Abstract
Opuntia polyacantha (prickly pear cactus) has been used extensively for its nutritional properties; however, less is known regarding medicinal properties of Opuntia tissues. In the present study, we extracted polysaccharides from O. polyacantha and used size-exclusion chromatography to fractionate the crude polysaccharides into four polysaccharide fractions (designated as Opuntia polysaccharides C-I to C-IV). The average Mr of fractions C-I through C-IV was estimated to be 733, 550, 310, and 168 kDa, respectively, and sugar composition analysis revealed that Opuntia polysaccharides consisted primarily of galactose, galacturonic acid, xylose, arabinose, and rhamnose. Analysis of the effects of Opuntia polysaccharides on human and murine macrophages demonstrated that all four fractions had potent immunomodulatory activity, inducing production of reactive oxygen species, nitric oxide, tumor necrosis factor α, and interleukin 6. Furthermore, modulation of macrophage function by Opuntia polysaccharides was mediated, at least in part, through activation of nuclear factor κB. Together, our results provide a molecular basis to explain a portion of the beneficial therapeutic properties of extracts from O. polyacantha and support the concept of using Opuntia polysaccharides as an immunotherapeutic adjuvant.
Keywords: Opuntia polyacantha, prickly pear cactus, polysaccharide, macrophage, reactive oxygen species, nitric oxide, cytokines
1. Introduction
Stimulation of the innate immune system with immunomodulators can increase host resistance to unforeseen pathogenic threats [1], and a number of innate system immunomodulators have been identified, including cytokines [2], substances isolated from microorganisms and fungi [3], and substances isolated from plants [4,5]. Indeed, a wide range of bioactive polysaccharides have been isolated from various medicinal plants, and these polysaccharides have been shown to possess immunomodulatory activity through their ability to modulate macrophage function (reviewed in [6]). Appropriate enhancement of innate immune functions by bioactive compounds can then augment host defense responsiveness [7]. Thus, because of their low toxicity and high potency, plant polysaccharides represent ideal candidates for therapeutics with immunomodulatory action.
Although the prickly pear cactus, Opuntia polyacantha (subfamily Opuntioideae, family Cactaceae), and other Opuntia species are currently consumed for their nutritional properties [8,9], tissues from these cacti have been used traditionally in the past for their pharmacological properties [8,9]. For example, fruits and stems of many Opuntia species have been used in folk medicine for burns, wounds, edema, bronchial asthma, hypertension, indigestion, and type II diabetes (reviewed in [10,11]). Extracts of fruits and stems from Opuntia species have been reported to exhibit hypoglycemic [12], antiulcer [13], antioxidant [14], anti-inflammatory activity [15], hepatoprotective [16], and neuroprotective [17] activities. While glycoproteins and low-molecular weight compounds, such as flavonoids, proanthocyanidins, carotenoids, betalains, beta-sterols, and alpha-pyrones have been suggested to be among the active principles of Opuntia species [13,15,18–22], little is known regarding the medicinal properties of polysaccharides from Opuntia, and essentially nothing is known regarding their potential immunomodulatory properties. Indeed, the main substance produced by these plants is mucilage composed primarily of water and polysaccharides, which helps prevent dehydration and freezing of their tissues.
Among the numerous Opuntia species, bioactive polysaccharides have been isolated and characterized primarily from one member, O. ficus-indica [12,23–26]. Polysaccharides from this species have been reported to exhibit hypoglycemic activity [12] and enhance re-epithelization in a model of dermal wounding [25]. Although macrophages play an important role in the cutaneous wound healing process (reviewed in [27]), the potential immunomodulatory effects of polysaccharides from Opuntia in this process were not evaluated. Based on previous studies showing that many plant polysaccharides enhance immune function by activation of macrophages (reviewed in [6]), we suggest that at least part of the effects of Opuntia polysaccharides on wound healing is through modulation of macrophage function. Thus, it is now important to address this question and further define the contribution of polysaccharides to the therapeutic properties of Opuntia tissues.
In the present study, we extracted polysaccharides from O. polyacantha and isolated four polysaccharide fractions (designated as C-I to C-IV). Analysis of immunomodulatory effects on murine and human monocyte/macrophages demonstrated that Opuntia polysaccharides activated the production of nitric oxide (NO), reactive oxygen species, and cytokines, and stimulated nuclear factor κB (NF-κB). The ability of O. polyacantha polysaccharides to modulate macrophage function suggests they may contribute to the therapeutic potential of tissues derived from this plant species.
2. Materials and methods
2.1. Reagents
β-glucosyl Yariv reagent [1,3,5-tri-(4-β-D-glucosopyranosyloxyphenyl-azo)-2,4,6-trihydroxybenzene] was from Biosupplies Australia (Parkville, Australia). Gum arabic was from Fluka BioChemica (Buchs, Switzerland) and 8-amino-5-chloro-7-phenylpyridol[3,4-d]pyridazine-1,4-(2H,3H)dione (L-012) was from Wako Chemicals (Richmond, VA). Sepharose 6B, galacturonic acid, galactose, arabinose, rhamnose, xylose, diphenylamine, aniline, anthrone, thiourea, trifluoroacetic acid (TFA), N-(1-naphthyl)ethylenediamine, sulfanilamide, phorbol-12-myristate-13-acetate (PMA), horseradish peroxidase, and lipopolysaccharide (LPS) from Escherichia coli K-235 were from Sigma Chemical Co. (St. Louis, MO). Dulbecco’s modified Eagle’s medium (DMEM) and endotoxin-free fetal bovine serum (FBS) were from Mediatech Inc., Herndon, VA). Human granulocyte macrophage colony-stimulating factor (GM-CSF) was from Calbiochem (San Diego, CA).
2.2. Purification and fractionation of polysaccharides
Stems of cactus O. polyacantha were collected near Big Timber, MT. The fresh O. polyacantha stems (1.8 kg) were washed with distilled H2O, homogenized, extracted with 6 L distilled H2O, and the aqueous extract was centrifuged at 2,000×g for 15 min. A four-fold volume of ethanol was added to the supernatant to precipitate polysaccharides overnight at 4°C. The precipitate was pelleted by centrifugation, re-dissolved in distilled H2O, sonicated for 10 min, and centrifuged at 11,000×g for 1 hr. The supernatant was then filtered through a 0.22 µm filter and concentrated in an Amicon concentrator with a 5 kDa PM5 membrane to obtain the crude polysaccharide extract (yield of 0.4% by weight).
The crude polysaccharide extract was fractionated by size-exclusion chromatography (SEC) on a Sepharose 6B column (2.5×92 cm) equilibrated with 0.01 M Tris-HCl buffer (pH 7.0) containing 0.15 M NaCl and eluted with the same buffer at a flow rate of 21 ml/hr. The carbohydrate elution profile was determined by the phenol–H2SO4 method, and absorbance was measured at 488 nm using a SpectraMax Plus microplate reader (Molecular Devices, Palo Alto, CA). The relevant fractions were pooled, concentrated, and lyophilized. For analysis of biological activity, the fractions were diluted in Hank’s balanced salt solution (HBSS) at the indicated concentrations (w/v) and filtered through sterile 0.22 µm filters.
2.3. Characterization of polysaccharide fractions
The pooled polysaccharide fractions were analyzed for protein content using a modified Lowry assay with bovine serum albumin as a standard, and carbohydrate content was determined by the phenol–H2SO4 method with glucose as a standard. For nuclear magnetic resonance (NMR) analysis, samples (5 mg) were dissolved in deuterium oxide (0.5 ml), and 1H NMR spectra were recorded on a Bruker DRX-600 spectrometer (Bruker BioSpin, Billerica, MA) at 20°C using 3-(trimethylsilyl)-propionic 2,2,3,3,-d4 acid sodium salt as an internal reference (δ 0.00 ppm).
The homogeneity and average Mr of the polysaccharide fractions were determined by high performance size-exclusion chromatography (HP-SEC) using a Shimadzu Class VP HPLC and Shodex OHpak SB-804 HQ column (8 mm × 300 mm) eluted with 50 mM sodium citrate buffer, pH 7.5, containing 0.15 M NaCl and 0.01% NaN3 at a flow rate of 0.3 ml/min. Peaks were detected using a refractive index detector (RID-10A; Shimadzu, Torrance, CA). Average Mr of the Opuntia polysaccharide fractions were estimated by comparison with retention times of pullulan standards P-800, 400, 200, 100, 50 and 20 (Phenomenex, Torrance, CA), which have Mr of 788, 404, 212, 112, 47.3, and 22.8 kDa, respectively. Reproducibility of the retention times was typically >98%.
The presence of arabinogalactan in the samples was detected by single radial gel diffusion in 1% agarose gels containing 100 µg/ml β-glucosyl Yariv reagent, which selectively interacts with and precipitates compounds containing type II arabinogalactan structures. Four µl of polysaccharide samples (10 mg/ml; w/v) were loaded into the wells, and the samples were incubated at 25°C for 24 hr in a humid atmosphere. A positive reaction was identified by a reddish circle around the well, and arabic gum (4 mg/ml) served as a positive control.
For monosaccharide composition analysis, samples were hydrolyzed at 100°C for 6 hr with 3 M TFA, and the resulting samples were separated by thin-layer chromatography (TLC) on Whatman silica gel 60 plates using monosaccharide standards for reference. The plates were developed with butanol/acetic acid/water (3:1:1), and bands were visualized by spraying the plates with aniline-diphenylamine reagent (2% aniline, 2% diphenylamine, and 8.5% H3PO4 acid in acetone) and heating at 100°C for 10 min. Individual monosaccharide bands were scraped off the plate, extracted with H2O, and quantified using a colorimetric method with monosaccharide standards. Briefly, the extracts were mixed with anthrone reagent (0.2% anthrone and 1% thiourea in H2SO4). After heating at 100°C for 10 min, absorbance was measured at 620 nm.
2.4. Endotoxin assays
A Limulus Amebocyte Lysate (LAL) assay kit (Cambrex, Walkersville, MD) was used to evaluate Opuntia polysaccharide samples for possible LPS contamination. To further evaluate the possible role of endotoxin, Opuntia polysaccharide fraction C-I was applied to a column containing Detoxi-Gel Endotoxin Removing Gel (Pierce, St. Louis, MO) and eluted with 0.05 M phosphate buffer containing 0.5 M NaCl to decrease ionic interactions of sample molecules with the affinity ligand. The concentration of polysaccharides in the eluted sample was adjusted to match that of the untreated fraction, as determined by the phenol–H2SO4 method, and both treated and untreated samples were analyzed for biological activity, as described below.
2.5. Cell cultures
Murine macrophage J774.A1 cells were cultured in DMEM supplemented with 10% (v/v) heat-inactivated, endotoxin-free FBS, 100 µg/ml streptomycin, and 100 U/ml penicillin. Cells were grown to confluence in sterile tissue culture flasks and gently detached by scraping. Human monocyte-macrophage MonoMac6 cells (DSMZ, Germany) were grown in RPMI 1640 supplemented with 10% (v/v) endotoxin-free FBS, 10 µg/ml bovine insulin, 100 µg/ml streptomycin, and 100 U/ml penicillin. Human monocytic THP1-Blue cells obtained from InvivoGen (San Diego, CA) were cultured in RPMI 1640 medium supplemented with 10% (v/v) endotoxin-free FBS, 100 µg/ml streptomycin, 100 U/ml penicillin, 100 µg/ml zeocin, and 10 µg/ml blasticidin S. These cells are stably transfected with a secreted embryonic alkaline phosphatase gene that is under the control of a promoter inducible by nuclear factor κB (NF-κB).
