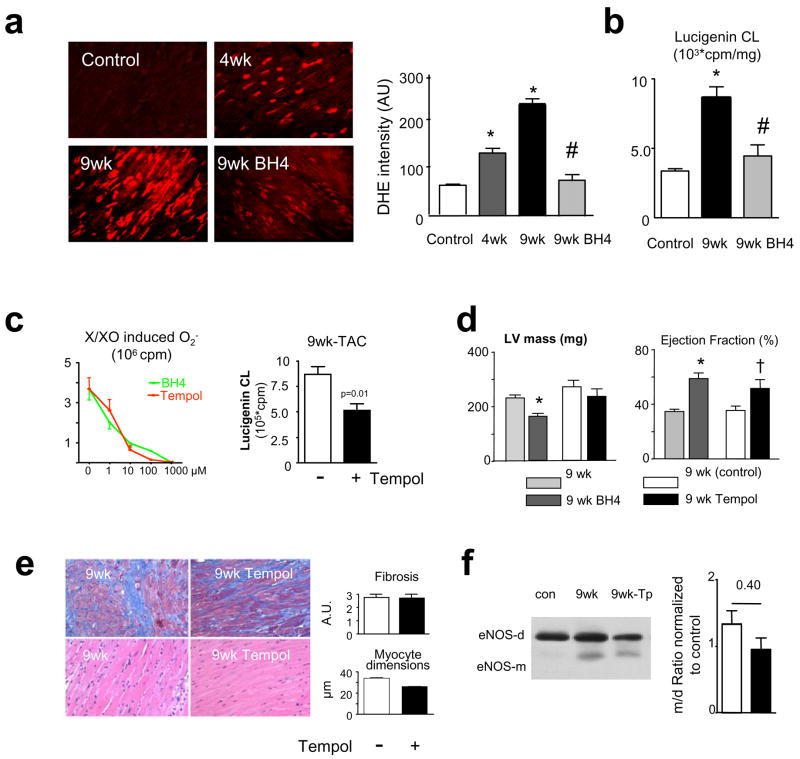
Another mechanism for BH4 efficacy is to target upstream signalling from NO, NO-ROS interaction, or ROS itself. Dihydroethidium (DHE) fluorescent microtopography (Fig 4a) revealed marked ROS generation at 4 and 9wks TAC that fell to near control levels with delayed BH4 treatment. This result was confirmed by lucigenin chemiluminescence (Fig 4b).
Figure 4.
a) BH4 reversed myocardial oxidant stress. Example of DHE fluorescence images show increased ROS at 4 and 9 wks TAC that is reversed with BH4 therapy. Summary data are to the right. b) Lucigenin chemilumenescence (CL) assay confirms a marked rise in O2− at 9 wks TAC that is reversed to control levels by BH4 treatment. c) Left: BH4 and Tempol have similar dose-dependent anti-oxidant effects in vitro. Assay used xanthine/xanthine oxidase O2− generator system. Right: O2−generation in myocardium of 9-wks TAC is reduced by delayed Tempol treatment, similar to that with BH4 (panel d). d) Tempol does not reduce LV mass, but ejection fraction borderline increased (p=0.08). (*:p<0.001 vs 9 wks TAC, †: p=0.08 vs TAC-untreated). c) Tempol does not reduce TAC-stimulated fibrosis, and more modestly reduces myocyte size compared to BH4 (p<0.0001). Upper panels are stained with Masson-trichrome, bottom with H/E. e) Tempol does not reverse eNOS uncoupling reflected in dimer/monomer ratios on immunoblot.
Given this potent anti-oxidant effect, we tested whether BH4 therapeutic benefits could be duplicated using a broad antioxidant. Using the same delayed treatment TAC protocol, mice received control diet, or food pre-mixed with the nitroxide Tempol (30–50mg/d), a superoxide dismutase mimetic that also suppresses hydroxyl, hydrogen peroxide, and other radicals22. Both Tempol and BH4 were equally effective in scavenging superoxide in vitro (Fig 4c, left), and Tempol reduced myocardial superoxide potently and similarly to BH4 in TAC hearts (Fig 4c, right; c.f. Fig 4b). Yet, Tempol did not reverse or prevent progressive hypertrophy (Fig 4d), or impact fibrosis from sustained TAC, and myocyte size declined less than with BH4 (Fig 4e). Tempol increased EF (Fig 4d) by reducing end-systolic dimensions (3.8±0.4 vs 2.8 ±0.4 mm, p<0.05), so some systolic improvement resulted, though it did not restore eNOS coupling (Fig 4f).
