Summary
Background
Highly elongated eukaryotic cells (e.g., neuronal axons, fungal hyphae, and pollen tubes) are generated through continuous apically restricted growth (tip growth), which universally requires tip-localized Rho GTPases. We used the oscillating pollen tube as a model system to determine the function and regulation of Rho GTPases in tip growth. Our previous work showed that the spatiotemporal dynamics of the apical cap of the activated ROP1 Rho GTPase is critical for tip growth in pollen tubes. However, the underlying mechanism for the generation and maintenance of this dynamic apical cap is poorly understood.
Results
A screen for mutations that enhance ROP1 overexpression-induced depolarization of pollen tube growth identified REN1 (ROP1 enhancer 1) in Arabidopsis, whose null mutations turn elongated pollen tubes into bulbous cells. REN1 encodes a novel Rho GTPase-activating protein (RhoGAP) required for restricting the ROP1 activity to the pollen tube tip. REN1 was localized to exocytic vesicles accumulated in the pollen tube apex as well as to the apical plasma membrane at the site of ROP1 activation. The apical localization of REN1 and its function in controlling growth polarity was compromised by disrupting ROP1-dependent F-actin and vesicular trafficking, which indicates that REN1 targeting and function is regulated by ROP1 downstream signaling.
Conclusions
Our findings suggest that the REN1 RhoGAP controls a negative feedback-based global inhibition of ROP1. This function provides a critical self-organizing mechanism, by which ROP signaling is spatially limited to the growth site and temporally oscillates during continuous tip growth. Similar spatiotemporal control of Rho GTPase signaling may also play an important role in cell polarity control in other systems, including tip growth in fungi and cell movement in animals.
Introduction
Tip growth, an extreme form of polar growth, is essential for the development and morphogenesis of many eukaryotic cells, such as animal neuronal axons, fungal hyphae, plant root hairs, and pollen tubes [1–3]. To form these elongated cells, the site of growth needs to be confined to the extreme apical area and to be continuously and rapidly regenerated over a long distance. The molecular mechanism underlying the confinement and dynamic maintenance of the tip growth site in these cells remains poorly understood.
Pollen tubes navigate through several female tissues by long-distance tip growth at an astonishing rate (< 1 cm/hr) and in an oscillatory manner [4–6]. This type of tip growth is manifested in in vitro-cultured pollen tubes, which are amenable to genetic manipulation and cell biological analyses at high spatiotemporal resolution [7–10]. Thus, the pollen tube is a favorite model system for the study of rapid tip growth and spatiotemporal control of polar growth. Because spatiotemporal dynamics of tip-localized Rho GTPase signaling is required for the pollen tube tip growth [8–11] and because Rho control of polar growth is conserved [1–5], findings of the Rho-based mechanism of pollen tube tip growth may provide important insights into polarized cell growth and long-distance tip growth in general.
Rho-family GTPases control a wide range of cellular processes such as cell polarity development, polar growth, cell morphogenesis, cell migration, and cell division [6, 12]. A common feature of Rho signaling in these processes is its localization to a specific domain of a cellular membrane system, commonly the plasma membrane (PM). Local activation of upstream activators in response to spatial cues and positive feedback regulation have been implicated in the asymmetry of Rho activation at the PM [13, 14], but the detailed mechanism underlying the dynamic spatial restriction of Rho activation remains poorly understood. Rho GTPase-activating proteins (RhoGAPs), which inactivate Rho by promoting the GTP hydrolysis, are critical for development and differentiation [15–17]. Emerging evidence indicates an essential role for RhoGAPs in the spatial restriction of Rho activation [16–18]. However, the mechanisms by which RhoGAPs spatially regulate Rho activity are not well understood. In this report, we reveal a new mechanism for RhoGAP-dependent spatial regulation of Rho GTPase using the pollen tube as a model system.
In Arabidopsis, ROP1, a pollen-specific member of the plant sole Rho GTPases (ROPs), is a central regulator of pollen tube tip growth [5, 19, 20]. Active ROP1 is localized to the apical PM as an apical cap, which defines the expanding region of the PM and drives tip growth by activating alternating increases in F-actin and Ca2+ levels [9, 11] (J-U Hwang and Z Yang, in revision). The ROP1-dependent F-actin dynamics control the apical targeting and exocytosis of vesicles required for tip growth [8]. Interestingly, ROP1-dependent F-actin and Ca2+ changes are also important for the generation and dynamics of the ROP1 apical cap [9]. ROP1 activation is initiated locally at the tip and propagated laterally, which is mediated by F-actin assembly at the tip through ROP1 effector RIC4 [9, 11]. In contrast, an increase in [Ca2+]cyt, resulting from the ROP1 activation of a second effector RIC3, is required for down-regulating the ROP1 apical cap by inducing F-actin disassembly [9, 11]. These results imply that the maintenance of dynamic tip-localized ROP1 activity is controlled by feedback regulations that depend on actin dynamics, but how these dynamics regulate those of the apical ROP1 activity remains mysterious.
In this study, we identified a novel RhoGAP, REN1 (ROP1 ENHANCER 1), which localizes to the apical cap and exocytic vesicles in the pollen tube tip. REN1 acts as a global inhibitor to restrict active ROP1 to the apical cap and to maintain the dynamics of the apical ROP1 cap. This function of REN1 is linked to F-actin-dependent feedback regulation of the apical ROP1 activity.
Results
ROP enhancer 1 (ren1) mutation causes depolarization of pollen tube growth
To identify new components in ROP1 signaling, we performed a sensitized mutant screen using a homozygous Arabidopsis line (12d.8), which expresses GFP-ROP1 under the control of pollen-specific LAT52 promoter [5, 21]. Cultured 12d.8 tubes displayed mild growth depolarization (i.e., shorter and wider tubes with slight tip swelling) (Figure 1 and Figure S1 available online). The 12d.8 line was mutagenized by T-DNA insertions [22] for suppressor or enhancer screens (Figure 1A). From ~3,800 T1 lines screened, we isolated one enhancer mutant, ren1 (ROP enhancer 1). Pollen grains from ren1 plants developed balloon-like tubes (Figure 1B) similar to those caused by the expression of constitutively active ROP1 mutant (CA-rop1) [19]. Pollen of ren1 was sterile, as demonstrated by reciprocally backcrossing ren1 to 12d.8 (Table S1 available online). Consequently, we failed to obtain a ren1 homozygote in T2 progenies.