All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. Cell number and viability were assessed microscopically using trypan blue exclusion.
2.6. Human monocytes-derived macrophages
Blood was collected from healthy donors in accordance with a protocol approved by the Institutional Review Board at Montana State University. Mononuclear cells were purified from the blood using dextran sedimentation, followed by Histopaque 1077 gradient separation and hypotonic lysis of red blood cells, as described previously [28]. Adherent monocytes were cultured for 7 days in RPMI-1640 containing 10% endotoxin-free FBS and 50 ng/ml GM-CSF to induce macrophage differentiation. Optimal conditions were maintained by refreshing the medium and cytokines every 3 days.
2.7. Analysis of NO production
J774.A1 cells were plated at a density of 1.5×105 cells/well in a final volume of 200 µl in 96-well flat-bottomed tissue culture plates and incubated in medium alone or medium containing various concentrations of polysaccharide fractions or E. coli LPS as a positive control. Cells were incubated at 37°C in the presence of 5% CO2 for 24 hr, and 100 µl of the cell culture supernatants were removed and analyzed for NO using a colorimetric method with NaNO2 as the standard. Briefly, supernatants were mixed with an equal volume of Griess reagent, which was prepared by mixing one part of 0.1% (w/v) N-(1-naphthyl)ethylenediamine with one part of 1% (w/v) sulfanilamide in 5% phosphoric acid. After 20 min, absorbance was measured at 540 nm using a SpectraMax Plus microplate reader.
2.8. Analysis of reactive oxygen species (ROS) production
Macrophage ROS production was analyzed using the chemiluminescent probe, L-012, which is highly sensitive for ROS generated in biologically complex systems. Murine J774.A1 macrophages (1.5×105 cells/well) were incubated with various concentrations of polysaccharide fractions or positive control (LPS) for 24 hr. After incubation, the culture supernatant was removed and replaced by an equal volume of HBSS supplemented with 25 µM L-012 and 5 µg/ml horseradish peroxidase with or without 100 nM PMA, as described previously [29]. Reactions were monitored on a Fluoroscan Ascent FL microtiter plate reader (ThermoElectron, Milford, MA) at 37°C. Chemiluminescence was measured every 2 min for 2 hr and is expressed as the integrated response over this time (arbitrary units).
2.9. Determination of TNF-α and IL-6
Cells were incubated for 24 hr in culture media supplemented with 3% (v/v) endotoxin-free FBS with or without Opuntia polysaccharide fractions or LPS as a positive control in 96-well plates at a density of 2×105 cells/well. Human monocyte-derived macrophages were plated in 96-well plates at a density of 6×104 cells/well. Human and murine TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA) kits (BD Biosciences Pharmigen) were used to detect TNF-α/IL-6 in the cell supernatants. Cytokine concentrations were determined by extrapolation from TNF-α/IL-6 standard curves, according to the manufacturer’s protocol.
2.10. Analysis of NF-κB activation
Activation of NF-κB was measured using an alkaline phosphatase reporter gene assay in THP1-Blue human monocytic cells. One week before an experiment, the cells were pre-activated overnight with PMA (50 ng/ml) to induce differentiation and plated in 96-well plates at a cell density of 2×105 cells/well. The cells were then incubated for 5 days with daily media changes. On day 7, Opuntia polysaccharide fractions or LPS (100 ng/ml) were added, the cells were incubated for 24 hr, and alkaline phosphatase activity was measured in cell supernatants using QUANTI-Blue mix (InvivoGen, San Diego, CA). Activation of NF-κB is reported as an absorbance at 655 nm and compared with positive control samples (LPS).
2.11. Cytotoxicity assay
Cytotoxicity was analyzed with a CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Inc., Madison, WI) according to the manufacturer’s protocol. Briefly, cells were cultured at a density of 3×104 cells/well with the polysaccharide fractions for 24 hr at 37°C and 5% CO2. Following treatment, the cells were allowed to equilibrate to room temperature for 30 min, substrate was added, and the samples were analyzed with a Fluoroscan Ascent FL.
2.12. Statistical analysis
Two-way analysis of variance (ANOVA) was performed on the indicated sets of data (GraphPad Prism Software, San Diego, CA). Pair-wise comparisons with differences at P<0.05 were considered to be statistically significant.
3. Results
3.1. Preparation and characterization of Opuntia polysaccharides
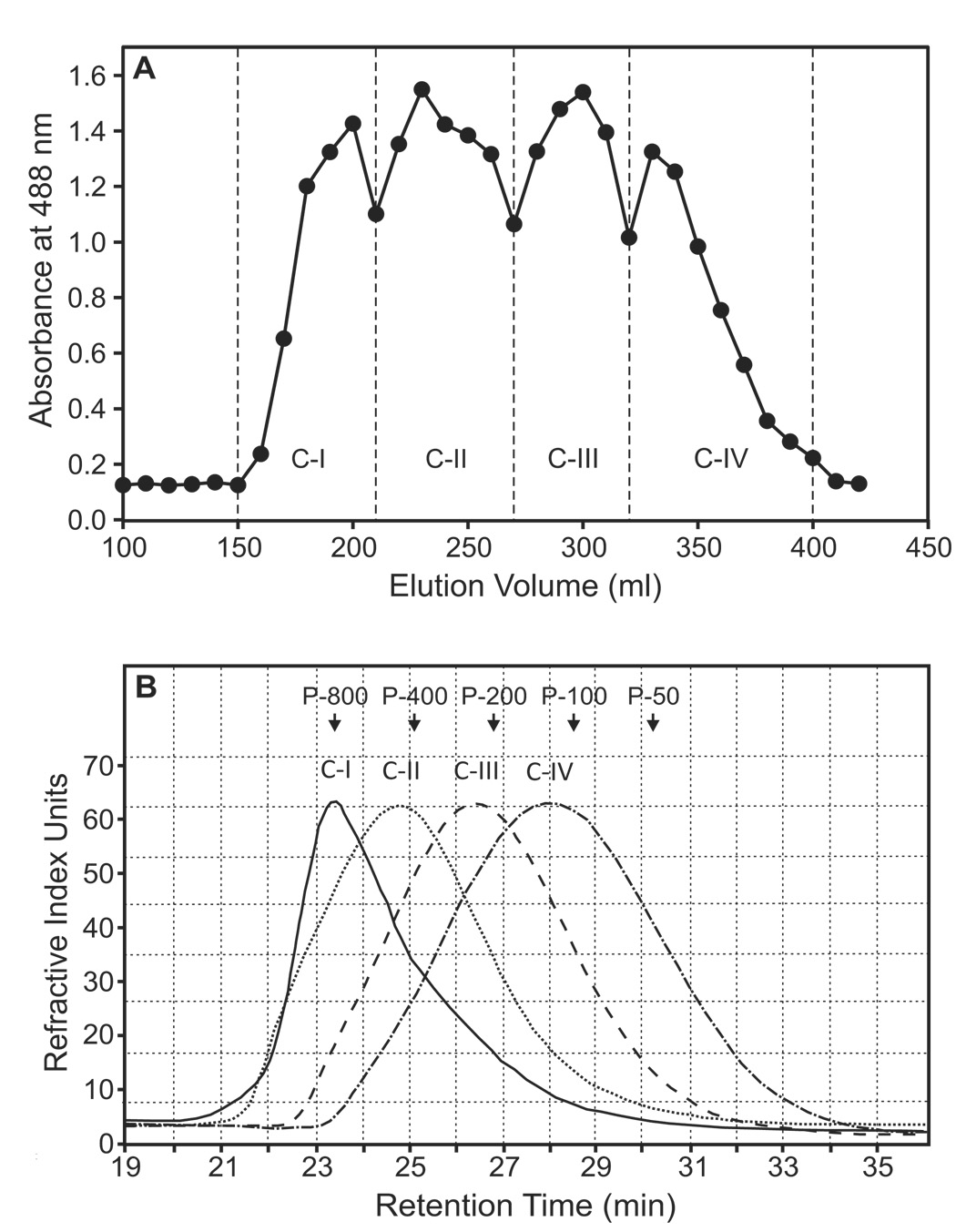
Polysaccharides from O. polyacantha were fractionated by preparative Sepharose 6B size-exclusion chromatography to obtain four main fractions, which were selected based on the total carbohydrate elution profile (designated as Opuntia polysaccharides C-I, C-II, C-III, and C-IV) (Fig. 1A). Each of the four fractions contained at least 96% carbohydrate and ≤ 2% protein (Table 1). Negligible levels of protein were also previously reported in mucilage of O. ficus-indica [30].
Figure 1.
Preparative and analytical size-exclusion chromatography of Opuntia polysaccharides. Panel A. The crude Opuntia polysaccharide extract was fractionated using Sepharose 6B column chromatography, and the eluate was combined to obtain the four fractions (designated C-I through C-IV). Total carbohydrate content of the fractions was determined as described (detected at 488 nm). Panel B. The Opuntia polysaccharide fractions were analyzed by HPSEC, and eluates were monitored with a refractive index detector, as described. The arrows indicate the peak retention times of the indicated pullulan standards used for calibration.
Table 1.
Biochemical properties of Opuntia polysaccharide fractions
| Polysaccharide Fractions | Molecular Weight (kDa) | Yariv Test | Carbohydrate Content (%) | Protein Content (%) |
|---|---|---|---|---|
| C-I | 733 | Negative | 96 | 2 |
| C-II | 550 | Negative | 99 | <1 |
| C-III | 310 | Positive | 98 | <1 |
| C-IV | 168 | Positive | 98 | <1 |
Analytical HP-SEC of the individual fractions showed that each fraction was represented by a single, generally symmetrical peak on the chromatogram (Fig. 1B), suggesting the polysaccharide fractions were relatively homogeneous. Based on calibration curves derived from analysis of pullulan standards, we determined that the average molecular weights of polysaccharide fractions C-I through C-IV were 733, 550, 310 and 168 kDa, respectively (Table 1). Analysis of the fractions using the Yariv test showed that fractions C-III and C-IV contained type II arabinogalactan; whereas, fractions C-I and C-II tested negative for the presence of this type of arabinogalactan (Table 1). Sugar composition analysis revealed that the Opuntia polysaccharides consisted primarily of galactose, xylose, arabinose, rhamnose, and galacturonic acid (Table 2).
Table 2.
Monosaccharide composition of Opuntia polysaccharide fractions
| Polysaccharide Fraction | Galactose (mol %) | Galacturonic Acid (mol %) | Xylose (mol %) | Arabinose (mol %) | Rhamnose (mol %) |
|---|---|---|---|---|---|
| C-I | 34 | 15 | 29 | 10 | 12 |
| C-II | 35 | 15 | 25 | 15 | 10 |
| C-III | 43 | 19 | 20 | 12 | 6 |
| C-IV | 48 | 18 | 17 | 10 | 6 |
Monosaccharides were identified and quantified based on TLC analysis of known standards.
