Abstract
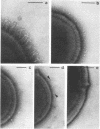
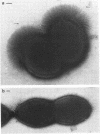
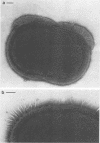
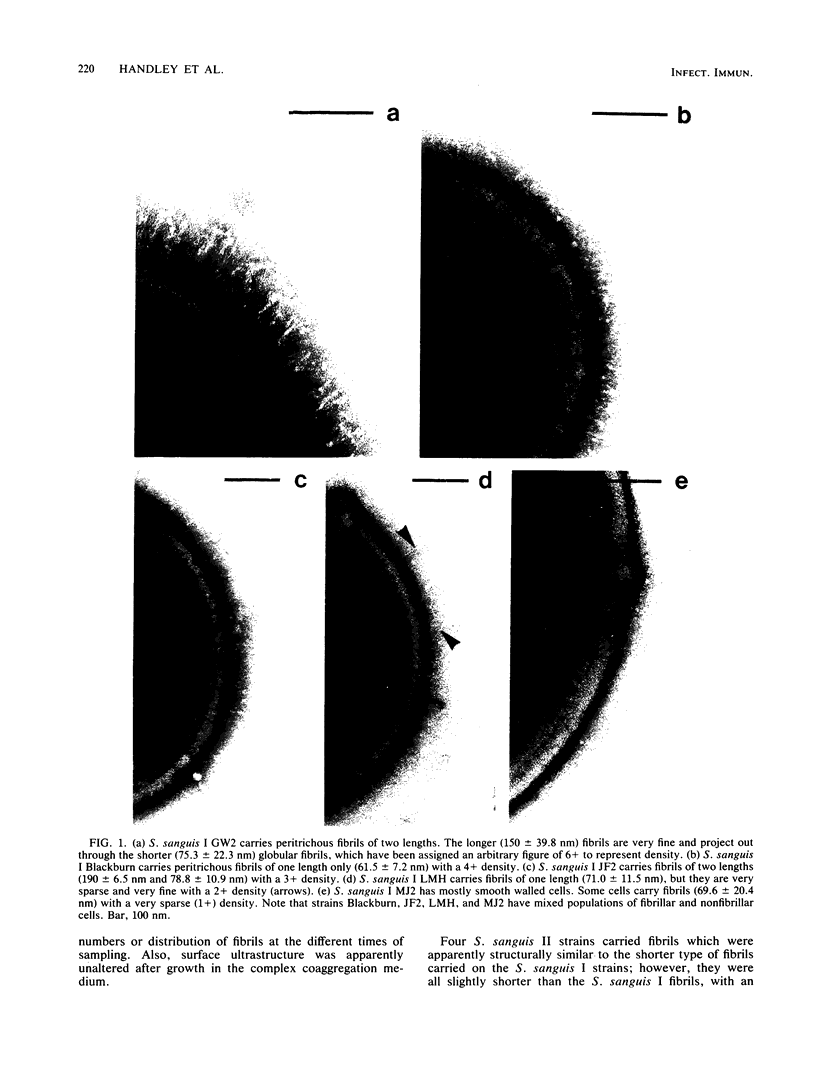
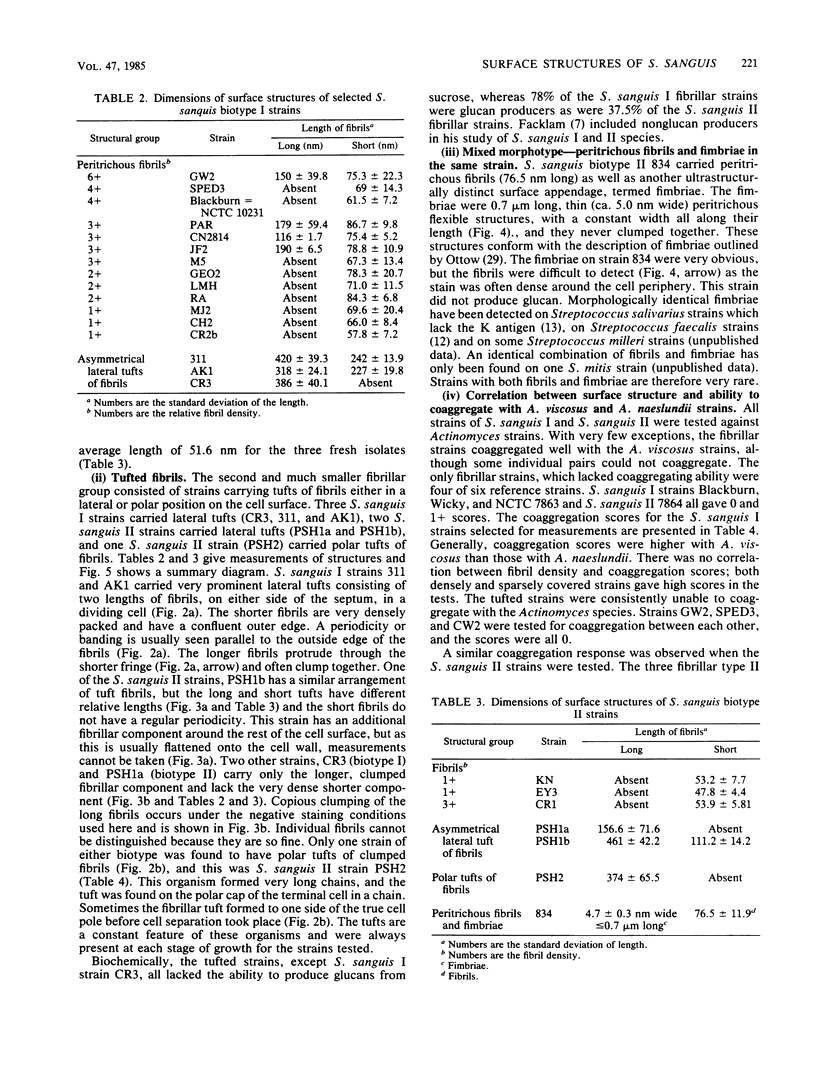
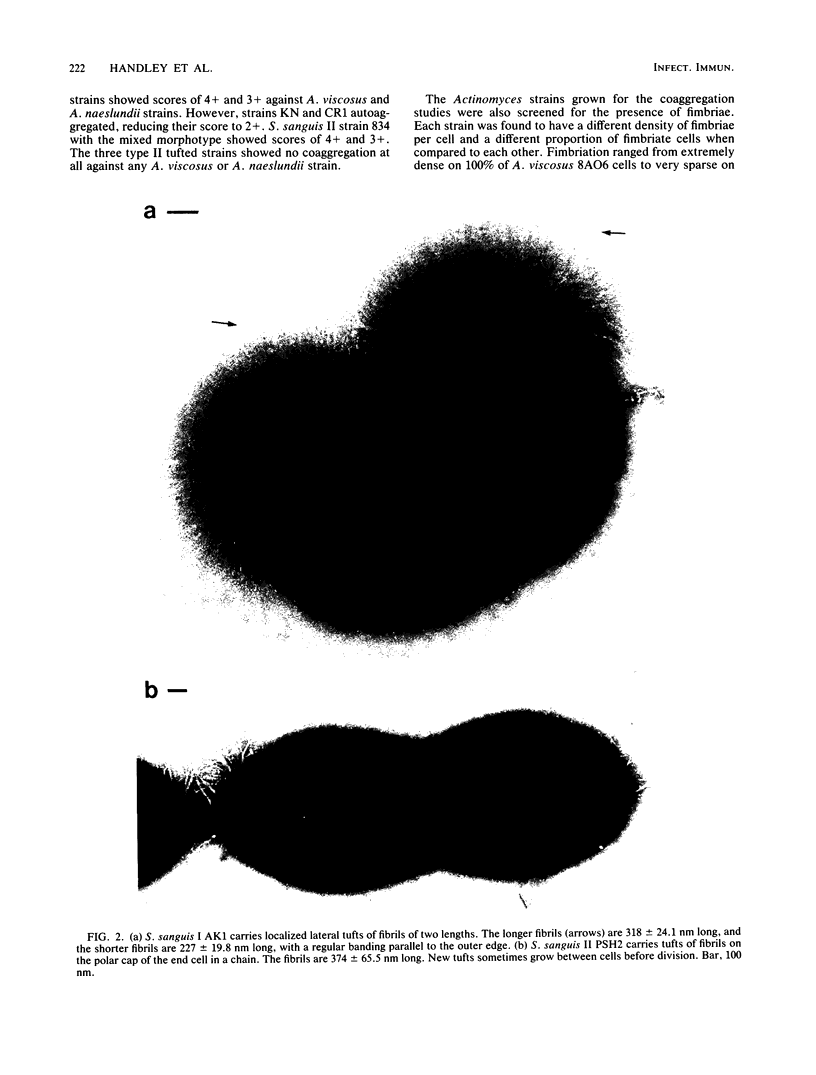
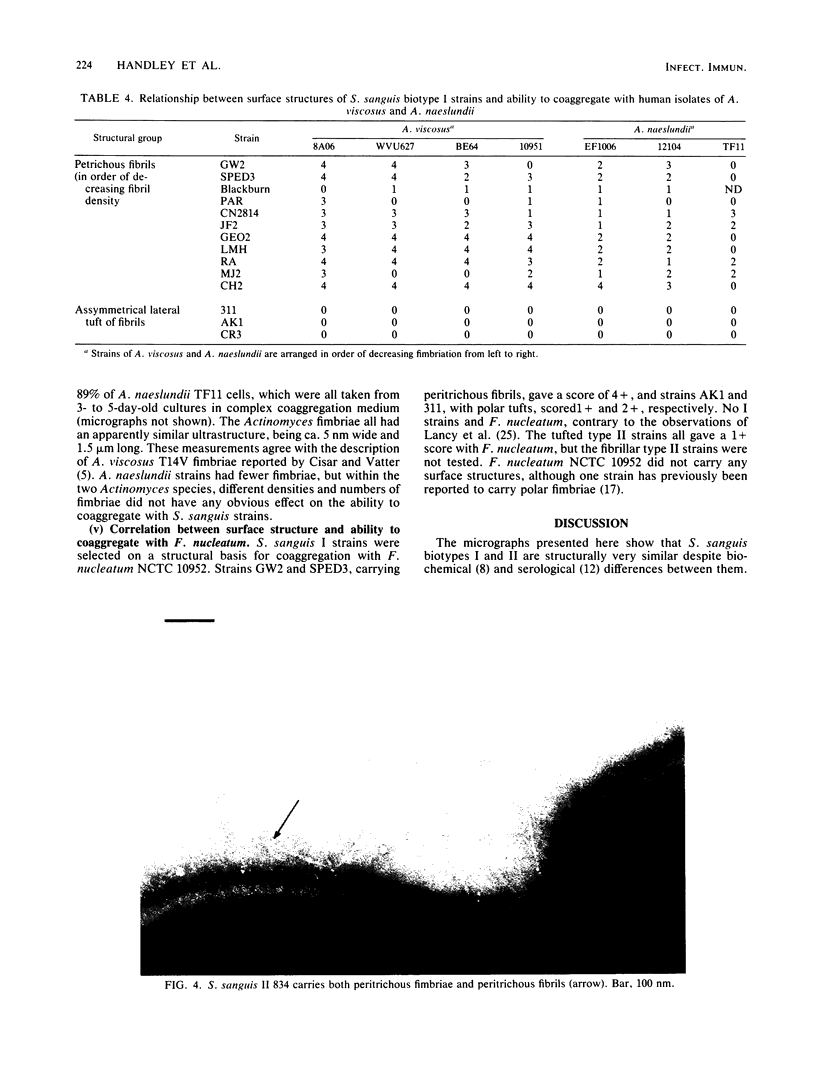
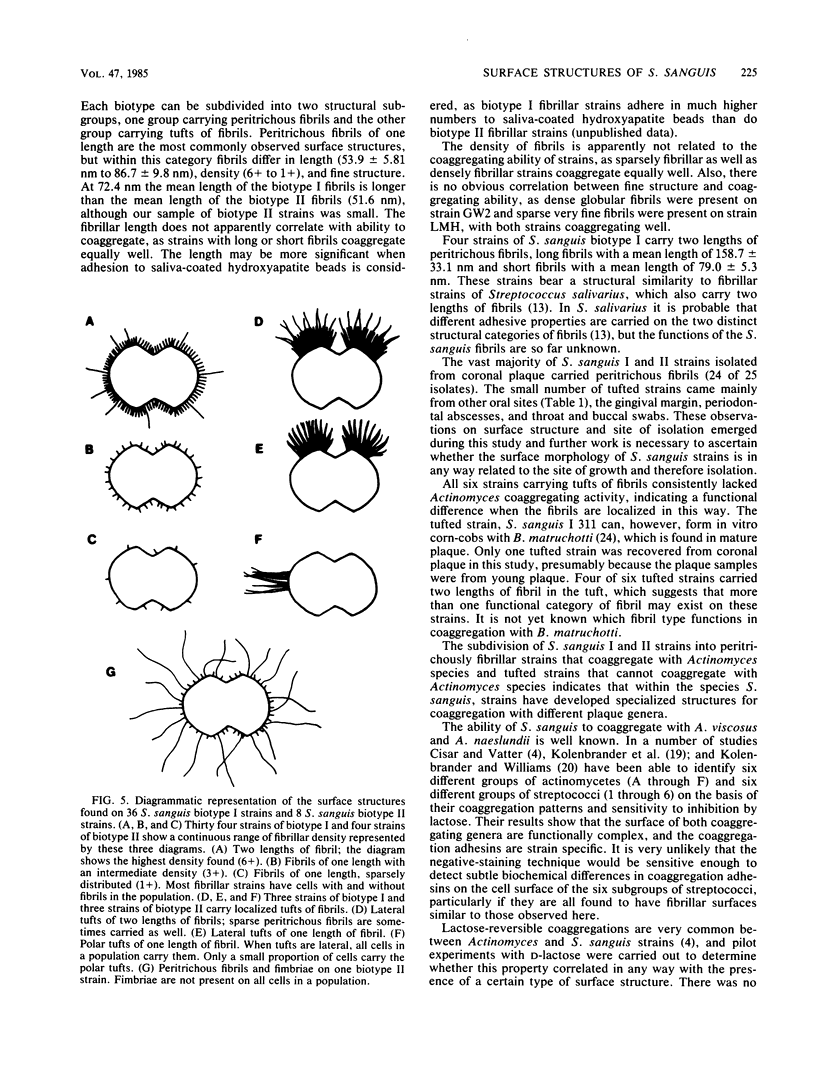
We screened 36 strains of Streptococcus sanguis biotype I and 8 strains of S. sanguis biotype II for the presence of surface structures and for their ability to coaggregate with Actinomyces viscosus, Actinomyces naeslundii, and Fusobacterium nucleatum. Negative staining under an electron microscope revealed detectable surface structures on all S. sanguis strains. The majority of strains (38 of 44) carried peritrichous fibrils, which have an irregular profile and no distinct width. They usually appeared as a fringe with a constant width around the cell. Strains selected for measurement had a fringe with an average length of 72.4 +/- 8.5 nm on biotype I strains and 51.6 +/- 3.3 nm on biotype II strains. Some fibrillar biotype I strains carried an additional, longer (158.7 +/- 33.1 nm) type of fibril projecting through the shorter fibrils. Fibrillar density was characteristic for each strain, ranging from very dense on all cells in a population to very sparse on a few cells in a population. A small group of six strains carried tufts of fibrils in a lateral or polar position on the cell. Either one or two lengths of fibril were present in the tuft depending on the