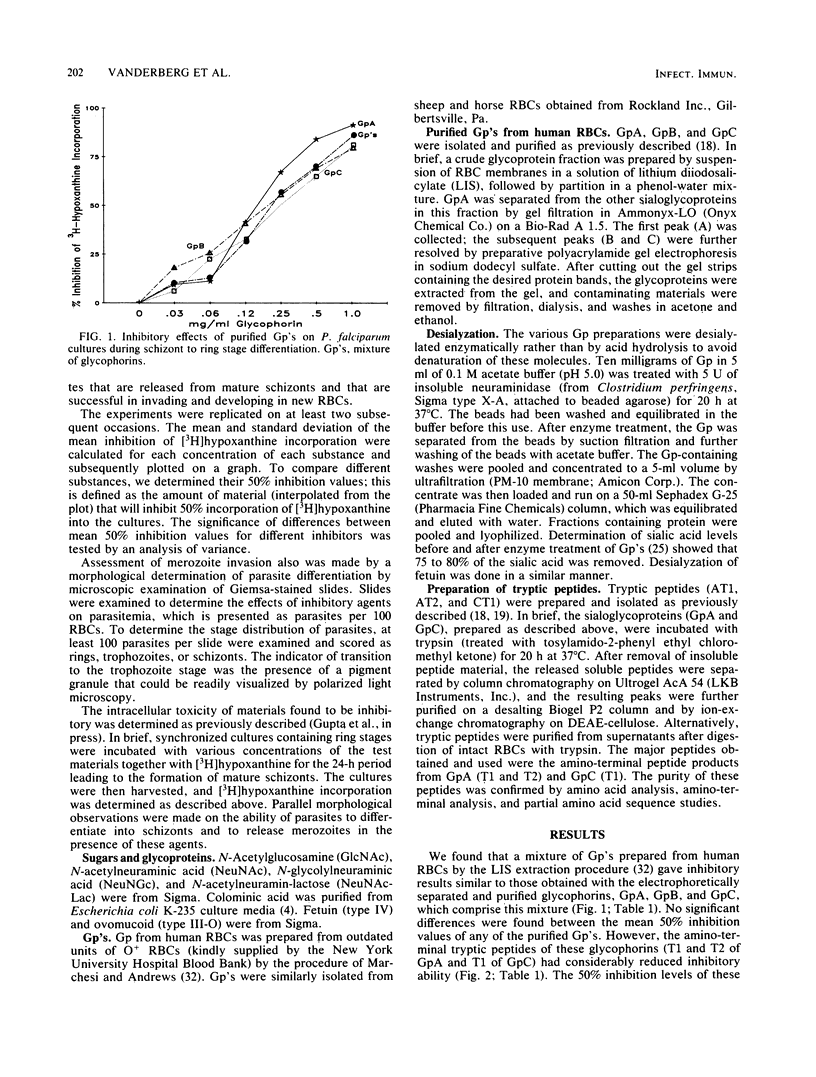
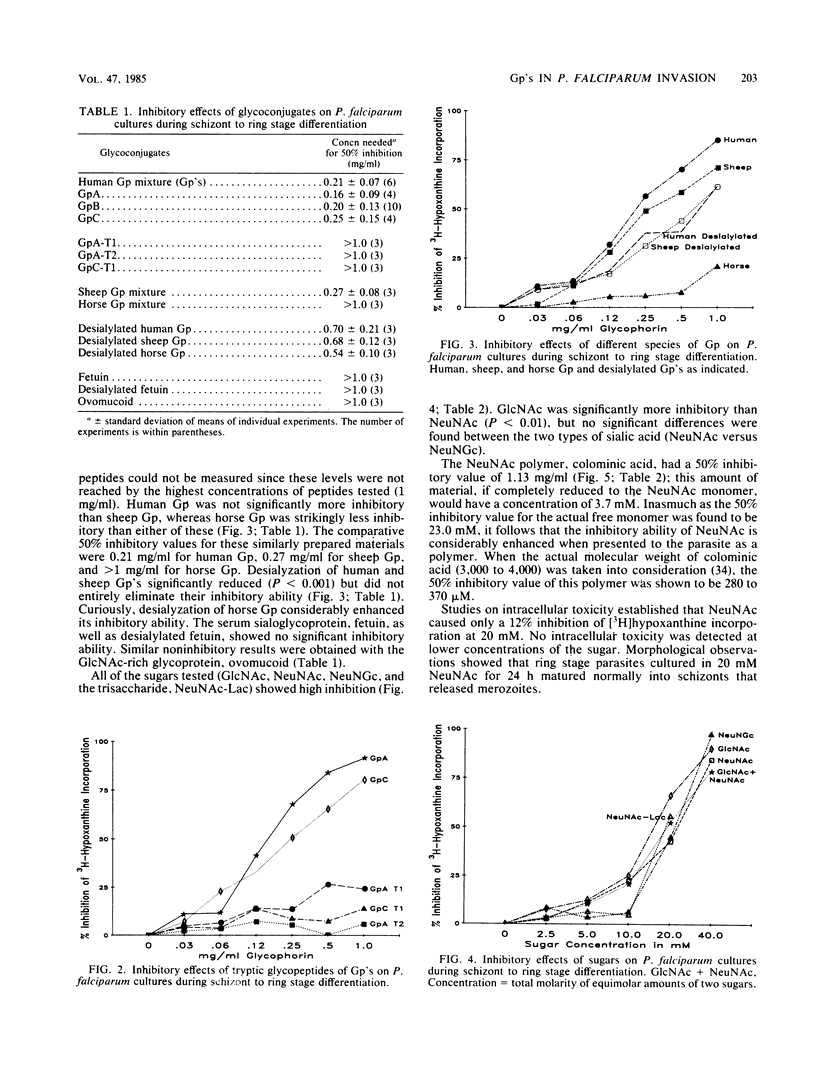
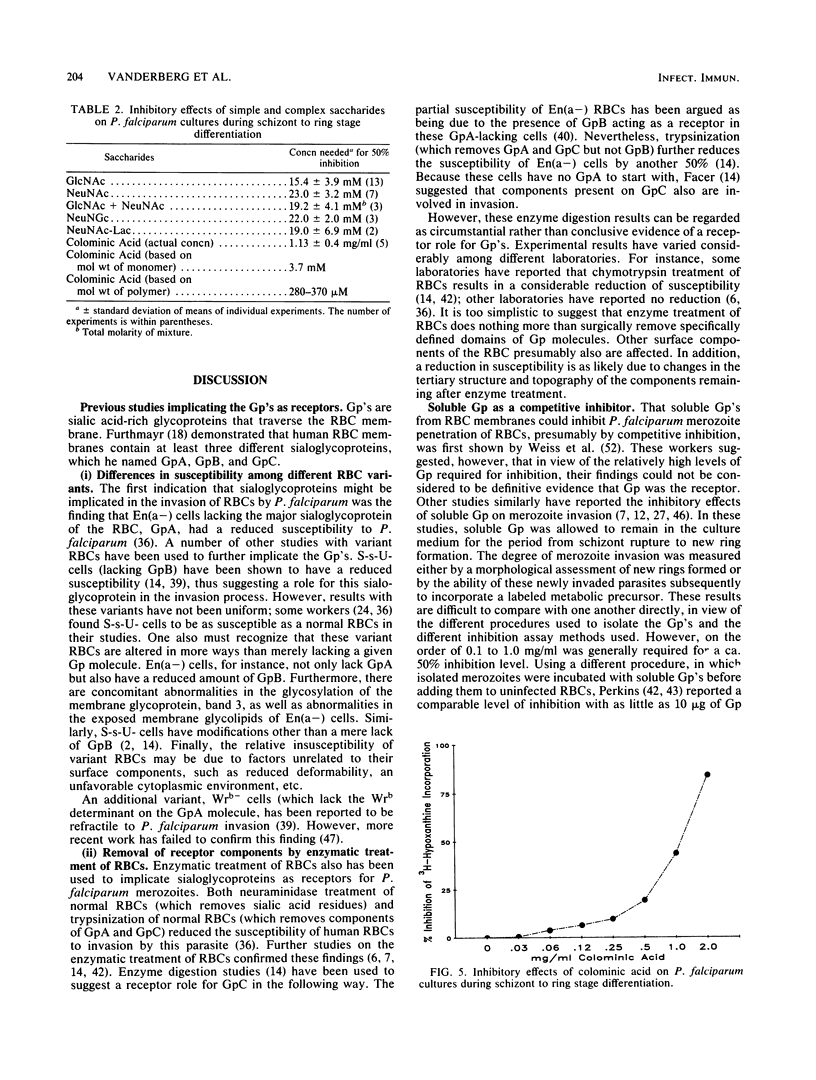
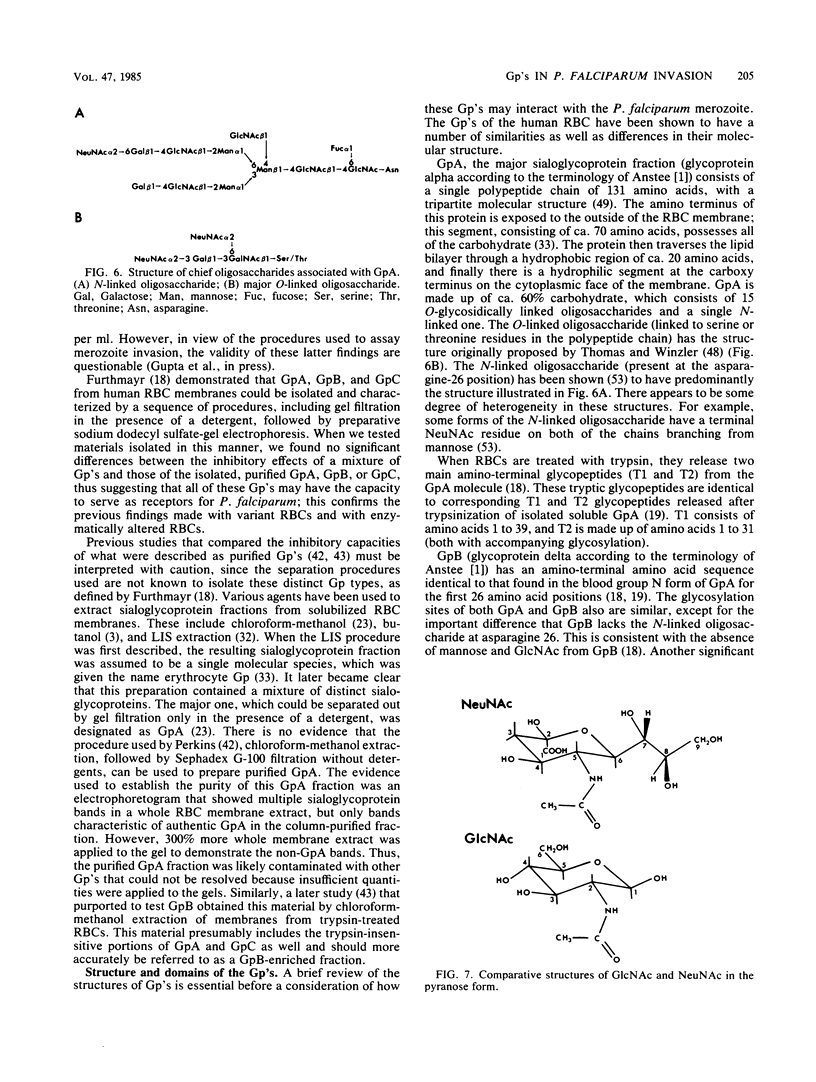
Abstract
Solubilized preparations of purified glycophorins and specific domains of these molecules were assessed for their effects as inhibitors of Plasmodium falciparum invasion of human erythrocytes in vitro. The ability of newly invaded merozoites to continue developing and incorporating [3H]hypoxanthine during a 24-h period after their invasion was used as an assay for merozoite invasion. Glycophorins A, B, and C were found to be equally effective as inhibitors. Previous studies had shown N-acetylglucosamine, a sugar component of glycophorins A and C but not B, to be an effective inhibitor. Accordingly, molecular domains common to all of the glycophorins were further assessed. Sialic acid was shown to act almost as effectively as N-acetylglucosamine, presumably because of the structural similarities between these sugars. The inhibitory ability of sialic acid is considerably enhanced when presented to the parasite in a clustered form, as in an oligosaccharide. The acetyl group of these sugars does not appear to play an essential role in this inhibition. How the P. falciparum merozoite recognizes and interacts with the sugar domains of the glycophorin molecule remains to be determined.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstee D. J., Mawby W. J., Tanner M. J. Abnormal blood-group-Ss-active sialoglycoproteins in the membrane of Miltenberger class III, IV and V human erythrocytes. Biochem J. 1979 Nov 1;183(2):193–203. doi: 10.1042/bj1830193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstee D. J., Tanner M. J. The distribution of blood-group antigens on butanol extraction of human erythrocyte 'ghosts'. Biochem J. 1974 Mar;138(3):381–386. doi: 10.1042/bj1380381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstee D. J. The blood group MNSs-active sialoglycoproteins. Semin Hematol. 1981 Jan;18(1):13–31. [PubMed] [Google Scholar]