To further probe differences between these therapies, gene-expression microarrays were performed (Supplemental Table 1). Quantitative RTPCR was performed on a subset of genes to confirm array results. 9-wks TAC principally stimulated genes controlling collagen synthesis/degradation, tissue growth factor-β signaling, and reduced expression of genes controlling metabolism. Intriguingly, none of these were significantly offset by BH4 or Tempol. Instead, BH4 increased expression of genes regulating lipid metabolism (e.g. fatty acid binding protein 1, apolipoprotein A-1, major urinary protein 1,2), and kallikrein signaling (e.g. plasminogen, fibrinogen). Only 8% of these genes were similarly affected by BH4 alone (without TAC), and none of these related to lipid metabolism. A more complete list of BH4 modified genes is provided in Supplemental Table 2. Tempol altered virtually none of the same genes as BH4, but modestly lowered expression of a different set e.g. phospholipase C -β4, γ1, AMP-kinase α2, GSK3-β, MAP4k3, PKG-1 and flavin containing monooxygenase 2 (the only gene with similar changes from BH4). Thus suppressing TAC-induced ROS, more broadly or by a NOS-targeted strategy, resulted in very different gene profiling and phenotype.
Non-endothelial BH4 is central for its anti-hypertrophic effects
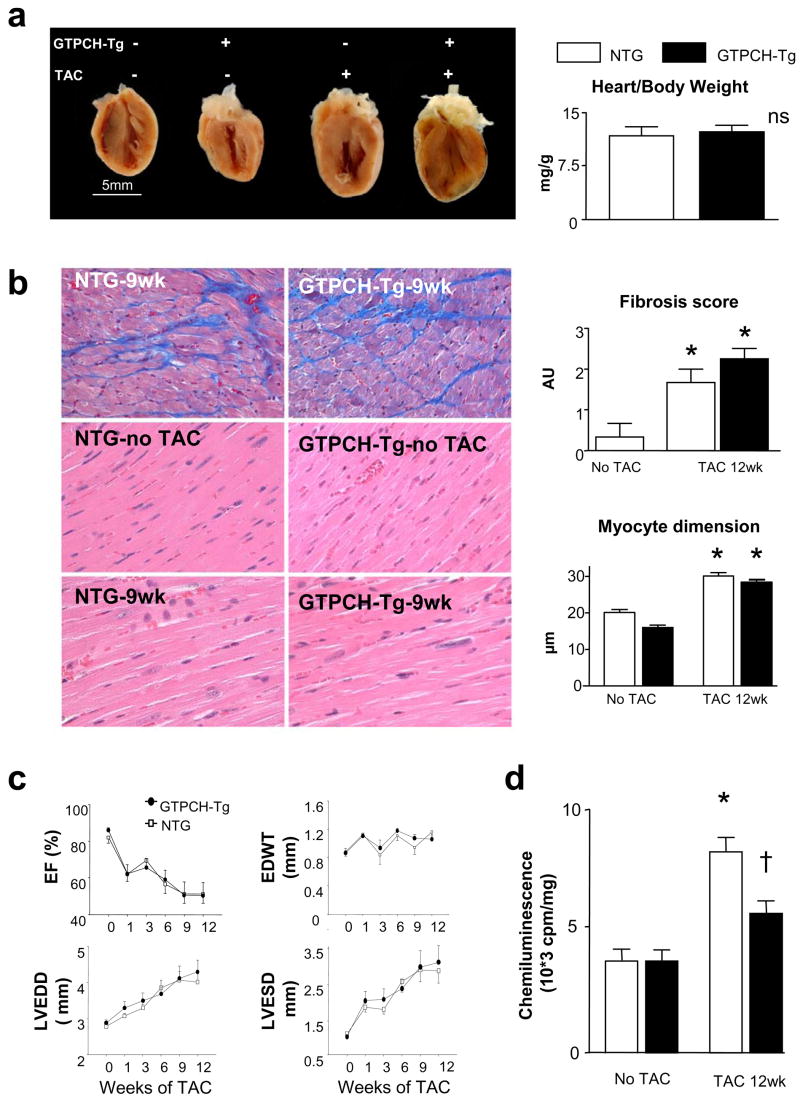
Exogenous BH4 can diffuse into both myocytes and the vascular endothelium, and as endothelial cells contain 80% of eNOS in the myocardium, this might be the presumed primary target. To test this, mice were studied that overexpressed GTP cyclohydrolase (GCH) only in endothelial cells using a Tei-2 promoter9. GCH is the rate-limiting enzyme involved in de-novo BH4 synthesis, and in this model, isolated myocyte BH4 levels are unaltered9, whereas total myocardial levels rise ~4 fold (5.2±3.5 to 19.3±4.9 pmol/mg protein), similar to that achieved by exogenous BH4 (40.5±19.1 pmol/mg protein). Intriguingly, chamber hypertrophy, fibrosis, myocyte enlargement, heart function, and dilation changed identically during 12-wks TAC in the hearts of GCH-Tg and littermate controls (Fig 5). However, superoxide declined in GCH-Tg myocardium (p<0.05), suggesting a role of endothelial NOS-uncoupling to myocardial ROS that is less associated with cardiac hypertrophic remodeling. These data indicate that the effectiveness of BH4 to ameliorate pressure-overload cardiac dysfunction and remodeling lies in its targeting of NOS uncoupling in myocytes (and perhaps fibroblasts) rather than in the endothelium.
Figure 5.
a) Mice overexpressing GCH in endothelial cells develop progressive hypertrophy with TAC similar to litter-mate (NTG) controls. Summary data for heart/body weight shown to right. b) Transgenic animals develop similar interstitial fibrosis (* p<0.05 vs no TAC) and myocyte enlargement (* p<0.001 vs no TAC) as controls. c) TAC-induced decline in ejection fraction (EF), and increase in wall thickness (WT), left ventricular end-diastolic and end-systolic dimensions (LVEDD, LVESV) were virtually identical in GCH-Tg and littermate controls. d) Superoxide increases significantly more in NTG than in GCH-Tg mice subjected to 12 weeks.