strain. One strain carried both peritrichous fibrils and fimbriae. Fimbriae are flexible structures with a constant width (4.5 to 5.0 nm) all along their length but very variable lengths (less than or equal to 0.7 micron) on each cell. S. sanguis I and II both included strains with peritrichous fibrils and tufts of fibrils, but the mixed morphotype strain was confined to biotype II. Fibrils were present on cells at all stages throughout the growth cycle for the strains tested. Freshly isolated fibrillar strains coaggregated consistently well with A. viscosus and A. naeslundii, although some fibrillar reference strains lacked the ability. In addition, all tufted strains could not coaggregate, but the strains with the mixed morphotype coaggregated well. Coaggregation with F. nucleatum was very strong for the fibrillar strains, but less strong for the tufted strains. We discuss the possible correlation between S. sanguis surface structure and ability to coaggregate.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton R. W. Adherence of oral streptococci to hydroxyapatite in vitro via glycerol-teichoic acid. Arch Oral Biol. 1980;25(2):111–114. doi: 10.1016/0003-9969(80)90085-0. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970 Dec;15(12):1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E. Surface fibrils (fimbriae) of Actinomyces viscosus T14V. Infect Immun. 1979 May;24(2):523–531. doi: 10.1128/iai.24.2.523-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Burger B. W. Microbial surface interactions: reduction of the haemagglutination activity of the oral bacterium Fusobacterium nucleatum by absorption with Streptococcus and Bacteroides. Arch Oral Biol. 1981;26(12):1015–1025. doi: 10.1016/0003-9969(81)90112-6. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Hamada S., Torii M., Tsuchitani Y., Kotani S. Isolation and immunobiological classification of Streptococcus sanguis from human tooth surfaces. J Clin Microbiol. 1980 Aug;12(2):243–249. doi: 10.1128/jcm.12.2.243-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley P. S., Carter P. L., Fielding J. Streptococcus salivarius strains carry either fibrils or fimbriae on the cell surface. J Bacteriol. 1984 Jan;157(1):64–72. doi: 10.1128/jb.157.1.64-72.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley P. S., Jacob A. E. Some structural and physiological properties of fimbriae of Streptococcus faecalis. J Gen Microbiol. 1981 Dec;127(2):289–293. doi: 10.1099/00221287-127-2-289. [DOI] [PubMed] [Google Scholar]
- Henriksen S. D., Henrichsen J. Twitching motility and possession of polar fimbriae in spreading Streptococcus sanguis isolates from the human throat. Acta Pathol Microbiol Scand B. 1975 Apr;83(2):133–140. doi: 10.1111/j.1699-0463.1975.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Hogg S. D., Handley P. S., Embery G. Surface fibrils may be responsible for the salivary glycoprotein-mediated aggregation of the oral bacterium Streptococcus sanguis. Arch Oral Biol. 1981;26(11):945–949. doi: 10.1016/0003-9969(81)90156-4. [DOI] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Aggregation of oral streptococci with Fusobacterium and Actinomyces. J Biol Buccale. 1974 Dec;2(4):347–362. [PubMed] [Google Scholar]