- BARRY G. T., GOEBEL W. F. Colominic acid, a substance of bacterial origin related to sialic acid. Nature. 1957 Jan 26;179(4552):206–206. doi: 10.1038/179206a0. [DOI] [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J Biol Chem. 1979 May 25;254(10):4000–4008. [PubMed] [Google Scholar]
- Breuer W. V., Ginsburg H., Cabantchik Z. I. An assay of malaria parasite invasion into human erythrocytes. The effects of chemical and enzymatic modification of erythrocyte membrane components. Biochim Biophys Acta. 1983 Jan 25;755(2):263–271. doi: 10.1016/0304-4165(83)90213-1. [DOI] [PubMed] [Google Scholar]
- Breuer W. V., Kahane I., Baruch D., Ginsburg H., Cabantchik Z. I. Role of internal domains of glycophorin in Plasmodium falciparum invasion of human erythrocytes. Infect Immun. 1983 Oct;42(1):133–140. doi: 10.1128/iai.42.1.133-140.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burness A. T., Pardoe I. U. A sialoglycopeptide from human erythrocytes with receptor-like properties for encephalomyocarditis and influenza viruses. J Gen Virol. 1983 May;64(Pt 5):1137–1148. doi: 10.1099/0022-1317-64-5-1137. [DOI] [PubMed] [Google Scholar]
- Buscher H. P., Casals-Stenzel J., Schaufer R. New sialic acids. Identification of N-glycoloyl-O-acetylneuraminic acids and N-acetyl-O-glycoloylneuraminic acids by improved methods for detection of N-acyl and O-acyl groups and by gas-liquid chromatography. Eur J Biochem. 1974 Dec 16;50(1):71–82. doi: 10.1111/j.1432-1033.1974.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Butcher G. A., Mitchell G. H., Cohen S. Letter: Mechanism of host specificity in malarial infection. Nature. 1973 Jul 6;244(5410):40–41. doi: 10.1038/244040a0. [DOI] [PubMed] [Google Scholar]
- Deas J. E., Lee L. T. Competitive inhibition by soluble erythrocyte glycoproteins of penetration by Plasmodium falciparum. Am J Trop Med Hyg. 1981 Nov;30(6):1164–1167. doi: 10.4269/ajtmh.1981.30.1164. [DOI] [PubMed] [Google Scholar]