DISCUSSION
The ability of exogenous BH4 to reverse advanced hypertrophic remodeling and ameliorate heart and myocyte function despite ongoing pressure-overload is unusual among existing therapies, and suggests that targeting un-coupled eNOS may be a potent and useful strategy for treating hypertrophic heart disease. Few experimental studies involving established advanced disease models have shown the capacity of an intervention to reverse the process. Much of the recent work has relied on genetically engineered models where the manipulation is generated at or before birth, and interventions initiated at or shortly after the induction of myocardial stress. In clinical trials, however, advanced disease is often required, making the present results particularly intriguing from a translational perspective. We specifically targeted pathologic hypertrophy coupled to cardiac decompensation, a period where ROS generation may be particularly important. Drugs such as angiotensin converting enzyme and receptor blockers blunt disease progression23, but the capacity of BH4 to reverse this pathobiology is striking and supports a detrimental role of NOS uncoupling and the nitroso-redox imbalance24 that ensues.
NOS uncoupling impairs NO synthesis and its downstream effecter signaling (i.e. cGMP and PKG) while concomitantly increasing ROS generation. Both aspects can trigger myocardial hypertrophy and remodeling3. Myocardial PKG signaling coupled to natriuretic peptides25,26 or modulation of cGMP catabolism18 blunts cardiac hypertrophy and can improve heart function. This is thought in part due to suppression of calcineurin and NFAT activation21, and other pathways are likely important as well18,27. Since NO stimulates soluble guanylate cyclase to generate cGMP and thus activate PKG, improved NOS function by BH4 could potentially involve this anti-hypertrophic mechanism. Yet enhanced PKG activity was not observed, and there are several potential reasons for this. BH4 did not hyper-stimulate NOS but returned its activity to normal control levels where basal PKG activation is typically low. PKG activity could have already been maximal, though this is unlikely since enhancing cGMP via PDE5a inhibition (e.g. sildenafil) during TAC can potently activate PKG further18. PKG was also activated more with 9-wk TAC alone over control (similar to results after 3-wk TAC18) despite reduced NOS activity, indicating that alternative mechanisms such as cGMP generated by natriuretic peptides (both ANP and BNP expression rose with TAC, supplemental Table 1) or oxidant stress28 could have played a role. While BH4 therapy might lower one source such as oxidant stress, it could raise another (i.e. eNOS), leaving net PKG activation unchanged.