Figure 1. Loss-of-function mutation in REN1 causes severe male gametophyte defect.
A) A schematic of the strategy for ROP1 enhancer and suppressor mutant screen. A homozygote Arabidopsis line of LAT52::GFP-ROP1 was subjected to T-DNA insertion mutagenesis. From basta-resistant T1 plants, we expected to identify ROP1 “enhancer” and “suppressor” mutants as shown. Some putative “suppressor” mutants might be false positive because of co-suppression between the endogenous ROP1 gene and the GFP-ROP1, which could be distinguished by reduction or loss of GFP fluorescence. Grey color indicates GFP-ROP1 expression.
B) Representative in vitro-germinated pollen tubes from ren1-1/+ (ren1-1) plants and two alleles (ren1-2 and ren1-3) compared with those of wild type (WT), 12d.8, and ren1 (ren1-1 LAT52::GFP-ROP1). A typical pollen tube from each mutant line is highlighted in yellow. Pollen grain (g) and pollen tube tip (t) are indicated. Bar=50 μm. The ren1-3 pollen tubes were cultured in a germination medium with 0.5 mM [CaCl2 + Ca(NO3)2], and others were cultured on standard germination medium for 6–8 hr.
C) Pollen grains from isolated dehisced WT, ren1-1, and 12d.8 anthers. Pollen grains were examined immediately following release from anthers onto the germination medium. A significant portion of pollen grains from ren1-1 anthers were found to have already developed swollen tubes (indicated by arrowheads). Bar = 50 μm.
D) T-DNA insertion loci of three alleles of ren1. Solid and grey blocks indicate exons and introns of REN1 (At4g24580), respectively. In ren1-1, two T-DNAs are inserted in the second intron in a tandem repeat. Arrows indicate the gene-specific primers used for RT-PCR analysis.
E) Suppression of ren1-1 by expression of full-length REN1 CDS. Comparison of in vitro pollen tube phenotype of WT, ren1-1/+ and ren1-1 LAT52::REN1 (ren1-1/ren1-1 LAT52::REN1/LAT52::REN1). Pollen was germinated for 8 hr on standard germination medium, and typical ren1-1 pollen tubes are indicated by arrows. Bar = 50 μm.
By backcrossing ren1 to the wild type (WT), from F2 progeny we isolated ren1-1, which lacks LAT52::GFP-ROP1. In ren1-1 plants (ren1-1/+), ~50% of pollen grains developed balloon-like tubes (Figure 1B). Compared to ballooning of ren1-1 LAT52::GFP-ROP1 tubes, that of ren1-1 tubes was delayed and less severe -- smaller balloons with a longer stalk at the base (Figure 1B and Figure S1B). Pollen grains of ren1-1 appeared morphologically normal but germinated precociously (Figure 1C). Within 1 hr, ~40% (73/187) of pollen from ren1-1/+ plants germinated and most produced swollen tubes, but only 3% (4/134) of WT pollen germinated. Surprisingly, ~15% (178/1223) of pollen from dehisced ren1-1/+ anthers found to have swollen tubes, not found in WT or 12d.8 pollen (Figure 1C). Swollen ren1-1 pollen tubes failed to adhere to and/or penetrate the stigma (Figure S1C), which accounts for the complete sterility of ren1-1 pollen (Table S2).
REN1 encodes a novel RhoGAP
The isolated genomic DNA fragment flanking the left border of the T-DNA revealed that the T-DNA was inserted in the second intron of At4g24580, a RhoGAP-like gene (Figure 1D). In addition, two SALK T-DNA insertion mutants for At4g24580 produced defect similar to that of ren1-1 (Figure 1B). SALK_014612 (ren1-2) had a T-DNA inserted in the 8th exon, which encodes part of the RhoGAP domain, and had a phenotype identical to that of ren1-1 (Figure 1B and Table S2). SALK_111625 (ren1-3) had a T-DNA inserted in the 19th exon, probably causing truncation at the C-terminus (677-920aa) (Figure S2). Pollen grains of ren1-3 developed moderately swollen tubes in vitro but appeared fertile (data not shown). Homozygote ren1-3 plants showed a reduced At4g24580 transcript level (data not shown). The mild defect of ren1-3 pollen may result from the C-terminal alteration and/or reduced transcript level. Finally, ren1-1 was complemented by the At4g24580 full-length CDS driven by the LAT52 promoter (Table S3, Figure 1E). The in vitro pollen tube growth of homozygous ren1-1 LAT52::At4g24580 plants was similar to that of WT tubes (N=2 lines, Figure 1E). Thus, a loss-of-function mutation in At4g24580 is responsible for the ren1 phenotype.
Microarray data suggest that REN1 is selectively expressed in mature pollen (https://www.genevestigator.ethz.ch/). Both RT-PCR analysis of REN1 and REN1 promoter::GUS expression confirmed the REN1 expression in mature pollen grains and pollen tubes (Figure S3). REN1 expression was high in WT and 12d.8 pollen but significantly reduced in pollen from heterozygote ren1 (ren1-1/+ LAT52::GFP-ROP1).
Sequencing the full-length REN1 cDNA revealed a 2,763-base-pair coding sequence, 54-bp longer than what is annotated in the TAIR database (http://www.arabidopsis.org/), which predicted the 14th intron splicing inaccurately. The REN1 ORF contains 23 exons and 22 introns (Figure 1D). The predicted REN1 protein contains an N-terminal pleckstrin homology (PH) domain and a central RhoGAP domain and two C-terminal coiled-coil (CC) domains (Figure S2). The RhoGAP domain is a conserved RhoGAP catalytic domain, which binds to Rho GTPase and promotes its GTP hydrolysis. The RhoGAP domain of REN1 shares an overall ~54% similarity with that of p190-RhoGAP and ~46% similarity with that of Arabidopsis RopGAP1 (Figure S2B). Thus REN1 is structurally distinct from the known RopGAP family members, which contain an N-terminal CRIB motif [23]. REN1 has two Arabidopsis homologs (At5g12150 and At5g19390) and homologs in rice, gape vine and Physcomitrella (a moss), which all share the overall REN1 structure (Figure S2 and data not shown). REN1 and its homologs may constitute a new plant RhoGAP family.