- Kelstrup J., Theilade J., Fejerskov O. Surface ultrastructure of some oral bacteria. Scand J Dent Res. 1979 Dec;87(6):415–423. doi: 10.1111/j.1600-0722.1979.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Inouye Y., Holdeman L. V. New Actinomyces and Streptococcus coaggregation groups among human oral isolates from the same site. Infect Immun. 1983 Aug;41(2):501–506. doi: 10.1128/iai.41.2.501-506.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. Isolation and characterization of coaggregation-defective mutants of Actinomyces viscosus, Actinomyces naeslundii, and Streptococcus sanguis. Infect Immun. 1982 Sep;37(3):1200–1208. doi: 10.1128/iai.37.3.1200-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C., Listgarten M., Rosan B. Serology of Streptococcus sanguis: localization of antigens with unlabeled antisera. Infect Immun. 1973 Sep;8(3):475–481. doi: 10.1128/iai.8.3.475-481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancy P., Jr, Appelbaum B., Holt S. C., Rosan B. Quantitative in vitro assay for "corncob" formation. Infect Immun. 1980 Aug;29(2):663–670. doi: 10.1128/iai.29.2.663-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancy P., Jr, Dirienzo J. M., Appelbaum B., Rosan B., Holt S. C. Corncob formation between Fusobacterium nucleatum and Streptococcus sanguis. Infect Immun. 