An alternative to cGMP/PKG mechanisms is the modulation of NO and NO-ROS interactions by BH4. This would include S-nitrosylation that can alter cardiac contractile regulation29, or local interaction of NOS-derived ROS with NO (forming peroxynitrite)30 perhaps in a particularly vulnerable sub-cellular compartment. Recoupling NOS would steer superoxide-derived ROS formation away from peroxide, which may be important. Restored NOS activity would not itself be expected to stimulate peroxynitrite in the absence of oxidant stress, as this is not observed in normal hearts, and if anything, BH4 appears to lower peroxynitrite in oxidant stress disorders31.
The present data support a growing notion that ROS signaling is compartmentalized32 and targeting specific oxidant generators may be more efficacious than broader anti-oxidant scavengers. Though both Tempol and BH4 provided similar in vitro and tissue (lucigenin) anti-oxidant effects, their impact on hypertrophy and fibrosis, and on NOS re-coupling were quite different. This is not likely due to an insufficient Tempol dose, as the dose used was fairly high (equivalent to 58 mM)19. It is also the highest dose mice will tolerate in their food due to the taste. Though clinical trials testing broad antioxidant strategies have been fairly unimpressive to date4,5, this may be analogous to continuously applying sponges to blot up water from an open faucet versus turning the faucet off. The latter, i.e. suppressing a strategic ROS source, might well provide more effective results.
There are other oxidant sources in the heart besides uncoupled NOS, though the impact of their inhibition on hypertrophic remodeling remains unclear. Xanthine oxidase (XO)-derived free radicals have been found to play a role in dilated cardiac failure, and allopurinol or its active metabolite oxypurinol which block XO improve myocardial efficiency, NO-ROS balance, and myofilament calcium-sensitization33. However, in clinical trials, these drugs did not improve symptoms or exercise capacity34. Furthermore, their role in hypertrophic disease has not been established. NADPH oxidases have also been widely studied35. Genetic studies in mice lacking NOX2 (gp91phox) found similar hypertrophic responses due to aortic banding as in controls36,37, but somewhat less fibrosis. Other NOX oxidases such as NOX4 may be important, though this remains to be confirmed. Importantly, small molecule inhibitors remain scant, and none are clinically viable or sufficiently selective at present. Lastly, ROS can derive from mitochondrial electron transport leakage which also may contribute to cardiac failure38, though involvement with pressure-overload hypertrophy remains to be established.
This study has several limitations. Though our data demonstrates that BH4 restores NOS coupling even in advance hypertrophic heart disease, it does not prove this is the sole or necessarily primary mechanism underlying the decline in ROS stimulation or amelioration of hypertrophic remodeling and cardiac function. However, the finding that BH4 administered from the onset of TAC did not suppress hypertrophy during the first week when control heart NOS activity was still preserved, yet prevented progression after that (when NOS uncoupling and reduced activity otherwise occurred) further supports such a link. A second limitation regards the comparison between BH4 and Tempol. As we did not employ a full dose-ranging study, the possibility remains that different pharmacology for the two compounds and/or alternative signalling not revealed by the data obtained, could explain some of the disparate effects.
The present findings suggest NOS-derived ROS plays a particularly important in decompensated hypertrophic heart disease. The existing clinical viability of BH4 as a drug, albeit for a different disorder at present15, should help facilitate clinical translation and testing of the present findings to human heart disease. One of the intriguing potential patient populations are individuals with heart failure and a preserved ejection fraction, also often termed diastolic heart failure. This disorder affects nearly half of all patients with heart failure world-wide, most often in elderly women with hypertension and ventricular hypertrophy39, and its prevalence is rising40. If the present findings can be translated to humans, BH4 might provide a novel and potent therapy to treat this common heart disease.
Acknowledgments
The authors thank Leslie-Anne Boxill, Azeb Haile, Norman Barker, and Pawel Kaminski, for their technical assistance in performing the study. The work was supported by Belgian-American Education Foundation (Collen), PhD-program (University of Antwerp, A.A.P.), and American Heart Association Fellowship Grant (ALM), AHA Scientist Development Grants (HCC, ET), HL081205 (SB), NIEHS center grant P30 ES 03819, HL31069, HL43023 and HL66331 (MSW); AG-18324, HL-47511, and HL-59408, and Abraham and Virginia Weiss Professorship (DAK).