REN1 down-regulates ROP1 GTPase signaling in pollen tubes
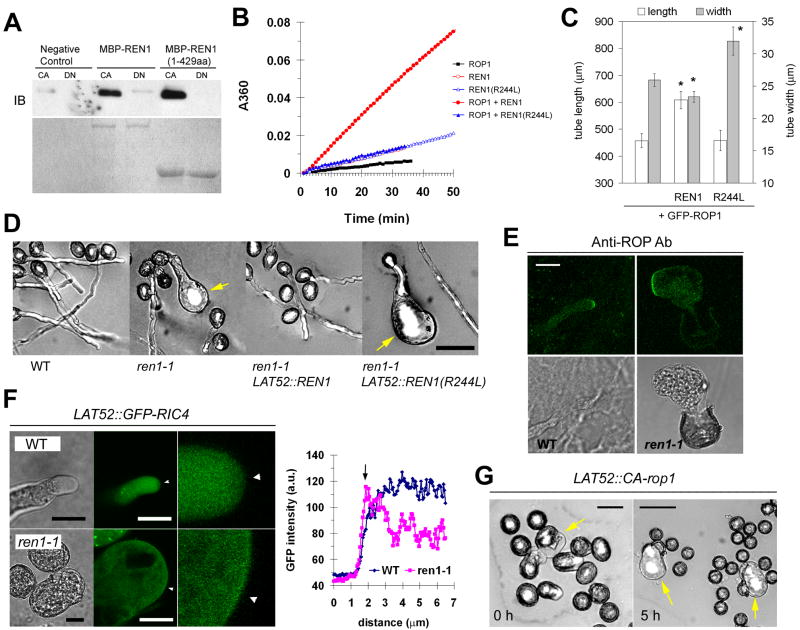
On the basis of the ren1 phenotype and the REN1 structure, we hypothesized that REN1 regulates the polarity of pollen tube growth by suppressing ROP1 activation to restrict ROP1 activity to the tube apex. To test this hypothesis, we first investigated whether REN1 activates ROP1 GTPase activity. As shown in Figure 2A, maltose-binding protein fused with REN1 (MBP-REN1) interacted preferentially with the GTP-bound active form of ROP1 (CA-rop1). The N-terminal part of REN1 [MBP-REN1 (1-429aa)] containing the RhoGAP domain bound GTP-loaded CA-rop1 more selectively, which is characteristic of a RhoGAP. The intrinsic GTPase activity of GST-ROP1 was greatly increased by His-tagged REN1 (His-REN1) (Figure 2B). A non-catalytic REN1 mutant [REN1(R244L)], with the catalytic Arg substituted by Leu, retained the full capacity to bind GST-ROP1 (data not shown) but failed to promote the GTP hydrolysis (Figure 2B). These results indicate that REN1 is a novel RhoGAP.
Figure 2. REN1 is a RhoGAP for ROP1.
A) REN1 interacts with GTP-bound ROP1. GST-CA-rop1 (CA) or GST-DN-rop1 (DN) were loaded with GTP or GDP, respectively, and incubated with MBP-REN1 or MBP-REN1 (1-429aa). CA- or DN-rop1-bound REN1 or REN1 deletion mutant were pulled down with amylose-conjugated agarose beads and quantified by western blotting. Upper panel shows western blotting reacted with anti-ROP antibody (IB). Lower panel is Coommassie blue-stained gel image for loading control.
B) REN1 promotes the GTP hydrolysis of ROP1. In vitro GTPase activity of ROP1 was measured as described in Wu et al (2000). The intrinsic GTPase activity of GST-ROP1 was minimal. To test GAP activity of REN1, His-REN1 or His-REN1(R244L) were added to GST-ROP1 as described in Experimental Procedures. His-REN1 or His-REN1(R244L) themselves do not induce GTP hydrolysis when tested in the absence of GST-ROP1. GST-ROP1 tends to show multiple turnover reactions rather than typical single turnover reaction, as observed on in vitro GAP activity assay for RopGAP1 [23]. In our assay condition, GDP-GTP exchange on ROP1 may be unusually fast and spontaneous.
C) Partial suppression of GFP-ROP1 overexpression-induced depolarized pollen tube growth by REN1 overexpression. When co-overexpressed with GFP-ROP1 in tobacco pollen tubes, REN1 partially suppressed the ROP1 overexpression-induced pollen tube phenotype, whereas REN1(R244L) slightly enhanced it. More than 20 tubes with significant GFP fluorescence were measured. The changes in GFP-ROP1 overexpression phenotype were not due to altered GFP-ROP1 expression levels, because similar GFP fluorescence was seen in tubes expressing REN1 and REN1(R244L). Asterisks indicate significant difference from control tubes expressing GFP-ROP1 alone (P<0.05). Representative result of three independent experiments is presented.
D) The GAP-dead mutant REN1(R244L) failed to complement ren1-1. Introduction of the LAT52::REN1(R244L) construct into ren1-1 plants did not increase basta-resistance segregation ratio of T2 ren1-1 plants (Table S3), nor did it restore ren1 pollen tubes to normality (N = 4 independent lines). Representative pollen tube images from wild type (WT), ren1-1 (ren1-1/+), ren1-1 LAT52::REN1 (ren1-1/ren1-1 LAT52::REN1/LAT52::REN) and ren1-1 LAT52::REN1(R244L)[ren1-1/+ LAT52::REN1(R244L)/LAT52::REN1(R244L)]. Pollen tubes were observed 5 h after placing pollens on to the standard germination medium. Bar, 50 μm.
E) ROP localization in ren1-1 pollen tubes compared with wild-type tubes (WT). ROP1 is delocalized to a broader region of PM in the ren1-1 pollen tube. Pollen tubes were chemically fixed and immunostained with anti-ROP antibody. Bar, 10 μm.
F) REN1 controls the tip-localized ROP1 activity in Arabidopsis pollen tubes.