- Dejter-Juszynski M., Harpaz N., Flowers H. M., Sharon N. Blood-group ABH-specific macroglycolipids of human erythrocytes: isolation in high yield from a crude membrane glycoprotein fraction. Eur J Biochem. 1978 Feb;83(2):363–373. doi: 10.1111/j.1432-1033.1978.tb12102.x. [DOI] [PubMed] [Google Scholar]
- Facer C. A. Erythrocyte sialoglycoproteins and Plasmodium falciparum invasion. Trans R Soc Trop Med Hyg. 1983;77(4):524–530. doi: 10.1016/0035-9203(83)90130-x. [DOI] [PubMed] [Google Scholar]
- Friedman M. J., Blankenberg T., Sensabaugh G., Tenforde T. S. Recognition and invasion of human erythrocytes by malarial parasites: contribution of sialoglycoproteins to attachment and host specificity. J Cell Biol. 1984 May;98(5):1672–1677. doi: 10.1083/jcb.98.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. J. Control of malaria virulence by alpha 1-acid glycoprotein (orosomucoid), an acute-phase (inflammatory) reactant. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5421–5424. doi: 10.1073/pnas.80.17.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Tomita M., Hamada A. Isolation and characterization of alkali-labile oligosaccharide units from horse glycophorin. J Biochem. 1980 Mar;87(3):687–693. doi: 10.1093/oxfordjournals.jbchem.a132797. [DOI] [PubMed] [Google Scholar]
- Furthmayr H. Glycophorins A, B, and C: a family of sialoglycoproteins. Isolation and preliminary characterization of trypsin derived peptides. J Supramol Struct. 1978;9(1):79–95. doi: 10.1002/jss.400090109. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Kahane I., Marchesi V. T. Isolation of the major intrinsic transmembrane protein of the human erythrocyte membrane. J Membr Biol. 1976 Mar 18;26(2-3):173–187. doi: 10.1007/BF01868872. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Marchesi V. T. Glycophorins: isolation, orientation, and localization of specific domains. Methods Enzymol. 1983;96:268–280. doi: 10.1016/s0076-6879(83)96025-1. [DOI] [PubMed] [Google Scholar]