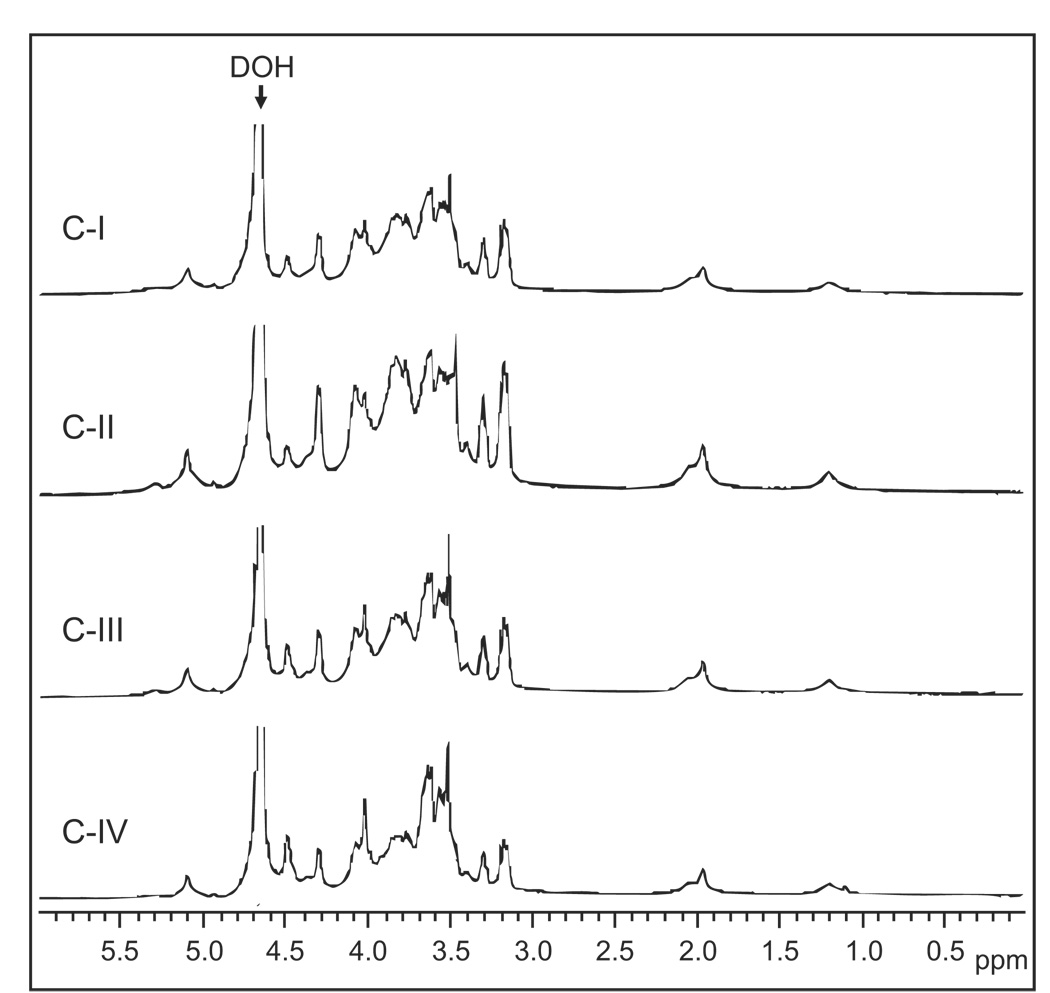
Very-high-field (600 MHz) 1H NMR was used to characterize the structure of the native Opuntia polysaccharides (Fig. 2). The spectra from all fractions were similar to each other, suggesting a common backbone structure, and resembled the spectra of polysaccharides isolated from O. ficus-indica [30]. The weak signals present at 3.38–3.45 ppm can be assigned to α-rhamnopyranose (α-Rha p), while the strong signals at 3.68–3.90 ppm are consistent with the presence of β-galactopyranose (β-Gal p) [31]. The signals at 4.05–5.05 ppm are consistent with the presence of α-arabinofuranose (α-Ara f) and α-galacturonopyranose (α-GalA p) residues [31,32].
Figure 2.
1H NMR spectra of Opuntia polysaccharide fractions. Fractions C-I through C-IV were dissolved in D2O, and spectra were recorded at 20°C.
3.2. Effect of Opuntia polysaccharides on macrophage NO and ROS production
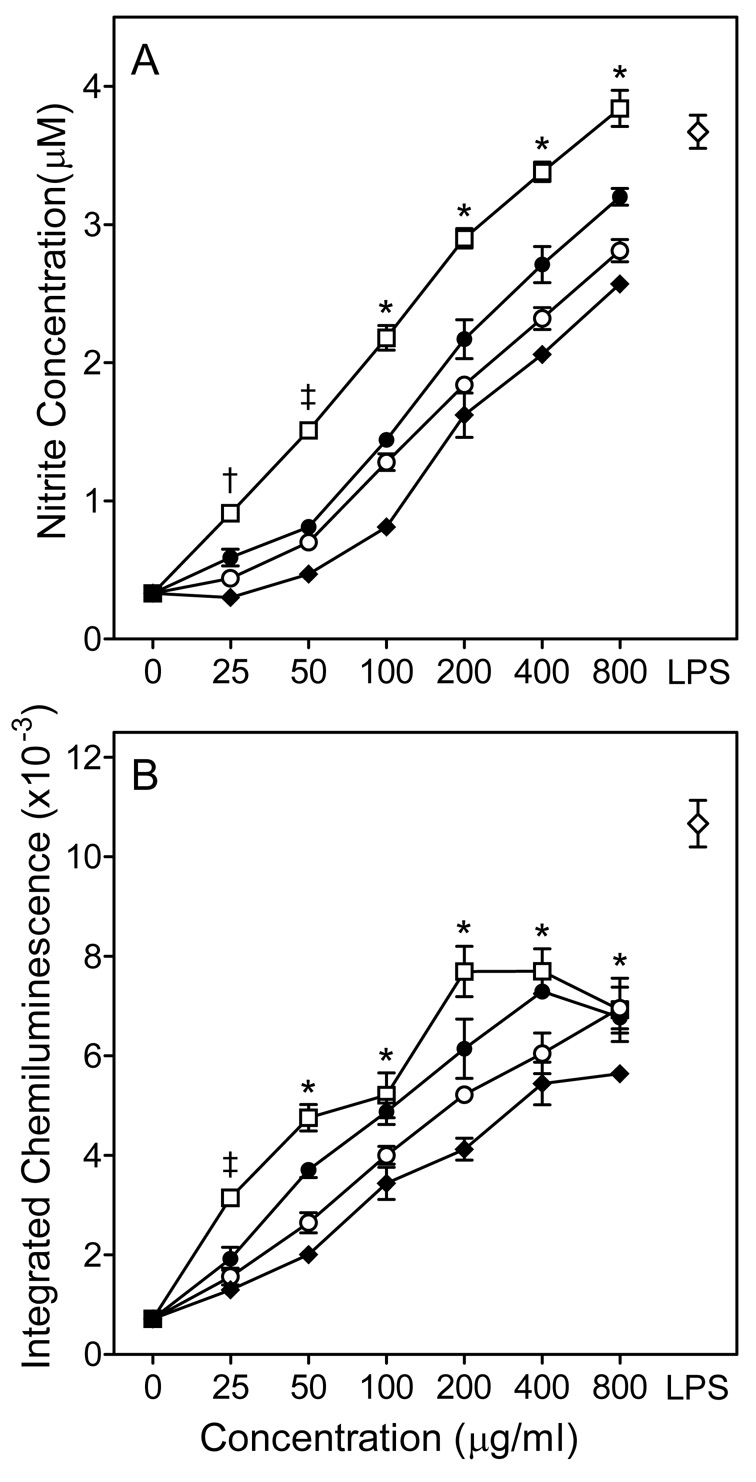
A minimum amount of NO was produced when murine J774.A1 macrophages were incubated with medium alone; whereas, treatment of these cells with any of the four Opuntia polysaccharide fractions resulted in a concentration-dependent increase in NO production (Fig. 3A), with fraction C-I being the most active. Indeed, NO production induced by fraction C-I at 800 µg/ml was comparable to that induced by 100 ng/ml LPS.
Figure 3.
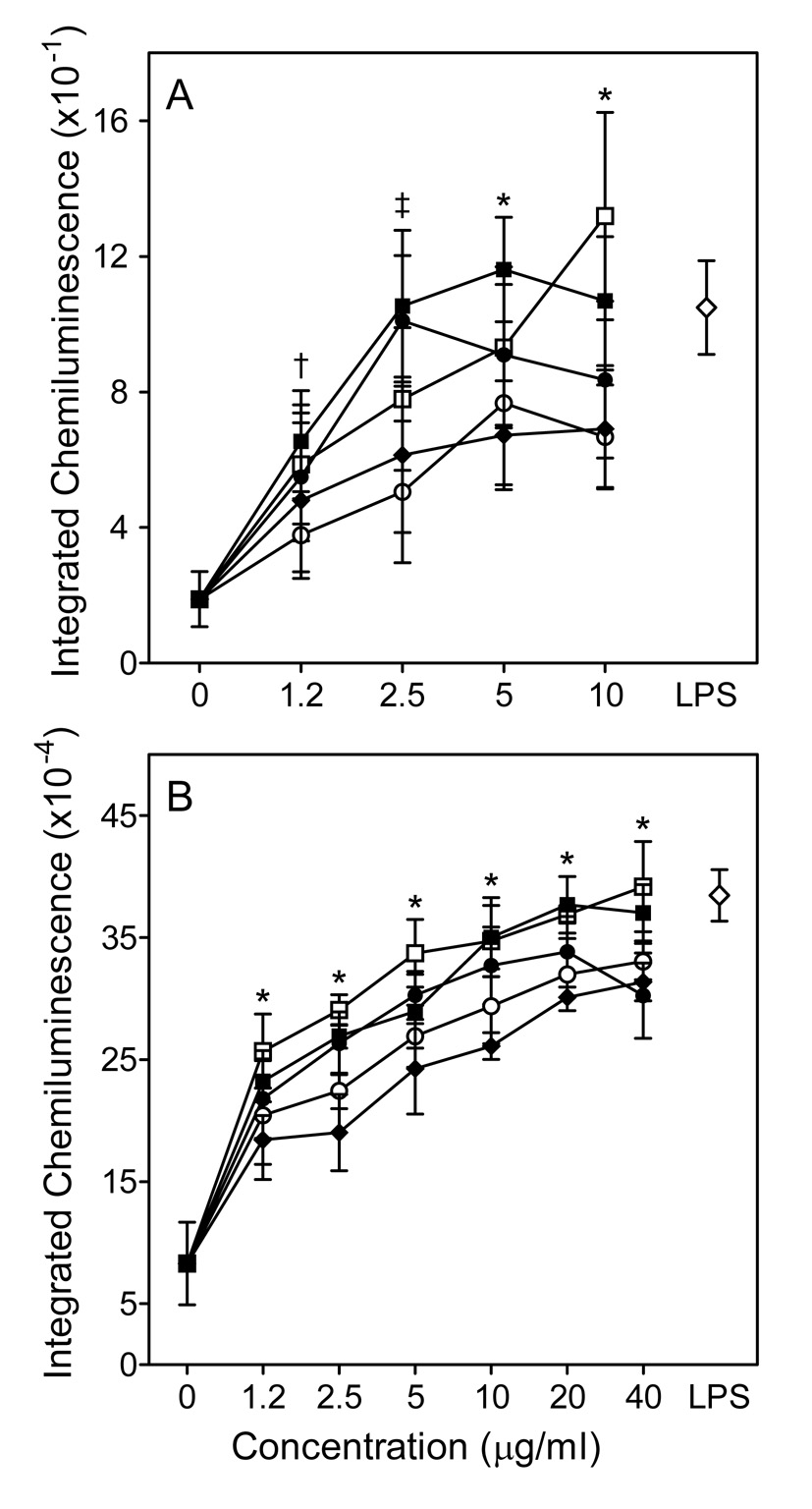
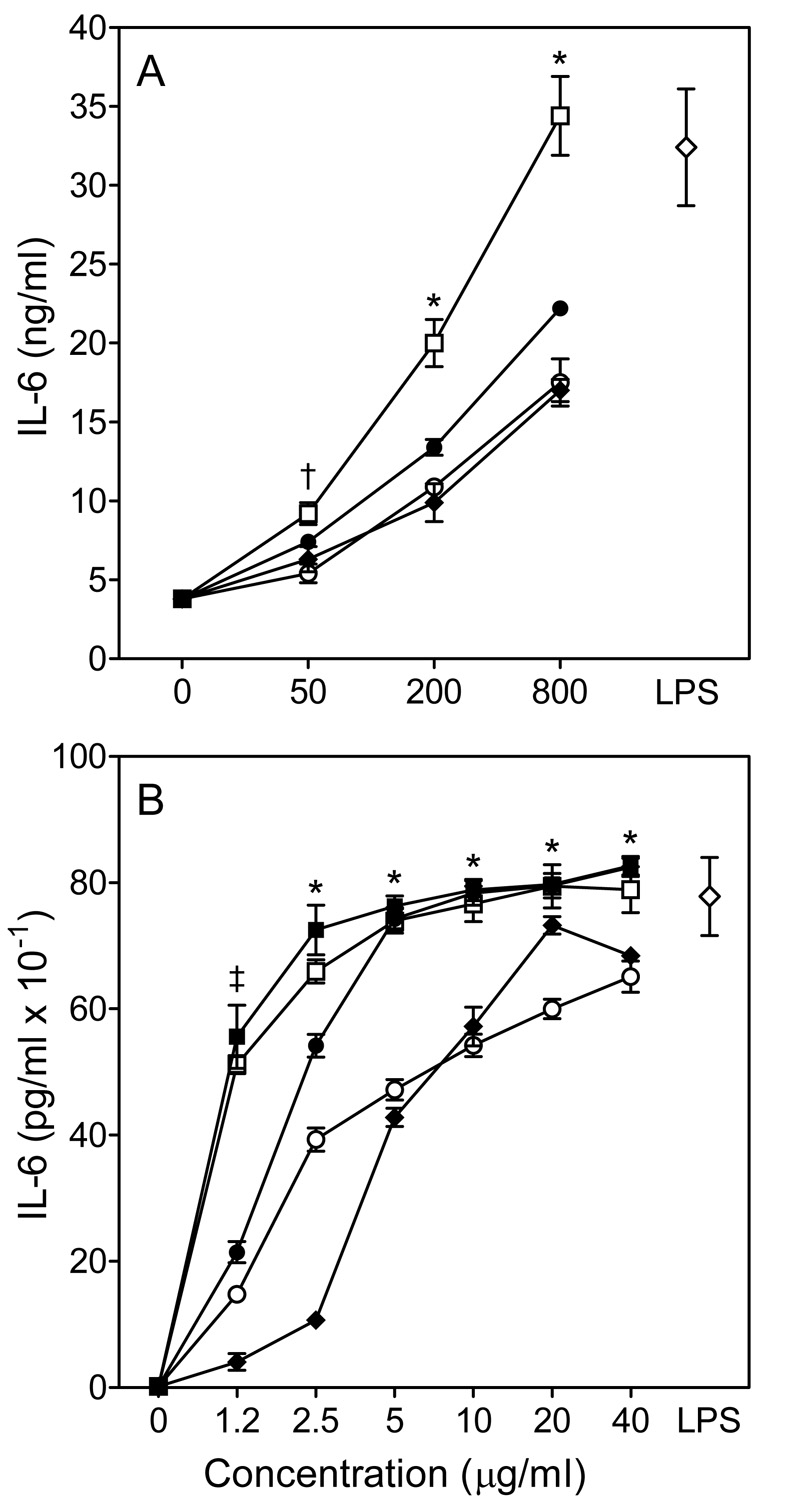
Effect of Opuntia polysaccharides on murine macrophage ROS and NO production. Murine J774.A1 macrophages were incubated for 24 hr with the indicated concentrations of polysaccharide fractions C-I (□), -II (●), C-III (○), C-VI (◆), or 100 ng/ml LPS (◇, positive control). NO production was quantified by measuring nitrite in the cell-free supernatants (Panel A), and chemiluminescence was monitored using an L-012 detection system, as described (Panel B). Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of two independent experiments. Statistically significant differences (P<0.05) between untreated cells and cells treated with fractions C-I (†, ‡, *), C-II (†, ‡, *), C-III (‡, *), and C-IV (*) are indicated.
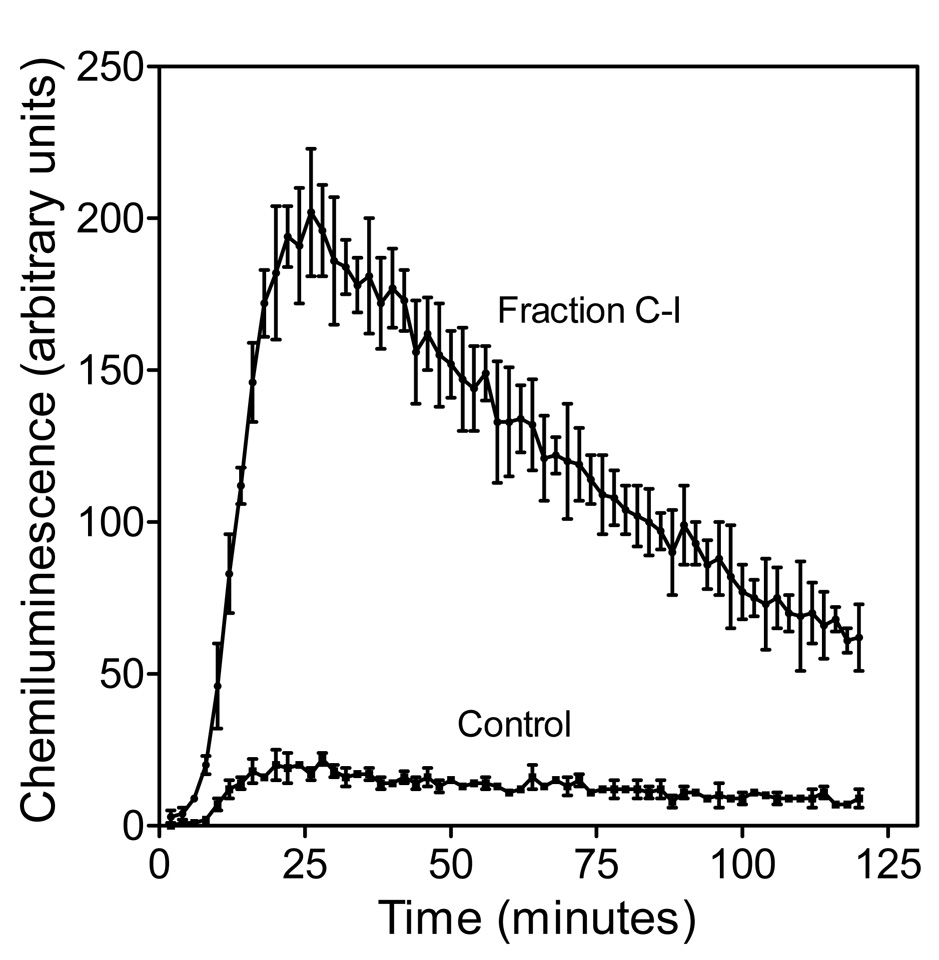