To visualize the in vivo ROP1 activity, we transformed Arabidopsis plants with LAT52::GFP-RIC4. RIC4 specifically binds with PM-localized active form of ROP1, which reflects the PM-localized ROP1 activity [11]. In pollen tubes of the WT background, GFP-RIC4 mainly localizes in the cytosol, forming some aggregates. In contrast, GFP-RIC4 apparently localizes in a broad area of PM in ren1-1 tubes. Bar = 10 μm. The rightmost images are zoomed to the PM area indicated with arrowheads. Inset: line-scans of GFP-RIC4 intensity profiles across the PM area indicated with arrowheads. The position of PM is indicated by an arrow. a.u., arbitrary unit
G) Similar to ren1-1, pollen grains expressing CA-rop1 germinated prematurely and developed severely swollen tubes. Pollen right after being released from the isolated anthers (0 h) and after 5 hr of incubation on the germination medium (5 h). Bar, 25 μm (0 h) and 50 μm (5 h), respectively.
We next investigated in vivo ROP1 regulation by REN1 with several experiments. First, REN1 coexpression suppressed the GFP-ROP1 overexpression-induced growth depolarization in tobacco pollen tubes (Figure 2C). In contrast, REN1(R244L) had an opposite effect, probably because of its dominant-negative action. Second, REN1(R244L) failed to functionally complement ren1-1 (Figure 2D and Table S3), which suggests that REN1 functions by down-regulating ROP1 activity.
Third, we investigated whether ROP1 signaling was enhanced and/or depolarized in ren1-1 by examining the localization of ROP1 and its effector protein. As compared with WT tubes, in ren1-1 tubes, ROP proteins were localized at the broader PM region (Figure 2E). RIC4 is an active ROP1 effector, and GFP-RIC4 localization to the PM reports ROP1 activity in tobacco pollen tubes [9, 11]. GFP-RIC4 localization to the PM was not clear in Arabidopsis pollen tubes, which may be due to its transient interaction with active ROP (Figure 2F). However, GFP-RIC4 was stably associated with a broad PM region in ren1-1 tubes (Figure 2F), which indicates the depolarized accumulation of excess active ROP1 in the PM.
Finally, we tested whether CA-rop1 expression mimics ren1-1. If ROP1 downregulation by REN1 is critical to prevent premature pollen germination and depolarized tube growth, the expression of GAP-insensitive CA-rop1 would mimic ren1-1. As previously reported [19], LAT52::CA-rop1 tubes were severely depolarized, as were ren1 tubes (Figure 1). Furthermore, a substantial portion of LAT52::CA-rop1 pollen grains developed swollen tubes even before their release from the anther (Figure 2G). Hence, REN1 downregulates ROP1 activity to restrict ROP1 activation at a proper time and site, thereby controlling pollen germination timing and pollen tube growth polarity.
REN1 is localized to the apical PM and exocytic vesicles
The apical PM restricted ROP1 signaling could be achieved by either lateral or global suppression of ROP1 activation. A lateral inhibitor of ROP1 is expected to be localized and active in the subapical PM region where active ROP1 is excluded. In contrast, a global inhibitor is expected to display an apical or uniform localization. The subcellular localization of REN1 was first determined by immunostaining with a REN1-specific antibody (Figure 3A). In WT tubes, REN1 was localized in the apical region in an inverted cone pattern, particularly enriched in close proximity to the apical PM, where endogenous ROP is localized and activated (Figures 3A and 2E). This localization pattern is reminiscent of the distribution of exocytic vesicles in an inverted cone pattern in the clear zone [8]. The anti-REN1 antibody gave only faint background staining in ren1-1 pollen tubes.
Figure 3. REN1 is localized to exocytic vesicles in the pollen tube apex.
A) REN1 is localized to the apex of pollen tubes. The localization of endogenous REN1 was observed by immunolocalization with specific anti-REN1 antibody, which was raised against the C-terminal region (530-920 aa) of REN1 and affinity purified. Representative images of ren1-1 (left) and wild-type (right) pollen tubes are presented.
B) GFP-REN1 shows similar localization in Arabidopsis pollen tubes. REN1 is localized in or close to the growth region in the apical PM, where active ROP1 is localized. Bar=5 μm. The intensity profiles of GFP-REN1 and immunolabeled endogenous ROP following the longitudinal axis (an arrow in the overlay image) are presented in the right. The position of PM is indicated by an arrow.
C-D) REN1 localization in the clear zone overlapped with that of FM4-64-labeled recycling exocytic vesicles (C) and YFP-RabA4D-labeled exocytic vesicles (D). FM4-64-contained medium was applied to GFP-REN1 pollen tubes 1 hr before observation. YFP-RabA4D pollen tubes were fixed and immunostained with anti-REN1 Ab and Alexa594-conjugated secondary Ab. Bars, 5 μm.
E) GFP-REN1 targeting to the tube apex was affected in raba4d pollen tubes. GFP-REN1 still localized to the cytosol near the tube apex but not in an inverted cone shape (arrow), which indicates that GFP-REN1-associated vesicle targeting is affected by raba4d mutation. However, GFP-REN1 association to the vegetative nuclear envelope was not altered (asterisk). Pollen of raba4d LAT52::GFP-REN1 was cultured in the germination medium with 0.001% borate, in which raba4d-induced growth polarity defect was more pronounced as compared with in the standard germination medium.
We also analyzed GFP-REN1 localization in Arabidopsis pollen. LAT52::GFP-REN1 largely, but not completely, complemented ren1-1, which indicates that GFP-REN1 is functional but not as fully functional as is native REN1 (Table S3). Consistent with the immunostaining results, GFP-REN1 accumulated in the pollen tube tip, with enrichment in and near the apical PM (Figures 3B and S4A). In addition, GFP-REN1 localization overlapped with endogenous ROP in the apical PM where the two proteins are expected to interact (Figures 3B and S4A). In growing tubes, GFP-REN1 appeared to be recruited to the growing region (Figure S4B). In un-germinated pollen grains, GFP-REN1 was evenly localized in the cortical region of the cytosol and was sometimes found in a ring structure. In pollen grains with an emerging tube, GFP-REN1 was enriched toward the initiation site (Figure S4C).