1983 Apr;40(1):303–309. doi: 10.1128/iai.40.1.303-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G. Isolation of a protein-containing cell surface component from Streptococcus sanguis which affects its adherence to saliva-coated hydroxyapatite. Infect Immun. 1981 Nov;34(2):428–434. doi: 10.1128/iai.34.2.428-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Reynolds H. S., Genco R. J. Characterization of tufted streptococci isolated from the "corn cob" configuration of human dental plaque. Infect Immun. 1980 Jan;27(1):235–245. doi: 10.1128/iai.27.1.235-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- Robinson P. J., Shapiro I. M. Effect of diphosphonates on root resorption. J Dent Res. 1976 Jan-Feb;55(1):166–166. doi: 10.1177/00220345760550011201. [DOI] [PubMed] [Google Scholar]
- Rosan B., Argenbright L. Antigenic determinant of the Lancefield group H antigen of Streptococcus sanguis. Infect Immun. 1982 Dec;38(3):925–931. doi: 10.1128/iai.38.3.925-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello A. D., Propas D., Oram V., van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Characterization of the adherence properties of Streptococcus salivarius. Infect Immun. 1980 Aug;29(2):459–468. doi: 10.1128/iai.29.2.459-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. D. Examination of the test for compressive strength applied to zinc oxide eugenol cements. J Dent Res. 1976 Jan-Feb;55(1):142–147. doi: 10.1177/00220345760550010701. [DOI] [PubMed] [Google Scholar]