In the absence of any treatment, murine J774.A1 macrophages generated very low levels of ROS (Fig. 4, control); whereas, a concentration-dependent enhancement of ROS production was observed in macrophages treated with 25–400 µg/ml doses of each polysaccharide fraction, with fraction C-I being the most active (Fig. 3B and 4). Thus, the individual polysaccharide fractions from O. polyacantha showed a similar pattern with respect to their ability to induce NO and ROS production by murine J774.A1 macrophages (C-I>C-II>C-III>C-IV).
Figure 4.
Kinetics of ROS Production by Murine Macrophages Stimulated with Opuntia Polysaccharides. J774.A1 cells were incubated for 24 hr with polysaccharide fraction C-I (800 µg/ml), and chemiluminescence was monitored, as described. Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of three independent experiments.
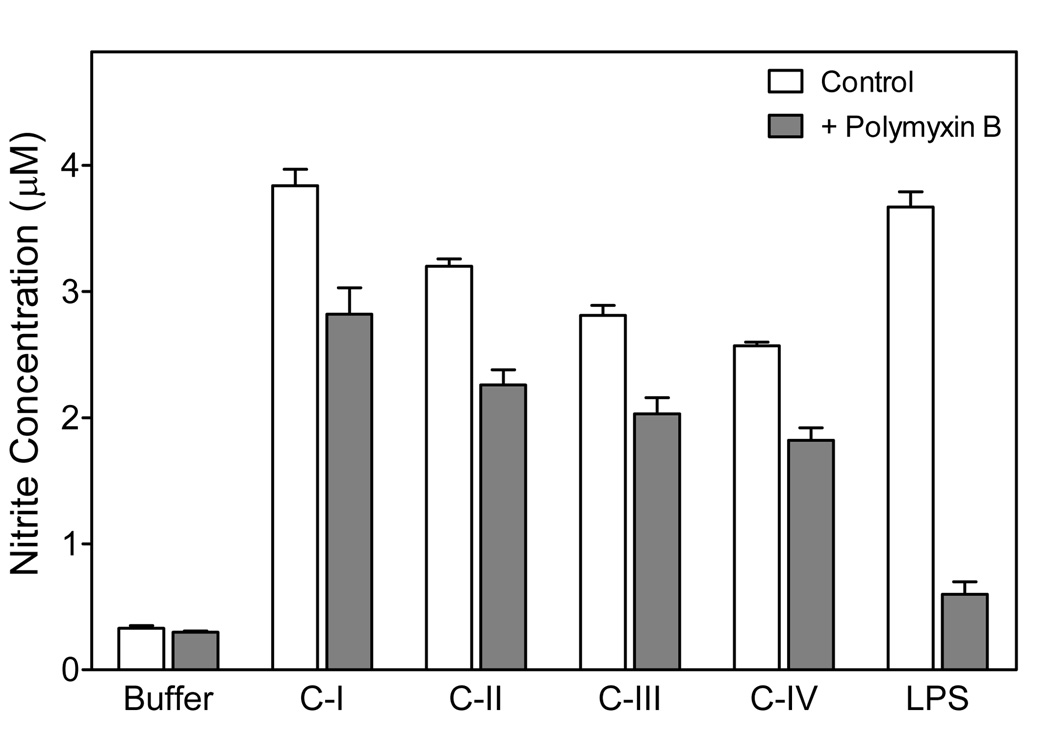
Since endotoxin (LPS) is often a contaminant in biological preparations, we evaluated possible contribution of endotoxin to the observed biological activity of Opuntia polysaccharides. All fractions caused a similar positive Limulus response (data not shown), suggesting polysaccharides present in our fractions were also activating the Limulus coagulation cascade (see Discussion). Thus, we evaluated NO production by macrophages treated with Opuntia polysaccharide fractions or LPS in the presence and absence of polymyxin B. As shown in Fig. 5, polymyxin B almost completely suppressed macrophage NO production induced by LPS (>90% inhibition). In comparison, polymyxin B only slightly reduced responses induced by the individual Opuntia polysaccharide fractions (<25% inhibition), suggesting that the observed responses were not due to LPS contamination. The small amount of inhibition observed with polymyxin B treatment is likely due to nonspecific effects of this compound on the cells (see Discussion). To eliminate this artifact, we pretreated Opuntia polysaccharide samples (fraction C-I) by elution through a column of endotoxin-removing gel so that the cells were not exposed directly to polymyxin B and found that treated polysaccharides were just as active as untreated samples. This control, which is present in subsequent assays, further demonstrates LPS contamination is not responsible for the responses observed (see Fig. 6–10).