We tested GFP-REN1 association with exocytic vesicles using different endomembrane markers. A lipophilic styryl dye FM4-64 stains recycling secretory vesicles in pollen tube tips in an inverted cone pattern [9, 24]. FM4-64 was applied to LAT52::GFP-REN1 tubes for 1 hr. FM4-64 signal overlapped with that of GFP-REN1 in growing tube tips (Figure 3C). REN1 also showed overlapping localization with RabA4D (Figure 3D), a pollen-specific Arabidopsis homolog of animal Rab11 known to localize to post-Golgi compartments, including exocytic vesicles in pollen tube tips, and control targeting of exocytic vesicles to the tip [8] (Szumlanski et al., manuscript submitted). In contrast, markers of the trans-Golgi network (SYP41-Venus) and endosomes (YFP-ARA7) were excluded from the apex when transiently expressed in tobacco pollen tubes (Figure S4D).
We next determined the effect of brefeldin A (BFA) on the distribution of FM4-64 signal and GFP-REN1. BFA inhibits the formation of exocytic vesicles but does not block the PM internalization through endocytosis in pollen tubes [24, 25]. If GFP-REN1 were associated with endocytic compartments, it would be integrated into aggregates of endomembranes together with internalized FM4-64 in the presence of BFA. With 1.5 hr of BFA treatment (0.1~2.5 ug/ml), internalized FM4-64 signal was found in aggregates in the tip cytoplasm (Figure S5). In most BFA-treated pollen tubes, GFP-REN1 lost the tip accumulation but was not incorporated in the FM4-64 aggregates (Figure S5). Some pollen tubes treated with 0.1 μg/ml BFA retained the tip accumulation of GFP-REN1. In these cells, internalized FM4-64 aggregates did not colocalize with GFP-REN1 either. These results clearly indicate that REN1 is not localized to endocytic compartments.
Finally, GFP-REN1 localization was altered in pollen tubes of a raba4d knockout line with defective tip-focused targeting of exocytic vesicles, thus resulting in growth depolarization (bulged pollen tubes) (Szumlanski et al., manuscript submitted). In raba4d tubes, GFP-REN1 was no longer concentrated in the tip in an inverted cone shape but appeared as a thin layer at the cell cortex (Figure 3E). Taken together, these results strongly suggest REN1’s association with exocytic vesicles at the tip of pollen tubes.
Association of REN1 with exocytic vesicles is required for REN1’s function in restricting ROP1 signaling
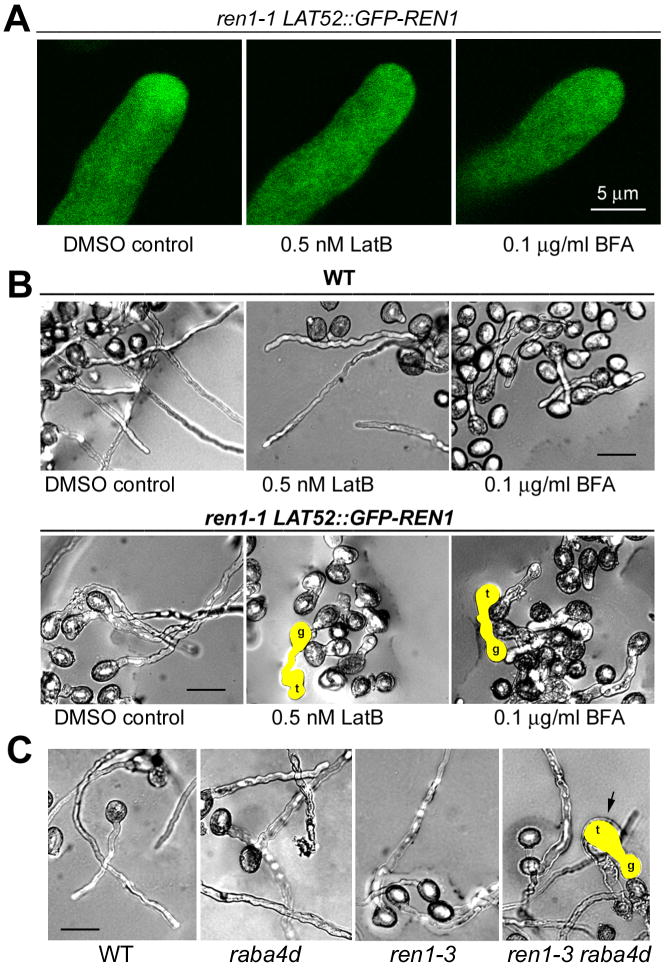
We next sought to understand the significance of REN1 association with exocytic vesicles. First, we determined whether chemical disruption of exocytic vesicle accumulation to the tip affected the REN1 function. Actin-disrupting drugs and BFA are known to disrupt the tip accumulation of exocytic vesicles [8, 25]. Latrunculin B (LatB) 0.5 nM weakly compromised the dynamic tip-localized F-actin but had no effect on the actin cables in pollen tubes [10]. It partially inhibited elongation but had no effect on the growth polarity of WT tubes (Figure 4). We similarly treated pollen tubes from a homozygous ren1-1 LAT52::GFP-REN1 line, in which LAT52::GFP-REN1 largely but not fully complemented the loss of native REN1 (Table S3). If F-actin-dependent targeting of exocytic vesicles is required for the function of REN1, a modest disruption of vesicle targeting by 0.5 nM LatB would severely compromise the REN1 function in the ren1-1 LAT52::GFP-REN1 tubes. This is analogous to synthetic lethality or the sensitized genetic screen aimed to identify functionally redundant components or pathways. Treating ren1-1 LAT52::GFP-REN1 tubes with 0.5 nM LatB for 20–30 min caused GFP-REN1 localization to change from the inverted cone pattern to a uniform distribution (10 of 12 tubes observed) (Figure 4A); tubes became short and swollen or kinky, a phenotype not seen in untreated control tubes or WT tubes similarly treated (Figure 4B). Similarly, treatment with BFA (0.1 ug/ml) caused ren1-1 LAT52::GFP-REN1 tubes to lose the apical localization of GFP-REN1 (9 of 11 tubes observed) (Figure 4A) and to become short and swollen (Figure 4B). In contrast, WT tubes so treated exhibited only some growth retardation. These results strongly support tip-directed targeting and exocytosis of REN1-containing vesicles being crucial for REN1’s function in limiting ROP1 activity to the tip.