- Furthmayr H. Structural comparison of glycophorins and immunochemical analysis of genetic variants. Nature. 1978 Feb 9;271(5645):519–524. doi: 10.1038/271519a0. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Tomita M., Marchesi V. T. Fractionation of the major sialoglycopeptides of the human red blood cell membrane. Biochem Biophys Res Commun. 1975 Jul 8;65(1):113–121. doi: 10.1016/s0006-291x(75)80068-4. [DOI] [PubMed] [Google Scholar]
- Hamaguchi H., Cleve H. Solubilization and comparative analysis of mammalian erythrocyte membrane glycoproteins. Biochem Biophys Res Commun. 1972 Apr 28;47(2):459–464. doi: 10.1016/0006-291x(72)90736-x. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Haynes J. D., McGinniss M. H., Miller L. H. Studies on the role of red blood cell glycoproteins as receptors for invasion by Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1982 Nov;6(5):303–315. doi: 10.1016/0166-6851(82)90063-9. [DOI] [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Jungery M., Boyle D., Patel T., Pasvol G., Weatherall D. J. Lectin-like polypeptides of P. falciparum bind to red cell sialoglycoproteins. Nature. 1983 Feb 24;301(5902):704–705. doi: 10.1038/301704a0. [DOI] [PubMed] [Google Scholar]
- Jungery M., Pasvol G., Newbold C. I., Weatherall D. J. A lectin-like receptor is involved in invasion of erythrocytes by Plasmodium falciparum. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1018–1022. doi: 10.1073/pnas.80.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLENK E., UHLENBRUCK G. Uber ein neuraminsäurehaltiges Mucoproteid aus Rindererythrocytenstroma. Hoppe Seylers Z Physiol Chem. 1958;311(4-6):227–233. [PubMed] [Google Scholar]
- LISOWSKA E. ISOLATION, STRUCTURE AND SEROLOGICAL PROPERTIES OF MUCOPROTEID FROM HORSE ERYTHROCYTES. Arch Immunol Ther Exp (Warsz) 1962;10:908–928. [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lisowska E. Periodate oxidation of the mucoid from horse erythrocytes. Arch Immunol Ther Exp (Warsz) 1966;14(4):484–490. [PubMed] [Google Scholar]