Figure 5.
Effect of polymyxin B on Opuntia polysaccharide- or LPS-induced macrophage NO production. The indicated Opuntia polysaccharide fractions polysaccharide fractions or LPS were preincubated with polymyxin B (20 µg/ml) for 1 hr. Murine J774.A1 cells were incubated for 24 h with untreated (open bars) and polymyxin B-treated (shaded bars) polysaccharide fractions (800 µg/ml) or LPS (100 ng/ml), and NO production was measured, as described. Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of two independent experiments.
Figure 6.
Effect of Opuntia polysaccharides on human macrophage ROS production. Human MonoMac6 macrophages were incubated for 24 hr with the indicated concentrations of polysaccharide fractions C-I (□), C-I pretreated with endotoxin-removing gel (■), C-II (●), CIII (○), C-VI (◆), or 100 ng/ml LPS (◇, positive control). Chemiluminescence was monitored using an L-012 detection system in unstimulated cells (Pane A) and cells stimulated with 100 nM PMA (Panel B). Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of two independent experiments. Statistically significant differences (P<0.05) between untreated cells and cells treated with fractions C-I (†, ‡, *), C-I pretreated with endotoxin-removing gel (†, ‡, *), C-II (†, ‡, *), C-III (*), and C-IV (‡, *) are indicated.
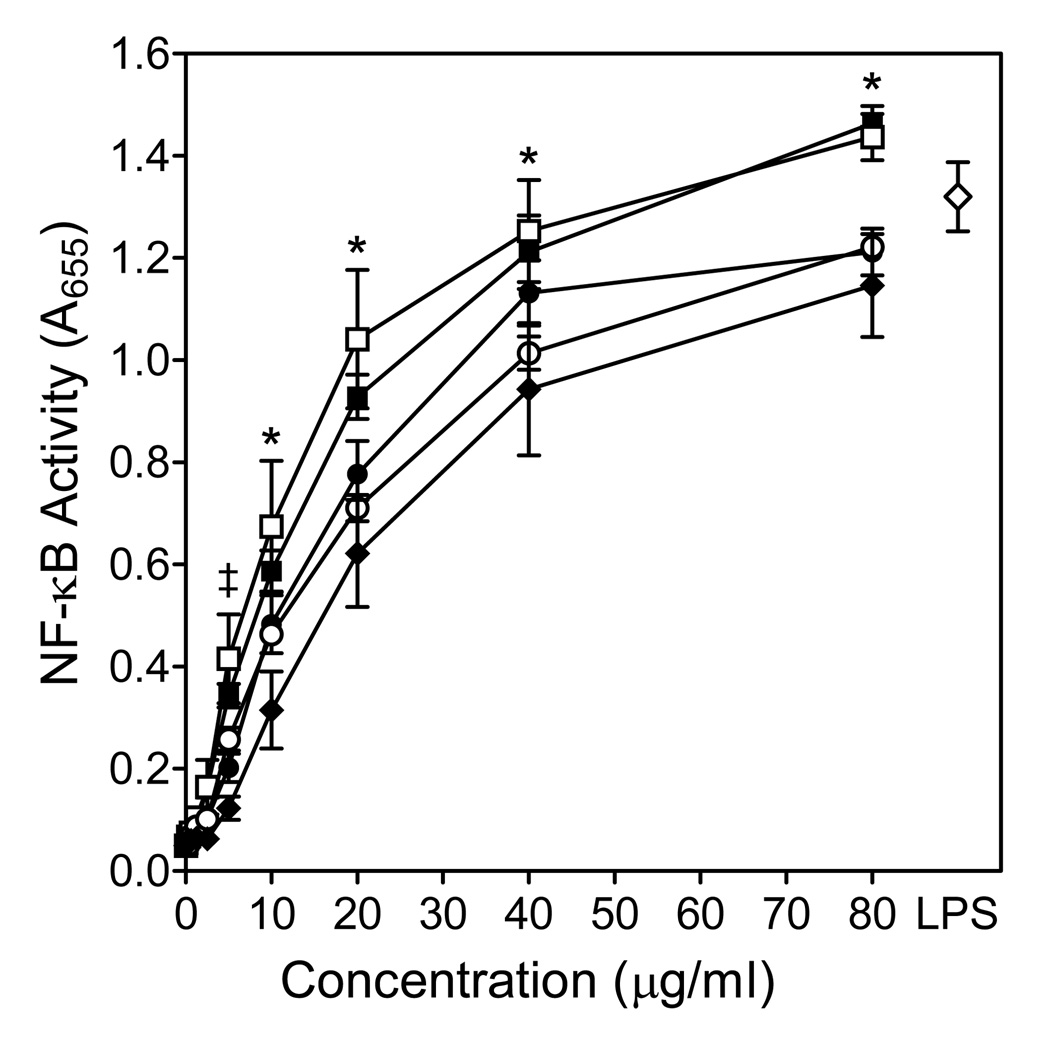
Figure 10.
Effects of Opuntia polysaccharides on NF-κB activation. Human THP-1 monocytes (5×105 cells/well) were incubated for 24 hr with Opuntia polysaccharide fractions C-I (□), C-I pretreated with endotoxin-removing gel (■), C-II (●), C-III (○), C-VI (◆), or 100 ng/ml LPS (◇, positive control). Alkaline phosphatase activity was analyzed spectrophotometrically (absorbance at 655 nm) in the cell supernatants, as described. Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of two independent experiments. Statistically significant differences (P<0.05) between untreated cells and cells treated with fractions C-I (‡, *), C-I pretreated with endotoxin-removing gel ( ‡, *), C-II (‡, *), C-III (‡, *), and C-IV (*) are indicated.
To determine whether human phagocytes also responded to Opuntia polysaccharides, we analyzed their ability to activate ROS production by human MonoMac6 macrophages. Opuntia polysaccharides stimulated low levels of ROS production by MonoMac6 cells in a concentration-dependent manner (Fig. 6A). Furthermore, Opuntia polysaccharides significantly enhanced PMA-mediated activation of ROS production by MonoMac6 cells (Fig. 6B), suggesting these polysaccharides can prime cells similar to LPS. Note, however, that pretreatment of fraction C-I with endotoxin-removing gel had no effect on its activity, indicating that the effect was not due to LPS contamination (Fig. 6). Overall, these data verify that Opuntia polysaccharides are not specific for murine phagocytes and can also activate human phagocytes.
3.3. Effect of Opuntia polysaccharides on macrophage TNF-α and IL-6 production
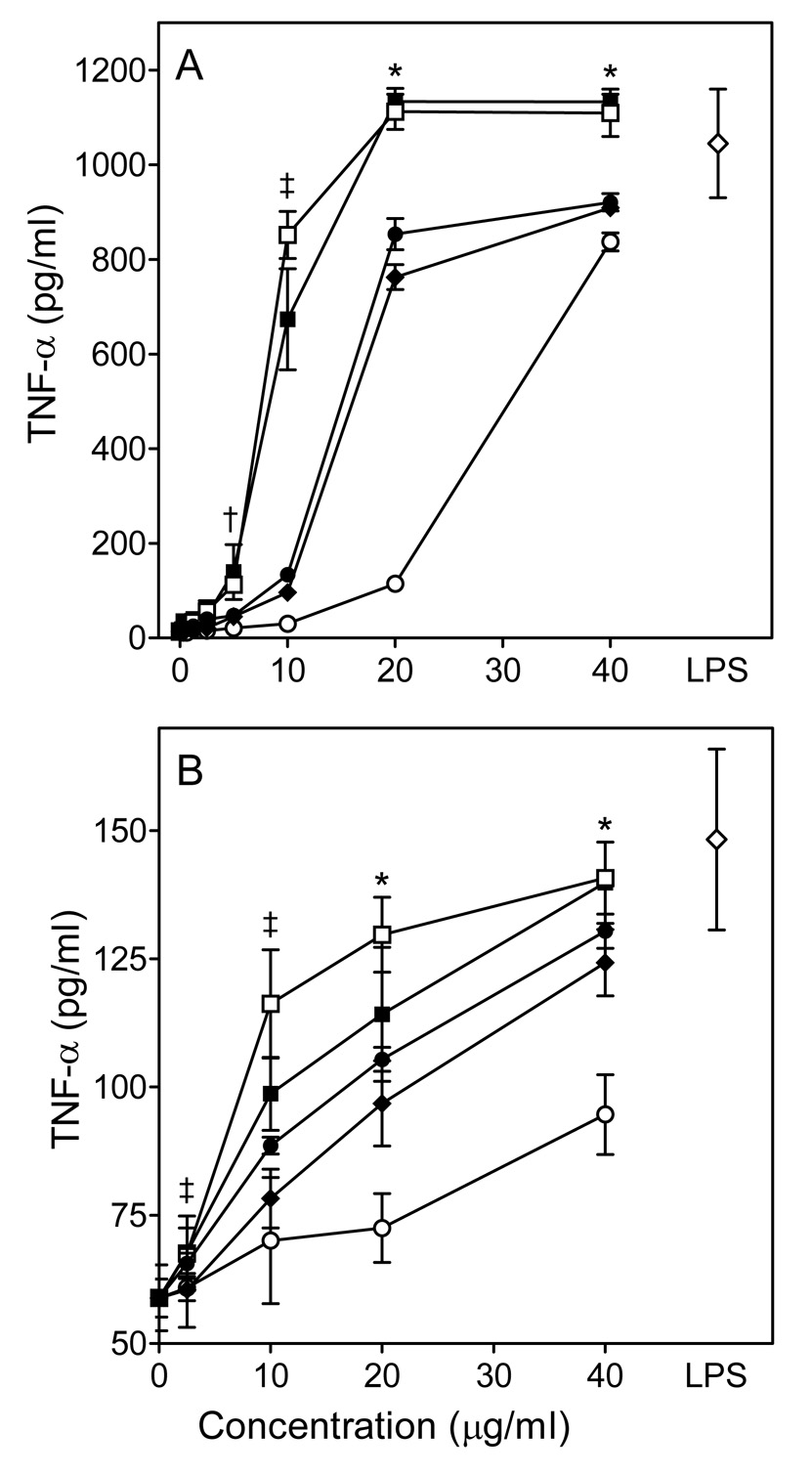
A number of immunomodulatory compounds can regulate cytokine production. Thus, we analyzed the effects of Opuntia polysaccharide fractions on macrophage TNF-α and IL-6 production. Untreated murine J774.A1 and human MonoMac6 macrophages produced very little TNF-α and IL-6; whereas, incubation of these cells with the Opuntia polysaccharide fractions significantly enhanced TNF-α and IL-6 production in a concentration-dependent manner (Fig. 7 and 8). These responses were quite robust, and the levels of TNF-α induced by 20–40 µg/ml of fraction C-I were comparable to those induced by 50 ng/ml LPS in both murine and human cells. Furthermore, cytokine production induced by fraction C-I was essentially the same for untreated samples and samples pretreated with endotoxin-removing gel, further demonstrating that LPS is not responsible for the observed effects (Fig. 7 and Fig. 8B).
Figure 7.