Figure 4. REN1 targeting to the pollen tube apex is mediated by dynamic F-actin and vesicular trafficking.
A) GFP-REN1 accumulation to the clear zone was disrupted with short-term treatments of 0.5 nM LatB or 0.1 μg/ml BFA. GFP-REN1-expressing pollen tubes (ren1-1 LAT52::GFP-REN1) were incubated with LatB or BFA-containing medium 20–30 min before observation. Bar, 5 μm.
B) Both LatB and BFA induced severe growth defect in ren1-1 LAT52::GFP-REN1 pollen tubes when added to the pollen germination medium. Representative pollen tubes are highlighted. However, LatB and BFA at the same concentration did not significantly affect wild-type (WT) pollen tube growth except that growth rate was reduced. Pollen grain (g) and pollen tube tip (t) are indicated. Bars, 50 μm.
C) ren1-3 and raba4d synergistically affected pollen tube growth. Pollen from ren1-3/+ raba4d/+ plants was cultured on the standard germination medium, on which pollen tubes from single ren1-3 or raba4d mutant did not display tip swelling. Six hours after incubation, the widest diameter for WT, ren1-3 and raba4d tubes was 7.9 ± 0.2 μm (mean ± s.e., N=91), 10.6 ± 0.2 μm (N=162) and 10.4 ± 0.2 μm (N=39), respectively. However, approximately 20% of pollen tubes from ren1-3/+ raba4d/+ flowers developed balloon-like tips (highlighted), with diameter ≥20 um (n=84/439). Bar, 50 μm.
Second, we tested the functional interaction between REN1 and RabA4D using a ren1-3 raba4d double mutant. REN1 function appeared to be moderately compromised in the ren1-3 mutant (Figure 1). However, several Rab11 homologs are expressed in pollen and may be functionally redundant to RabA4D. If RabA4D functionally interacts with REN1, a vesicle targeting defect in raba4d would have a synergistic effect with ren1-3 on REN1 function. In standard germination medium, ren1-3 or raba4d tubes displayed a nearly normal shape, but tubes from the ren1-3/+ raba4d/+ double mutant developed balloon-like tips (Figure 4C), which demonstrates that a mild REN1 defect in ren1-3 was synergistically enhanced by moderately compromised targeting of exocytic vesicles in raba4d.
Finally, we determined the structural requirement for REN1 localization to exocytic vesicles. The C-terminal part of REN1 containing CC regions shows some homology with motifs found in vesicle trafficking components such as BinAmphiphysinRvs (BAR) and syntaxin-N-terminal domains. The REN1ΔC mutant lacking the CC regions, [REN1 (1-429aa)], still selectively interacted with active ROP1 and promoted its GTPase activity in vitro (Figure 2A and Figure S6A), which suggests that the CC regions are not essential for the RhoGAP activity. However, YFP-REN1ΔC did not show the apical localization in pollen tubes (Figure S6B), and LAT52::REN1ΔC could neither restore the male fertility defect nor suppress the pollen tube phenotype in ren1-1 (Table S3 and data not shown). Thus, localization of REN1 to exocytic vesicles might depend on the C-terminal part containing CC domains, which is essential for REN1 control of ROP1 activity in pollen tubes.
The apical localization of REN1 oscillates ahead of tip growth
Since ROP1 signaling-dependent actin dynamics control the apical accumulation of exocytic vesicles and their tip-targeted exocytosis [8, 10, 11], the above results (Figure 4) strongly support REN1 regulation of ROP1 being feedback-regulated by ROP1 signaling. This model predicts that REN1 is targeted to the tip after ROP1 activation and participates in ROP1 inactivation in oscillating pollen tubes. GFP-REN1 accumulation to the tip was analyzed from the time-series CLSM images of Arabidopsis LAT52::GFP-REN1 tubes. From GFP-REN1 intensity profiles along the tube longitudinal axis (Figure 5A), we obtained the mean GFP intensity values within 2 μm from the tip margin (I-tip). I-tip oscillated slightly ahead of tip growth (Figure 5B and C), with peaks ahead of growth peaks by ~30°, i.e., by 2.9 ± 2.3 sec (mean ± s.e.) in periods of 34.4 ± 2.4 sec (N=4). In tobacco pollen tubes, ROP activity peaks are about 90° ahead of those of tip growth, whereas the lowest ROP activity is 90° behind the tip growth peak [9]. Assuming a similar phasal relationship between ROP1 activity and tip growth in Arabidopsis, these results suggest that the apical localization of REN1 oscillates behind ROP1 activation and ahead of ROP1 deactivation.
Figure 5. REN1-vesicle targeting to the tip oscillates ahead of tip growth.
A-C) The tip localization of GFP-REN1 oscillates with growth rate in growing Arabidopsis pollen tubes. GFP-REN1 localization was observed by time-lapse imaging of Arabidopsis pollen tubes (ren1-1 LAT52::GFP-REN1). The GFP intensity of a 2-μm wide region was line scanned along the longitudinal axis of an Arabidopsis pollen tube (A). From the resulting intensity profiles, the mean GFP intensity of the area within 2 μm from the cell margin was calculated (shaded area in the intensity profile). The net tip growth was calculated by net advance of the cell margin between two consecutive images. Cell margin was determined where GFP intensity turns above the background signal. Representative GFP-REN1 signal oscillation in the tip is presented with an oscillation curve of tip growth (B) and in false colored sequential images (C, red, high fluorescence and blue, low fluorescence). Numbers at the bottom (C) are the elapsed time (seconds). Bar, 5 μm.