- MCGUIRE E. J., BINKLEY S. B. THE STRUCTURE AND CHEMISTRY OF COLOMINIC ACID. Biochemistry. 1964 Feb;3:247–251. doi: 10.1021/bi00890a017. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Dvorak J. A., Shiroishi T., Durocher J. R. Influence of erythrocyte membrane components on malaria merozoite invasion. J Exp Med. 1973 Dec 1;138(6):1597–1601. doi: 10.1084/jem.138.6.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Haynes J. D., McAuliffe F. M., Shiroishi T., Durocher J. R., McGinniss M. H. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J Exp Med. 1977 Jul 1;146(1):277–281. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Sene C., Maget-Dana R., Delmotte F. Sugar-lectin interactions: how does wheat-germ agglutinin bind sialoglycoconjugates? Eur J Biochem. 1980 Feb;104(1):147–153. doi: 10.1111/j.1432-1033.1980.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Jungery M. Glycophorins and red cell invasion by Plasmodium falciparum. Ciba Found Symp. 1983;94:174–195. doi: 10.1002/9780470715444.ch11. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Jungery M., Weatherall D. J., Parsons S. F., Anstee D. J., Tanner M. J. Glycophorin as a possible receptor for Plasmodium falciparum. Lancet. 1982 Oct 30;2(8305):947–950. doi: 10.1016/s0140-6736(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Wainscoat J. S., Weatherall D. J. Erythrocytes deficiency in glycophorin resist invasion by the malarial parasite Plasmodium falciparum. Nature. 1982 May 6;297(5861):64–66. doi: 10.1038/297064a0. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Wilson R. J., Smalley M. E., Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann Trop Med Parasitol. 1978 Feb;72(1):87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- Perkins M. E. Binding of glycophorins to Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1984 Jan;10(1):67–78. doi: 10.1016/0166-6851(84)90019-7. [DOI] [PubMed] [Google Scholar]