Effect of Opuntia polysaccharides on macrophage TNF-α production. Murine J774.A1 (Panel A) or human MonoMac6 (Panel B) macrophages were incubated for 24 hr with the indicated concentrations of polysaccharide fractions C-I (□), C-I pretreated with endotoxin-removing gel (■), (C-II (●), C-III (○), C-VI (◆), or 50 ng/ml LPS (◇, positive control). Cell-free supernatants were collected, and extracellular TNF-α was quantified by ELISA. Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of three independent experiments. Statistically significant differences (P<0.05) between untreated cells and cells treated with fractions C-I (†, ‡, *), C-I pretreated with endotoxin-removing gel (†, ‡, *), C-II (‡, *), C-III (*), and C-IV (‡, *) are indicated.
Figure 8.
Effect of Opuntia polysaccharides on macrophage IL-6 production. Murine J774.A1 (Panel A) and human MonoMac6 (Panel B) macrophages were incubated for 24 hr with polysaccharide fractions C-I (□), C-I pretreated with endotoxin-removing gel (■), C-II (●), CIII (○), C-VI (◆), or 100 ng/ml LPS (◇, positive control). Cell-free supernatants were collected, and extracellular IL-6 was quantified by ELISA. For both panels, values are the mean ± S.D. of triplicate samples from one experiment, which is representative of two independent experiments. Statistically significant differences (P<0.05) between untreated cells and cells treated with fractions C-I (†, ‡, *), C-I pretreated with endotoxin-removing gel (†, ‡, *), C-II (†, ‡, *), C-III (‡, *), and C-IV (†,*) are indicated.
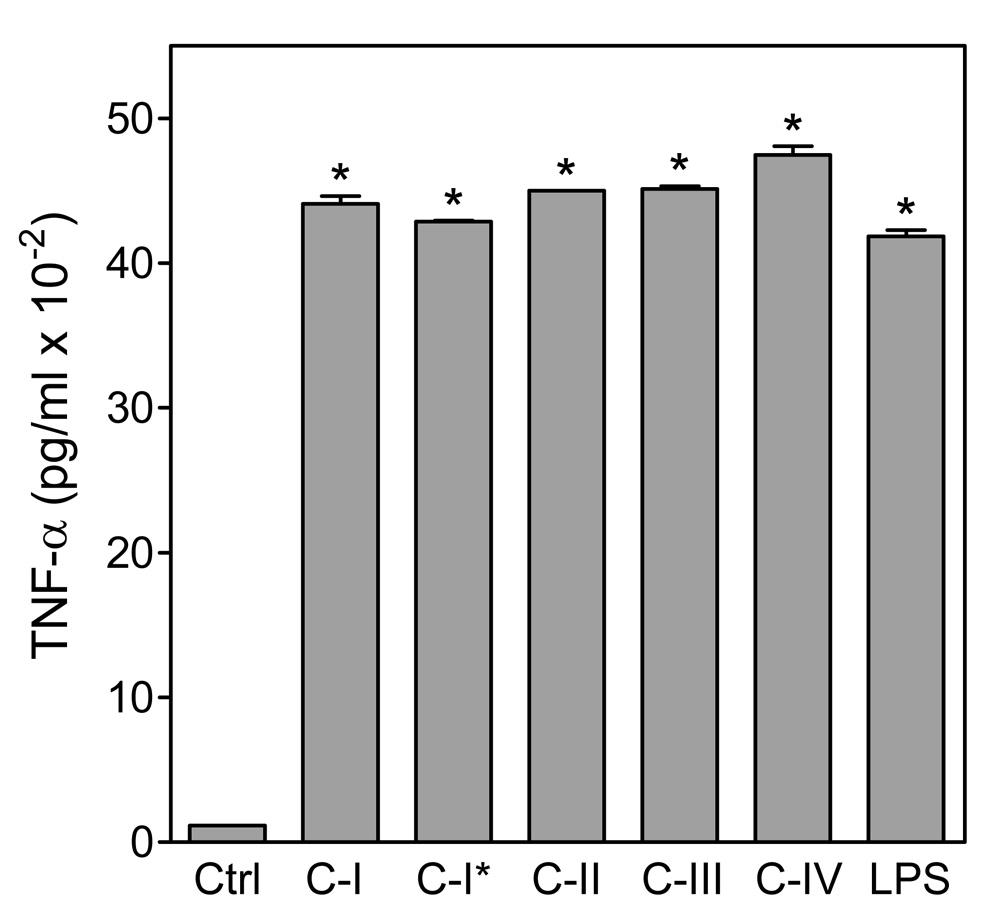
The effect of Opuntia polysaccharides on macrophage TNF-α production was also confirmed in human primary monocyte-derived macrophages. All fractions induced TNF-α production by monocyte-derived macrophages (Fig. 9). Again, responses were not significantly different between untreated and polymyxin gel-pretreated samples of fraction C-I. Thus, these results indicate that Opuntia polysaccharides induce a significant up-regulation of TNF-α and IL-6 synthesis in murine as well as in human macrophages.
Figure 9.
Effect of Opuntia polysaccharides on TNF-α production by human monocyte-derived macrophages. Human monocyte-derived macrophages were incubated for 24 hr with the indicated Opuntia polysaccharide fractions polysaccharide fractions (200 µg/ml) or 100 ng/ml LPS (positive control). C-I* indicates fraction C-I pretreated with endotoxin-removing gel. Cell-free supernatants were collected, and extracellular TNF-α was quantified by ELISA. Values are the mean ± S.D. of three replicates, which is representative of two independent experiments from different donors. Statistically significant differences (P<0.05) between untreated cells and treated cells are indicated (*).
3.4. Effect of Opuntia polysaccharides on nuclear factor kappa B (NF-κB) activation
To evaluate signaling pathways involved in the immunomodulatory activity of Opuntia polysaccharides, we utilized a transcription factor-based bioassay for NF-κB activation in human THP-1 monocytes. All fractions, including fraction C-I pretreated with endotoxin-removing gel, dose-dependently stimulated NF-κB directed alkaline phosphatase expression in THP-1 human monocytes (Fig. 10), indicating that these polysaccharides potently activate the NF-κB pathway. Indeed, alkaline phosphatase release induced by fractions C-I and C-II at concentration of 80 µg/ml was even greater than that induced by 100 ng/ml LPS. Note also that the activity of untreated fraction C-I was not significantly different than that of fraction C-I pretreated with endotoxin-removing gel (Fig. 10).
3.5. Effect of Opuntia polysaccharides on cell viability
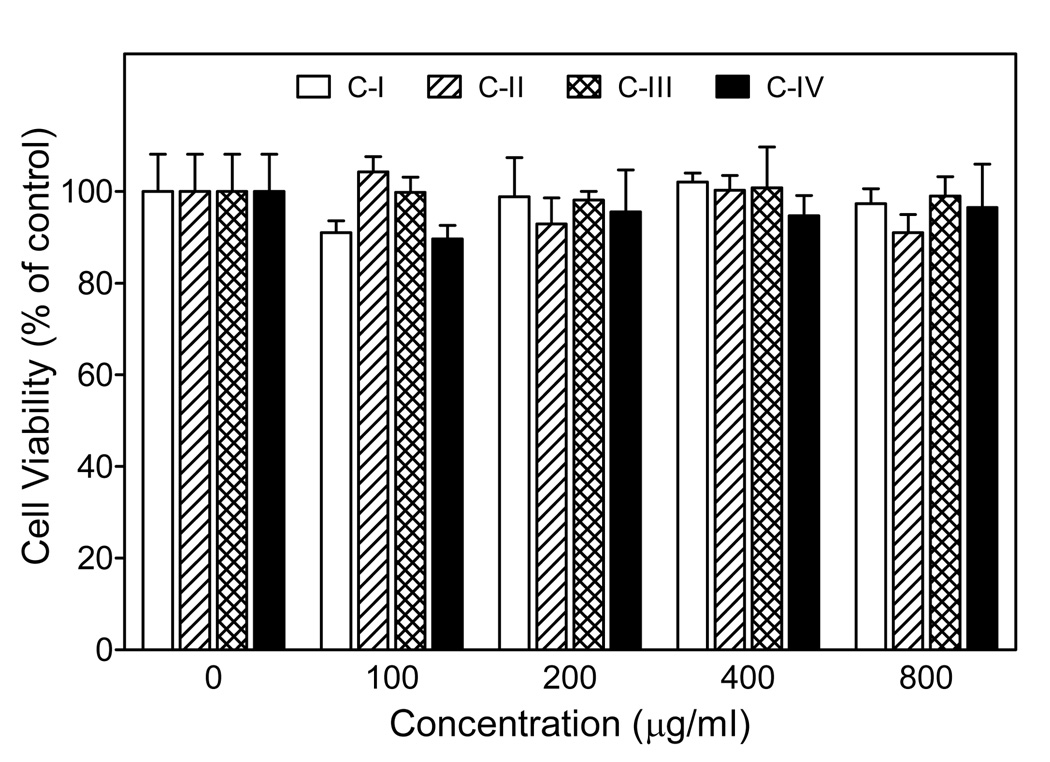
Although our functional assays suggested that Opuntia polysaccharides were relatively non-toxic, we evaluated the potential cytotoxic effect of the polysaccharides to determine if the results might be influenced by background toxicity. Using a cytotoxicity assay, we determined that none of the fractions significantly affected proliferation/viability of J774.A1 cells over the entire concentration range of polysaccharide fractions tested (100–800 µg/ml), verifying that these polysaccharides were not toxic to these cells (Fig. 11).
Figure 11.
Effects of Opuntia polysaccharides on cell viability. Murine J774.A1 macrophages were incubated for 24 hr with the indicated concentrations of Opuntia polysaccharide fractions, and cell viability was determined using a luminescent cell viability assay kit, as described. Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of two independent experiments.
4. Discussion
Since ancient times, fruits and stems of different species of the genus Opuntia have been used in remedies for treating a number of medical problems. For example, the stems of O. polyacantha has been used to treat infections and wounds [33], which suggests that O. polyacantha extracts might have immune-enhancing effects. Despite the wide-spread use of O. polyacantha, little is known regarding the active components responsible for its therapeutic properties. Previous studies indicated that some of the biological properties of O. polyacantha were due to the presence glycoproteins and low-molecular weight compounds, such as flavonoids, proanthocyanidins, carotenoids, betalains, β-sterols, and α-pyrones [13,15,18–22]. In the present report, we provide evidence suggesting that polysaccharides of O. polyacantha have potent immunomodulatory properties that can enhance monocyte/macrophage function and suggest that this may contribute, at least in part, to the therapeutic potential of O. polyacantha tissues.
We isolated four polysaccharide fractions from the stems of O. polyacantha and provide initial structural and pharmacological characterization. Average molecular weights of the fractions were 733, 550, 310, and 168 kDa for fractions C-I through C-IV, respectively, and sugar composition was similar to the monosaccharide composition of polysaccharides isolated from another cactus species, O. ficus-indica [30,34]. Analysis of the fractions using the Yariv test showed that two relatively high-molecular weight Opuntia polysaccharide fractions C-I and C-II lacked arabinogalactan; whereas, the other fractions (C-III and C-IV) tested positive for the presence of type II arabinogalactan (Table 1). Type II arabinogalactans have a β-(1,3)-linked galactan backbone with side chains containing arabinose and galactose residues and have been reported to possess a variety of biological activities [4]. Indeed, in polysaccharide fractions C-III and C-IV, galactose was the dominant monosaccharide and represented >40 mol% of the total sugars in these fractions. However, the most potent biological properties were associated with fractions C-I and C-II. Thus, type II arabinogalactans do not appear to be the main structures responsible for macrophage-activating properties of the Opuntia polysaccharides. This observation is supported by our previous studies on other plant-derived polysaccharides [35,36].