D) A model for REN1 regulation of ROP1 activity in the pollen tube tip. Left, Localized ROP1 activation (red circles) in the tube apical PM laterally amplifies, probably via a positive feedback mechanism (1–2). Active ROP1 induces the assembly of F-actin (indicated by lines) and promotes accumulation of REN1-associated exocytotic vesicles in the tube apex (2). Once vesicles fuse to the PM, REN1 is targeted to the PM and inactivates PM-localized active ROP1 (3). This activity prevents excess ROP1 activation and depolarization of tip growth. After ROP1 inactivation, REN1 returns to the cytosol, thus allowing ROP1 activity to increase again (4). The REN1-mediated negative feedback mechanism is activated behind the apical ROP activation by ~60° and prevents excess ROP activation in the apical PM (Right).
To confirm the phasal relationship between the apical REN1 localization and ROP activity, we used tobacco pollen tubes because ROP1 activity oscillation is well characterized [9]. With GFP-REN1 overexpressed in tobacco pollen tubes, its accumulation to the tip was barely detectable (Figure S7). In these tubes, REN1 binding sites may be saturated with the native REN1 homolog, thus causing excessive cytosolic accumulation of GFP-REN1, which would mask its tip accumulation. Indeed, co-expression with a small amount of ROP1 clearly enhanced GFP-REN1 localization to the apical PM (Figure S7). In these tubes, mean GFP-REN1 intensity values at the apical PM (I-tip-pm) were compared with tip growth (Figure S7 D-E). Peaks of I-tip-pm were ahead of growth peaks by ~25° (2.5 ± 3.7 sec in periods of 36 ± 3.5 sec), similar to the observation in Arabidopsis pollen tubes. Therefore, the peaks of REN1 targeting to the apical PM are behind those of the tip-localized ROP1 activity by about 60° (Figure 5D), which further supports a role for REN1 in the negative feedback regulation of ROP1 signaling.
Discussion
In cell systems with rapid prolonged tip growth [26, 27], the restriction of Rho GTPase signaling to the growth site by negative regulators appears to be a common mechanism for the maintenance of the growth polarity. For example, loss of RhoGAPs or expression of constitutively active Cdc42 GTPase induces growth depolarization in filamentous fungi [27, 28]. One mechanism for RhoGAP-mediated spatial restriction of Rho signaling is by localizing RhoGAP to the region of the cell where the Rho GTPase activity is excluded [16, 29–32]. For instance, in the one-cell embryo of C. elegans, the CYK-4 RhoGAP localizes to the posterior cortex, creating a gradient of RhoA-mediated actomyosin to the anterior [16]. In pollen tubes, similar lateral inhibitor function was proposed for NtRhoGAP1, a tobacco RopGAP distinct from REN1 [23], on the basis of the subapical localization of overproduced NtRhoGAP1 [31]. Knocking out pollen-expressed RopGAPs, however, does not affect growth polarity in Arabidopsis pollen tubes [31] (J-U Hwang and Z Yang, in revision), which indicates that RopGAPs do not have a major role in limiting ROP signaling to the pollen tube tip.
Our results demonstrate a novel mechanism for RhoGAP action in spatially limiting Rho GTPase signaling, which we refer to as global inhibition. REN1 is localized to the apical PM and to tip-localized exocytic vesicles. In the apical PM where active ROP1 is present, REN1 globally inactivates ROP1 there. Tip-targeted fusion of exocytic vesicles, which contain REN1 and accumulate in an inverted cone pattern, allows REN1 to target to the apical PM. Since Rho GTPase regulates vesicular trafficking [8, 33], the global inhibition mode of action, which depends on vesicular trafficking-based delivery of RhoGAPs to the site of Rho activation, may facilitate feedback-based self-organization of Rho GTPase signaling medicated by RhoGAP at the cell apex.
We hypothesize that in vivo REN1 activity is tightly regulated by tip-localized ROP1 signaling (Figure 5). Consequently, REN1 is a key component of the self-organizing mechanism for the oscillatory tip growth. Our previous studies suggest that ROP1/RIC4-dependent F-actin assembly participates in positive feedback activation of ROP1, which contributes to lateral amplification of active ROP1 that is countered with a negative feedback regulation of ROP1 [9] (J-U Hwang and Z Yang, revision). Our current results suggest that active ROP1 signaling is also required for the REN1 function to initiate a negative feedback loop of ROP1 regulation. Active ROP1 drives the tip-targeted exocytosis of REN1-associated vesicles by inducing tip-localized F-actin assembly and cytosolic calcium increase [8, 11]. The apical F-actin-dependent accumulation of REN1-associated exocytic vesicles oscillates ahead of the cytosolic calcium increase that depends on ROP1 activation of RIC3 [8, 11] (Figure 5D). This initial calcium increase may induce actin disassembly [11] and vesicle fusion to the apical PM [8]. This polar exocytosis brings REN1 to the apical PM, where active ROP1 is localized.
The PM-localized REN1 may not be active until a further calcium increase to a threshold level, which may trigger REN1 activation on the PM. This scenario is consistent with previous observations that below a threshold level, calcium stimulates pollen tube tip growth, and above a threshold level, it inhibits growth, which is influenced by the ROP1/RIC3 signaling strength [11, 19]. Furthermore, the potential requirement for a threshold level of calcium in direct activation of REN1 could explain why F-actin-mediated vesicle targeting can participate in both positive (presumably by targeting positive ROP1 regulators) and negative (targeting REN1) feedback regulation of ROP1 signaling.
The REN1-dependent negative feedback loop limits the lateral amplification of active ROP1 and subsequently diminishes the apical ROP1 activity to the basal level. The ROP1 activity decrease, in turn, results in a decrease of REN1 activity, thus allowing ROP1 activity to increase again. Such alternating and interlinking positive and negative feedback regulations of ROP1 can explain how the active ROP1 apical cap is maintained at a proper size and how the tip-localized ROP1 activity oscillates.
In summary, we have demonstrated that by localizing to the pollen tube tip, REN1 RhoGAP globally inhibits the lateral propagation of ROP1 activity, restricting active ROP1 to the tip. The tip localization and function of REN1 require ROP1 signaling targets, including dynamic apical F-actin and tip-focused exocytosis, consequently generating a negative feedback loop of ROP1 regulation. Several observations suggest the requirement of negative regulation of Rho for restricting Rho signaling in the apical region in non-plant cell systems with tip growth or cell migration [28, 29, 34, 35]. It would be interesting to determine whether a negative feedback-mediated global inhibition of Rho GTPase by RhoGAPs is a common mechanism for cell polarity control in various eukaryotic systems.