- Perkins M. Inhibitory effects of erythrocyte membrane proteins on the in vitro invasion of the human malarial parasite (Plasmodium falciparum) into its host cell. J Cell Biol. 1981 Sep;90(3):563–567. doi: 10.1083/jcb.90.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman S., Lee Y. C., Vanderberg J. P. Effects of neoglycoproteins on penetration of Plasmodium falciparum merozoites into erythrocytes in vitro. J Parasitol. 1984 Apr;70(2):213–216. [PubMed] [Google Scholar]
- Schulman S., Oppenheim J. D., Vanderberg J. P. Assay of erythrocyte components as inhibitors of Plasmodium falciparum merozoite invasion of erythrocytes. Am J Trop Med Hyg. 1983 Jul;32(4):666–670. doi: 10.4269/ajtmh.1983.32.666. [DOI] [PubMed] [Google Scholar]
- Schulman S., Vanderberg J. P. Wright's b determinant, monoclonal antibodies, and Plasmodium falciparum merozoite invasion. Lancet. 1984 Oct 20;2(8408):934–935. doi: 10.1016/s0140-6736(84)90693-7. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J Biol Chem. 1969 Nov 10;244(21):5943–5946. [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W., Tershakovec M., Lyandvert L., Stanley H., Lanners N., Gubert E. Clones of the malaria parasite Plasmodium falciparum obtained by microscopic selection: their characterization with regard to knobs, chloroquine sensitivity, and formation of gametocytes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6527–6530. doi: 10.1073/pnas.78.10.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. M., Oppenheim J. D., Vanderberg J. P. Plasmodium falciparum: assay in vitro for inhibitors of merozoite penetration of erythrocytes. Exp Parasitol. 1981 Jun;51(3):400–407. doi: 10.1016/0014-4894(81)90127-2. [DOI] [PubMed] [Google Scholar]
- Yoshima H., Furthmayr H., Kobata A. Structures of the asparagine-linked sugar chains of glycophorin A. J Biol Chem. 1980 Oct 25;255(20):9713–9718. [PubMed] [Google Scholar]
- Zolg J. W., MacLeod A. J., Dickson I. H., Scaife J. G. Plasmodium falciparum: modifications of the in vitro culture conditions improving parasitic yields. J Parasitol. 1982 Dec;68(6):1072–1080. [PubMed] [Google Scholar]