Macrophages play critical roles in host defense, including phagocytosis of pathogens and apoptotic cells, production of cytokines, and proteolytic processing and presentation of foreign antigens (reviewed in [37]). Thus, the identification of agents that can modulate macrophages is of significant interest. Indeed, a variety of plant polysaccharides have been reported to exhibit beneficial pharmacological effects via their ability to modulate macrophage function (reviewed in [6]). In this study, all Opuntia polysaccharides contained potent macrophage immunomodulatory activity, as demonstrated by induction of effector molecules, such as NO, ROS, and cytokines. In addition, treatment with Opuntia polysaccharides before PMA resulted in a significantly enhanced response, which is indicative of a priming effect. Many immunomodulatory compounds, including LPS, can prime phagocytes for enhanced ROS production, and it is generally thought that priming plays a key role in the host-defense process and may be essential for host survival against microbial pathogens [38]. Note that macrophage ROS production plateaued or even decreased slightly at higher polysaccharide concentrations, especially with murine macrophages. Based on our previous studies showing that Artemisia polysaccharides can scavenge ROS [36], we suggest that this effect may be due, at least in part, to ROS scavenging by residual Opuntia polysaccharides present in the wells (<10% of the initial concentrations), since the cells were not washed when the medium was replaced with fresh medium containing the detection reagents. Indeed, we found that all of the Opuntia polysaccharide fractions exhibited ROS scavenging activity in an enzymatic ROS-generating system, with the high-molecular weight fractions C-I and C-II being the most active (data not shown). Thus, wells treated with the highest polysaccharide concentrations would have the greatest residual polysaccharide concentrations and the greatest ROS scavenging effect, which would explain the apparent plateau in ROS production. Nevertheless, further studies are needed to fully evaluate the ROS scavenging properties of Opuntia polysaccharides.
The macrophage immunomodulatory activity of Opuntia polysaccharides seems to be positively correlated with the average molecular weight of the polysaccharides, with higher molecular weight fractions being the most active. This may be a common feature of plant polysaccharides that modulate macrophage function, as we found previously that biological activity correlated with molecular weight for polysaccharides isolated from Juniperus scopolorum [39], Tanacetum vulgare [35], and Artemisia tripartite [36]. Likewise, biological activity of mannans from Aloe vera [40] and β-glucans from mushrooms [41] was found to correlate with increased size. Overall, these observations suggest that Opuntia and other plant-derived polysaccharides may activate macrophages via receptor(s) or other surface structures, although the nature of these surface targets is currently unknown. The potent macrophage-stimulatory effect of high molecular weight polysaccharides may also be related to their highly repetitive structures, which could cross-link receptors or other membrane targets in a multivalent fashion [40]. Clearly, further studies are necessary to identify the cellular target of these polysaccharides and understand the relationship between their molecular weight and biological activity. For example, it is not clear how such plant-derived polysaccharides are metabolized in the gut and whether this affects their bioavailability. Traditionally, extracts from Opuntia species have been used topically for treatment of wounds and burns, as well as internally for a variety of diseases [10,11]. Thus, bioavailability of various components of Opuntia extracts may vary, depending on the site of application and other metabolic processes.
A number of plant-derived polysaccharides have been shown to induce production of TNF-α and/or IL-6 in macrophages (e.g., see [6,35,36]). However, this is first report to demonstrate induction of these cytokines by Opuntia polysaccharides. Among the pro-inflammatory cytokines, IL-6 is one of the most important mediators of fever and the acute-phase response [42]. TNF-α also plays an important role as a key cytokine in immune and inflammatory reactions. TNF-α has direct in vitro and in vivo cytostatic and cytocidal effects and, together with IL-6, is also considered as a major immune and inflammatory mediator [42]. One of the most prominent characteristics of TNF-α is its ability to cause apoptosis of tumor-associated endothelial cells, resulting in tumor necrosis [43]. TNF-α also plays a pivotal role in host defense and can act on monocytes and macrophages in an autocrine manner to enhance various function responses and induce the expression of a number of other immunoregulatory and inflammatory mediators [44]. Furthermore, TNF-α has been reported to exert in anti-influenza effect, which is greater that of γ- and α- interferons [45]. In addition to enhancement of cytokine production, Opuntia polysaccharides also activated macrophage NO and ROS production. These reactive oxidants play key roles in host defense and other physiological processes and are also involved in the regulation of apoptosis and immune homeostasis (reviewed in [46,47]). Overall, our data suggest modulation of the production of cytokines and reactive oxygen/nitrogen species by macrophages likely contributes to the therapeutic effects of O. polyacantha extracts and provides further evidence that the Opuntia polysaccharides have potent immunomodulatory properties.
Opuntia polysaccharides potently activated transcription factor NF-κB in THP-1 human monocytes. Activation of NF-κB controls multiple genes in phagocytes, and target genes regulated by NF-κB include proinflammatory cytokines, chemokines, inflammatory enzymes, adhesion molecules, receptors, and inhibitors of apoptosis (reviewed in [48]). Therefore, the ability to activate phagocyte NF-κB signaling provides further evidence that Opuntia polysaccharides possess immunomodulatory properties and confirms that human phagocytes respond to these compounds. In support of this finding, a number of polysaccharides have been reported previously to activate NF-κB. For example, high molecular weight polysaccharides from Aloe barbadensis increased NF-κB-directed luciferase expression in THP-1 cells [49]. Likewise, high Mr polysaccharides from Spirulina platensis, Aphanizomenon flos-aquae, and Chlorella pyrenoidosa were found to activate NF-κB, leading to increased immune cytokine mRNA levels [50]. Clearly, activation of NF-κB plays a critical role in the transcriptional regulation of TNF-α and inducible NO synthase (iNOS) genes [51]. In addition, TNF-α [52] and NO [53] generated upon cell activation can further activate the NF-κB pathway. Thus, it is reasonable that Opuntia polysaccharide fractions dose-dependently stimulated both macrophage TNF-α, IL-6 and NO production and also activated NF-κB in a similar pattern. In future studies, it will be interesting to determine whether these polysaccharides enhanced production of proinflammatory mediators (e.g., TNF-α and NO) through activation of NF-κB and/or vice versa.
Endotoxin (LPS) is a known immunomodulator and is often a contaminant in biological preparations. Therefore, a number of approaches were utilized to evaluate and prevent possible LPS contamination. Since the crude extract used for isolation of the Opuntia polysaccharide fractions was prepared from intact, uninjured green stems of O. polyacantha after washing in ultrapure water, bacterial contamination of the stems was unlikely. Furthermore, isolation was conducted under conditions that minimized the possibility of bacterial contamination, and the fractions were filtered through 0.22 µm filters prior to biological assays. However, further analyses were performed to directly evaluate this issue. Initially, we evaluated Opuntia polysaccharide fractions using the Limulus-based endotoxin assay. We found that all four fractions caused a positive Limulus response. This is not surprising, as a number of plant polysaccharides have been shown to react in the Limulus assay, presumably through their ability to mimic LPS [39,54,55]. Thus, we evaluated the effects of polymyxin B on polysaccharide activity. This compound can bind to and inactivate LPS, and we found that inclusion of polymyxin B only had little effect on macrophage NO production induced by Opuntia polysaccharides, while it completely inhibited activation by LPS. The partial inhibition of the polysaccharide activity observed after polymyxin B treatment is likely due to nonspecific effects of this agent. For example, polymyxin B has been reported to cause down-regulation of phospholipid-sensitive Ca2+-dependent protein kinase [56] and inhibition of iNOS gene expression and cytokine production in murine macrophages [57]. In addition, polymyxin B is a surface-active peptide that may interact with sites on the plasma membrane functionally related to the targets of plant polysaccharides [58]. Polymyxin B is a cationic peptide that could also directly bind to and/or neutralize active anionic groups in plant polysaccharides. Because of these issues, we performed additional control experiments using polysaccharides eluted through a column of endotoxin-removing gel and showed that polysaccharides treated with endotoxin-removing gel were as active as untreated samples. Therefore, we are confident that the biological activity in our samples is due to the Opuntia polysaccharides and not a contaminating artifact.
In summary, the present study demonstrates that high molecular weight polysaccharides from stems of O. polyacantha are not cytotoxic and activate macrophages, resulting in modulation of NO, ROS, and cytokine production. Our data provide a molecular basis to explain at least part of the beneficial therapeutic effects reported for extracts of O. polyacantha, and suggest that macrophage stimulation by the Opuntia polysaccharides might contribute enhance resistance to infection. Further studies are now in progress to determine which receptor(s) are essential to the expression of the various immunomodulatory effects ascribed to Opuntia polysaccharides and other structural features conferring biological activity.