Supplementary Material
Acknowledgments
We thank members of the Yang group for helpful discussion. This work is supported by funding from National Institute of Health (R01 GM081451-01), National Science Foundation (MCB-0111082), Department of Energy (DE-FG02-04ER15555) to ZY, and a Department of Energy Grant (DE-FG02-03ER15412) to EN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wendland J. Comparison of morphogenetic networks of filamentous fungi and yeast. Fungal Genet Biol. 2001;34:63–82. doi: 10.1006/fgbi.2001.1290. [DOI] [PubMed] [Google Scholar]
- 2.Cole RA, Fowler JE. Polarized growth: maintaining focus on the tip. Curr Opin Plant Biol. 2006;9:579–588. doi: 10.1016/j.pbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 4.Franklin-Tong VE. Signaling and the modulation of pollen tube growth. Plant Cell. 1999;11:727–738. doi: 10.1105/tpc.11.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Y, Vernoud V, Fu Y, Yang Z. ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J Exp Bot. 2003;54:93–101. doi: 10.1093/jxb/erg035. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z. Cell Polarity Signaling in Arabidopsis. Annu Rev Cell Dev Biol. 2008;24 doi: 10.1146/annurev.cellbio.23.090506.123233. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palanivelu R, Preuss D. Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol. 2006;6:7. doi: 10.1186/1471-2229-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YJ, Szumlanski A, Nielsen E, Yang Z. Rho-GTPase-dependent F-actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J Cell Sci. 2008;24:1155–1168. doi: 10.1083/jcb.200801086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang JU, Gu Y, Lee YJ, Yang Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell. 2005;16:5385–5399. doi: 10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Y, Wu G, Yang Z. Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J Cell Biol. 2001;152:1019–1032. doi: 10.1083/jcb.152.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol. 2005;169:127–138. doi: 10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- 15.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins N, Saam JR, Mango SE. CYK-4/GAP provides a localized cue to initiate anteroposterior polarity upon fertilization. Science. 2006;313:1298–1301. doi: 10.1126/science.1130291. [DOI] [PubMed] [Google Scholar]
- 17.Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, Colwill K, Starostine A, Metalnikov P, Pawson T. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125:535–548. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 18.Knaus M, Pelli-Gulli MP, van Drogen F, Springer S, Jaquenoud M, Peter M. Phosphorylation of Bem2p and Bem3p may contribute to local activation of Cdc42p at bud emergence. Embo J. 2007;26:4501–4513. doi: 10.1038/sj.emboj.7601873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Lin Y, Heath RM, Zhu MX, Yang Z. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell. 1999;11:1731–1742. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Wu G, Ware D, Davis KR, Yang Z. Arabidopsis Rho-related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant Physiol. 1998;118:407–417. doi: 10.1104/pp.118.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twell D, Yamaguchi J, Wing RA, Ushiba J, McCormick S. Promoter analysis of genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Genes Dev. 1991;5:496–507. doi: 10.1101/gad.5.3.496. [DOI] [PubMed] [Google Scholar]
- 22.Weigel D, Ahn JH, Blazquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrandiz C, Kardailsky I, Malancharuvil EJ, Neff MM, Nguyen JT, Sato S, Wang ZY, Xia Y, Dixon RA, Harrison MJ, Lamb CJ, Yanofsky MF, Chory J. Activation tagging in Arabidopsis. Plant Physiol. 2000;122:1003–1013. doi: 10.1104/pp.122.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu G, Li H, Yang Z. Arabidopsis RopGAPs are a novel family of rho GTPase-activating proteins that require the Cdc42/Rac-interactive binding motif for rop-specific GTPase stimulation. Plant Physiol. 2000;124:1625–1636. doi: 10.1104/pp.124.4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parton RM, Fischer-Parton S, Watahiki MK, Trewavas AJ. Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J Cell Sci. 2001;114:2685–2695. doi: 10.1242/jcs.114.14.2685. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Kong L, Hao H, Wang X, Lin J, Samaj J, Baluska F. Effects of brefeldin A on pollen germination and tube growth. Antagonistic effects on endocytosis and secretion. Plant Physiol. 2005;39:1692–1703. doi: 10.1104/pp.105.069765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–1016. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- 27.Wendland J, Philippsen P. Determination of cell polarity in germinated spores and hyphal tips of the filamentous ascomycete Ashbya gossypii requires a rhoGAP homolog. J Cell Sci. 2000;113(Pt 9):1611–1621. doi: 10.1242/jcs.113.9.1611. [DOI] [PubMed] [Google Scholar]
- 28.Ushinsky SC, Harcus D, Ash J, Dignard D, Marcil A, Morchhauser J, Thomas DY, Whiteway M, Leberer E. CDC42 is required for polarized growth in human pathogen Candida albicans. Eukaryot Cell. 2002;1:95–104. doi: 10.1128/EC.1.1.95-104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito K, Fujimura-Kamada K, Hanamatsu H, Kato U, Umeda M, Kozminski KG, Tanaka K. Transbilayer phospholipid flipping regulates Cdc42p signaling during polarized cell growth via Rga GTPase-activating proteins. Dev Cell. 2007;13:743–751. doi: 10.1016/j.devcel.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Anderson DC, Gill JS, Cinalli RM, Nance J. Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science. 2008;320:1771–1774. doi: 10.1126/science.1156063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 Spatially Restricts Signaling of RAC/Rop to the Apex of Pollen Tubes. Plant Cell. 2006;18:3033–3046. doi: 10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 33.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Alberts P, Rudge R, Irinopoulou T, Danglot L, Gauthier-Rouviere C, Galli T. Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane. Mol Biol Cell. 2006;17:1194–1203. doi: 10.1091/mbc.E05-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Y, Li N, Wu S, Zhou Y, Shan Y, Zhang Q, Ding C, Yuan Q, Zhao F, Zeng R, Zhu X. Nudel binds Cdc42GAP to modulate Cdc42 activity at the leading edge of migrating cells. Dev Cell. 2008;14:342–353. doi: 10.1016/j.devcel.2008.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.