References
- 1.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.McKinsey TA, Kass DA. Small-molecule therapies for cardiac hypertrophy: moving beneath the cell surface. Nature Reviews Drug Discovery. 2007;6:1–18. doi: 10.1038/nrd2193. [DOI] [PubMed] [Google Scholar]
- 3.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 4.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 6.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 7.Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- 8.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 11.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorren AC, Mayer B. Tetrahydrobiopterin in nitric oxide synthesis: a novel biological role for pteridines. Curr Drug Metab. 2002;3:133–157. doi: 10.2174/1389200024605154. [DOI] [PubMed] [Google Scholar]
- 13.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–2444. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem. 1999;68:355–381. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- 15.Levy HL, Milanowski A, Chakrapani A, Cleary M, Lee P, Trefz FK, Whitley CB, Feillet F, Feigenbaum AS, Bebchuk JD, Christ-Schmidt H, Dorenbaum A. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet. 2007;370:504–510. doi: 10.1016/S0140-6736(07)61234-3. [DOI] [PubMed] [Google Scholar]
- 16.Stuehr DJ. Structure-function aspects in the nitric oxide synthases. Annu Rev Pharmacol Toxicol. 1997;37:339–359. doi: 10.1146/annurev.pharmtox.37.1.339. [DOI] [PubMed] [Google Scholar]
- 17.Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 18.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JB, Xavier S, DeLuca AM, Sowers AL, Cook JA, Krishna MC, Hahn SM, Russo A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 20.Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, Moens AL, Champion HC, Kass DA. Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation. 2007;115:2159–2167. doi: 10.1161/CIRCULATIONAHA.106.643536. [DOI] [PubMed] [Google Scholar]
- 21.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, Molkentin JD, Drexler H, Wollert KC. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci U S A. 2002;99:11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. The chemistry and biology of nitroxide compounds. Free Radic Biol Med. 2007;42:1632–1650. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 24.Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006;114:1531–1544. doi: 10.1161/CIRCULATIONAHA.105.605519. [DOI] [PubMed] [Google Scholar]
- 25.Knowles JW, Esposito G, Mao L, Hagaman JR, Fox JE, Smithies O, Rockman HA, Maeda N. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest. 2001;107:975–984. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiedler B, Feil R, Hofmann F, Willenbockel C, Drexler H, Smolenski A, Lohmann SM, Wollert KC. cGMP-dependent protein kinase type I inhibits TAB1-p38 mitogen-activated protein kinase apoptosis signaling in cardiac myocytes. J Biol Chem. 2006;281:32831–32840. doi: 10.1074/jbc.M603416200. [DOI] [PubMed] [Google Scholar]
- 28.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 29.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 30.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kase H, Hashikabe Y, Uchida K, Nakanishi N, Hattori Y. Supplementation with tetrahydrobiopterin prevents the cardiovascular effects of angiotensin II-induced oxidative and nitrosative stress. J Hypertens. 2005;23:1375–1382. doi: 10.1097/01.hjh.0000173520.13976.7d. [DOI] [PubMed] [Google Scholar]
- 32.Madamanchi NR, Moon SK, Hakim ZS, Clark S, Mehrizi A, Patterson C, Runge MS. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol. 2005;25:950–956. doi: 10.1161/01.ATV.0000161050.77646.68. [DOI] [PubMed] [Google Scholar]
- 33.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cleland JG, Coletta AP, Clark AL. Clinical trials update from the Heart Failure Society of America meeting: FIX-CHF-4, selective cardiac myosin activator and OPT-CHF. Eur J Heart Fail. 2006;8:764–766. doi: 10.1016/j.ejheart.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 36.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 37.Maytin M, Siwik DA, Ito M, Xiao L, Sawyer DB, Liao R, Colucci WS. Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation. 2004;109:1168–1171. doi: 10.1161/01.CIR.0000117229.60628.2F. [DOI] [PubMed] [Google Scholar]
- 38.Tsutsui H, Ide T, Kinugawa S. Mitochondrial oxidative stress, DNA damage, and heart failure. Antioxid Redox Signal. 2006;8:1737–1744. doi: 10.1089/ars.2006.8.1737. [DOI] [PubMed] [Google Scholar]
- 39.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta LE, Najjar SS, Kasuya H. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventiruclar hypertrophy in the urban Baltimore community. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 40.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]