Acknowledgements
We would like to thank Jerry and Linda Iverson (Big Timber, MT) for providing prickly pear cactus from their ranch and Dr. Scott Busse, Montana State University, Bozeman, MT for help in running NMR samples. This work was supported in part by Department of Defense grant W9113M-04-1-0001, National Institutes of Health grants P20 RR-020185 and U54 AI-065357, National Institutes of Health contract HHSN266200400009C, an equipment grant from the M.J. Murdock Charitable Trust, and the Montana State University Agricultural Experimental Station. The U.S. Army Space and Missile Defense Command, 64 Thomas Drive, Frederick, MD 21702 is the awarding and administering acquisition office. The content of this report does not necessarily reflect the position or policy of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballow M, Nelson R. Immunopharmacology: immunomodulation and immunotherapy. JAMA. 1997;278:2008–2017. doi: 10.1001/jama.278.22.2008. [DOI] [PubMed] [Google Scholar]
- 2.Villinger F. Cytokines as clinical adjuvants: how far are we? Expert Rev Vaccines. 2003;2:317–326. doi: 10.1586/14760584.2.2.317. [DOI] [PubMed] [Google Scholar]
- 3.Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 4.Paulsen BS. Plant polysaccharides with immunostimulatory activities. Curr Organic Chem. 2001;5:939–950. [Google Scholar]
- 5.Kayser O, Masihi KN, Kiderlen AF. Natural products and synthetic compounds as immunomodulators. Expert Rev Anti Infect Ther. 2003;1:319–335. doi: 10.1586/14787210.1.2.319. [DOI] [PubMed] [Google Scholar]
- 6.Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6:317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Finlay BB, Hancock REW. Can innate immunity be enhanced to treat microbial infections? Nat Rev Microbiol. 2004;2:497–504. doi: 10.1038/nrmicro908. [DOI] [PubMed] [Google Scholar]
- 8.Stintzing FC, Carle R. Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol Nutr Food Res. 2005;49:175–194. doi: 10.1002/mnfr.200400071. [DOI] [PubMed] [Google Scholar]
- 9.Feugang JM, Konarski P, Zou DM, Stintzing FC, Zou CP. Nutritional and medicinal use of Cactus pear (Opuntia spp.) cladodes and fruits. Front Biosci. 2006;11:2574–2589. doi: 10.2741/1992. [DOI] [PubMed] [Google Scholar]
- 10.Morton JF. Mucilaginous plants and their uses in medicine. J Ethnopharmacol. 1990;29:245–266. doi: 10.1016/0378-8741(90)90036-s. [DOI] [PubMed] [Google Scholar]
- 11.Lopez AD. Review: Use of the fruits and stems of the prickly pear cactus (Opuntia spp.) into human food. Food Sci Technol Int. 1995;1:65–74. [Google Scholar]
- 12.Alarcon-Aguilar FJ, Valdes-Arzate A, Xolalpa-Molina S, Banderas-Dorantes T, Jimenez-Estrada M, et al. Hypoglycemic activity of two polysaccharides isolated from Opuntia ficusindica and O. streptacantha. Proc West Pharmacol Soc. 2003;46:139–142. [PubMed] [Google Scholar]
- 13.Galati EM, Mondello MR, Giuffrida D, Dugo G, Miceli N, et al. Chemical characterization and biological effects of Sicilian Opuntia ficus indica (L.) mill. fruit juice: antioxidant and antiulcerogenic activity. J Agric Food Chem. 2003;51:4903–4908. doi: 10.1021/jf030123d. [DOI] [PubMed] [Google Scholar]
- 14.Lee JC, Kim HR, Kim J, Jang YS. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem. 2002;50:6490–6496. doi: 10.1021/jf020388c. [DOI] [PubMed] [Google Scholar]
- 15.Park EH, Kahng JH, Lee SH, Shin KH. An anti-inflammatory principle from cactus. Fitoterapia. 2001;72:288–290. doi: 10.1016/s0367-326x(00)00287-2. [DOI] [PubMed] [Google Scholar]
- 16.Ncibi S, Ben Othman M, Akacha A, Krifi MN, Zourgui L. Opuntia ficus indica extract protects against chlorpyrifos-induced damage on mice liver. Food Chem Toxicol. 2008;46:797–802. doi: 10.1016/j.fct.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 17.Lee MH, Kim JY, Yoon JH, Lim HJ, Kim TH, et al. Inhibition of nitric oxide synthase expression in activated microglia and peroxynitrite scavenging activity by Opuntia ficus indica var. saboten. Phytother Res. 2006;20:742–747. doi: 10.1002/ptr.1942. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed MS, El Tanbouly ND, Islam WT, Sleem AA, El Senousy AS. Antiinflammatory flavonoids from Opuntia dillenii (Ker-Gawl) Haw. flowers growing in Egypt. Phytother Res. 2005;19:807–809. doi: 10.1002/ptr.1708. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J, Li Y, Chen Z, Min Z, Lou F. Two novel C29-5β-sterols from the stems of Opuntia dillenii. Steroids. 2006;71:1073–1077. doi: 10.1016/j.steroids.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Oh PS, Lim KT. Glycoprotein (90 kDa) isolated from Opuntia ficus-indica var. saboten MAKINO lowers plasma lipid level through scavenging of intracellular radicals in triton WR-1339-induced mice. Biol Pharm Bull. 2006;29:1391–1396. doi: 10.1248/bpb.29.1391. [DOI] [PubMed] [Google Scholar]
- 21.Sreekanth D, Arunasree MK, Roy KR, Reddy TC, Reddy GV, et al. Betanin a betacyanin pigment purified from fruits of Opuntia ficus-indica induces apoptosis in human chronic myeloid leukemia Cell line-K562. Phytomedicine. 2007;14:739–746. doi: 10.1016/j.phymed.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Qiu YK, Zhao YY, Dou DQ, Xu BX, Liu K. Two new alpha-pyrones and other components from the cladodes of Opuntia dillenii. Arch Pharm Res. 2007;30:665–669. doi: 10.1007/BF02977624. [DOI] [PubMed] [Google Scholar]
- 23.Majdoub H, Roudesli S, Deratani A. Polysaccharides from prickly pear peel and nopals of Opuntia ficus-indica: extraction, characterization and polyelectrolyte behaviour. Polymer International. 2001;50:552–560. [Google Scholar]
- 24.Habibi Y, Mahrouz M, Marais MF, Vignon MR. An arabinogalactan from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr Res. 2004;339:1201–1205. doi: 10.1016/j.carres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Trombetta D, Puglia C, Perri D, Licata A, Pergolizzi S, et al. Effect of polysaccharides from Opuntia ficus-indica (L.) cladodes on the healing of dermal wounds in the rat. Phytomedicine. 2006;13:352–358. doi: 10.1016/j.phymed.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Panico AM, Cardile V, Garufi F, Puglia C, Bonina F, et al. Effect of hyaluronic acid and polysaccharides from Opuntia ficus indica (L.) cladodes on the metabolism of human chondrocyte cultures. J Ethnopharmacol. 2007;111:315–321. doi: 10.1016/j.jep.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Wilson K. Wound healing: the role of macrophages. Nurs Crit Care. 1997;2:291–296. [PubMed] [Google Scholar]
- 28.Gauss KA, Nelson-Overton LK, Siemsen DW, Gao Y, DeLeo FR, et al. Role of NF-kappa B in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. J Leukoc Biol. 2007;82:729–741. doi: 10.1189/jlb.1206735. [DOI] [PubMed] [Google Scholar]
- 29.Schepetkin IA, Kirpotina LN, Khlebnikov AI, Quinn MT. High-throughput screening for small-molecule activators of neutrophils: Identification of novel N-formyl peptide receptor agonists. Mol Pharm. 2007;71:1061–1074. doi: 10.1124/mol.106.033100. [DOI] [PubMed] [Google Scholar]
- 30.Matsuhiro B, Lillo LE, Saenz C, Urzua CC, Zarate O. Chemical characterization of the mucilage from fruits of Opuntia ficus indica. Carbohydrate Polymers. 2006;63:263–267. [Google Scholar]
- 31.Polle AY, Ovodova RG, Shashkov AS, Ovodov YS. Some structural features of pectic polysaccharide from tansy, Tanacetum vulgare L. Carbohydrate Polymers. 2002;49:337–344. doi: 10.1023/a:1021810010222. [DOI] [PubMed] [Google Scholar]
- 32.Gane AM, Craik D, Munro SL, Howlett GJ, Clarke AE, et al. Structural analysis of the carbohydrate moiety of arabinogalactan-proteins from stigmas and styles of Nicotiana alata. Carbohydr Res. 1995;277:67–85. doi: 10.1016/0008-6215(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 33.Moerman D. Native American Ethnobotany. Portland: Timber Press; 1998. [Google Scholar]
- 34.Nobel PS, Cavelier J, Andrade JL. Mucilage in cacti - Its apoplastic capacitance, associated solutes, and influence on tissue water relations. J Exp Botany. 1992;43:641–648. [Google Scholar]
- 35.Xie G, Schepetkin IA, Quinn MT. Immunomodulatory activity of acidic polysaccharides isolated from Tanacetum vulgare L. Int Immunopharmacol. 2007;7:1639–1650. doi: 10.1016/j.intimp.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie G, Schepetkin IA, Siemsen DW, Kirpotina LN, Wiley JA, et al. Fractionation and characterization of biologically-active polysaccharides from Artemisia tripartita. Phytochemistry. 2008;69:1359–1371. doi: 10.1016/j.phytochem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Swain SD, Rohn TT, Quinn MT. Neutrophil priming in host defense: role of oxidants as priming agents. Antioxid Redox Signal. 2002;4:69–83. doi: 10.1089/152308602753625870. [DOI] [PubMed] [Google Scholar]
- 39.Schepetkin IA, Faulkner CL, Nelson-Overton LK, Wiley JA, Quinn MT. Macrophage immunomodulatory activity of polysaccharides isolated from Juniperus scopolorum. Int Immunopharmacol. 2005;5:1783–1799. doi: 10.1016/j.intimp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Leung MY, Liu C, Zhu LF, Hui YZ, Yu B, et al. Chemical and biological characterization of a polysaccharide biological response modifier from Aloe vera L. var. chinensis (Haw.) Berg. Glycobiology. 2004;14:501–510. doi: 10.1093/glycob/cwh050. [DOI] [PubMed] [Google Scholar]
- 41.Okazaki M, Adachi Y, Ohno N, Yadomae T. Structure-activity relationship of (1-->3)-β-D-glucans in the induction of cytokine production from macrophages, in vitro. Biol Pharm Bull. 1995;18:1320–1327. doi: 10.1248/bpb.18.1320. [DOI] [PubMed] [Google Scholar]
- 42.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8 Suppl 2:S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lejeune FJ, Lienard D, Matter M, Ruegg C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006;6:6. [PubMed] [Google Scholar]
- 44.Baugh JA, Bucala R. Mechanisms for modulating TNFα in immune and inflammatory disease. Curr Opin Drug Discov Devel. 2001;4:635–650. [PubMed] [Google Scholar]
- 45.Seo SH, Webster RG. Tumor necrosis factor α exerts powerful anti-influenza virus effects in lung epithelial cells. J Virol. 2002;76:1071–1076. doi: 10.1128/JVI.76.3.1071-1076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 47.Quinn MT, Ammons MC, DeLeo FR. The expanding role of NADPH oxidases in health and disease: no longer just agents of death and destruction. Clin Sci. 2006;111:1–20. doi: 10.1042/CS20060059. [DOI] [PubMed] [Google Scholar]
- 48.Brasier AR. The NF-κB regulatory network. Cardiovasc Toxicol. 2006;6:111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- 49.Pugh N, Ross SA, ElSohly MA, Pasco DS. Characterization of Aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J Agric Food Chem. 2001;49:1030–1034. doi: 10.1021/jf001036d. [DOI] [PubMed] [Google Scholar]
- 50.Pugh N, Ross SA, ElSohly HN, ElSohly MA, Pasco DS. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001;67:737–742. doi: 10.1055/s-2001-18358. [DOI] [PubMed] [Google Scholar]
- 51.Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Grivennikov SI, Kuprash DV, Liu ZG, Nedospasov SA. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: From simple paradigms to complex mechanisms. Int Rev Cytol. 2006;252:129–161. doi: 10.1016/S0074-7696(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 53.Persichini T, Cantoni O, Suzuki H, Colasanti M. Cross-talk between constitutive and inducible NO synthase: an update. Antioxid Redox Signal. 2006;8:949–954. doi: 10.1089/ars.2006.8.949. [DOI] [PubMed] [Google Scholar]
- 54.Royce CL, Pardy RL. Endotoxin-like properties of an extract from a symbiotic, eukaryotic Chlorella-like green alga. J Endotoxin Res. 1996;3:437–444. [Google Scholar]
- 55.Tanaka S, Aketagawa J, Takahashi S, Shibata Y, Tsumuraya Y, et al. Activation of a limulus coagulation factor G by (1->3)-β-D-glucans. Carbohydr Res. 2005;218:167–174. doi: 10.1016/0008-6215(93)80008-3. [DOI] [PubMed] [Google Scholar]
- 56.Mazzei GJ, Katoh N, Kuo JF. Polymyxin B is a more selective inhibitor for phospholipids-sensitive Ca2+-dependent protein kinase than for calmodulin-sensitive Ca2+-dependent protein kinase. Biochem Biophys Res Commun. 1982;109:1129–1133. doi: 10.1016/0006-291x(82)91894-0. [DOI] [PubMed] [Google Scholar]
- 57.Jun CD, Choi BM, Kim HM, Chung HT. Involvement of protein kinase C during taxol-induced activation of murine peritoneal macrophages. J Immunol. 1995;154:6541–6547. [PubMed] [Google Scholar]
- 58.Raynor RL, Zheng B, Kuo JF. Membrane interactions of amphiphilic polypeptides mastoparan, melittin, polymyxin B, and cardiotoxin. Differential inhibition of protein kinase C, Ca2+/calmodulin-dependent protein kinase II and synaptosomal membrane Na,K-ATPase, and Na+ pump and differentiation of HL60 cells. J Biol Chem. 1991;266:2753–2758. [PubMed] [Google Scholar]