Abstract
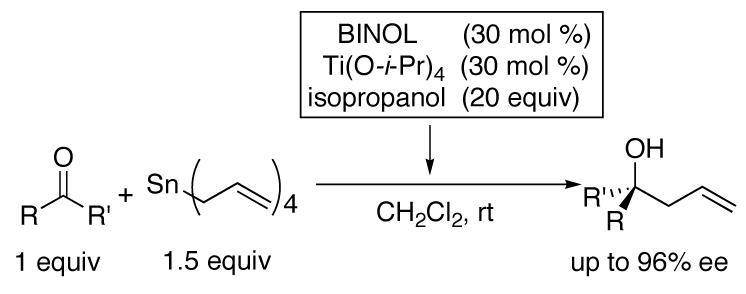
The first catalytic asymmetric methallylation of ketones is reported. The catalyst, which is generated from titanium tetraisopropoxide, H8-BINOL, isopropanol, and tetramethallylstannane, reacts with ketones in acetonitrile to afford tertiary homoallylic alcohols in fair to excellent yields (55-99%) and fair to high enantioselectivities (46-90%). Ozonolysis of the resulting products provides access to chiral β-hydroxy ketones, which are not readily prepared from direct asymmetric aldol reaction of acetone with ketones.
Catalytic asymmetric additions of carbon-based nucleophiles to carbonyl groups constitute an important class of C-C bond-forming reactions that are of great value in synthetic organic chemistry.1-5 In comparison to aldehydes, additions to ketones have proven to be more challenging due to their reduced reactivity and lower binding affinity to metals.1-5 Furthermore, the catalyst must differentiate between the lone pairs of the carbonyl oxygen to achieve high enantioselectivity. This task becomes very difficult when the groups R’ and R of the ketone R’RCO are similar in size. Nonetheless, successful catalysts for additions to ketones are beginning to emerge, providing access to tertiary alcohols with high enantioselectivities.6-24
We have been involved in the development of catalytic asymmetric additions of carbon-based nucleophiles to ketones,6,9,7,10,11,8 including the allylation of ketones. We,12,13 among other groups,20,16,15,14,18,17,19 have recently reported catalytic asymmetric allylation of ketones with high enantioselectivity. Despite the level of attention focused on the asymmetric allylation of ketones, related methallylation of these substrates remains unexplored. The paucity of methods for the asymmetric methallylation of ketones is surprising given the utility and versatility of the resulting homoallylic alcohols. For example, subsequent oxidative cleavage of the double bond of the methallylation products generates β-hydroxy ketones equivalent to those of the asymmetric cross aldol reaction between ketones and acetone. The direct aldol approach to enantioenriched tertiary aldol products remains challenging (Scheme 1).25
Scheme 1.
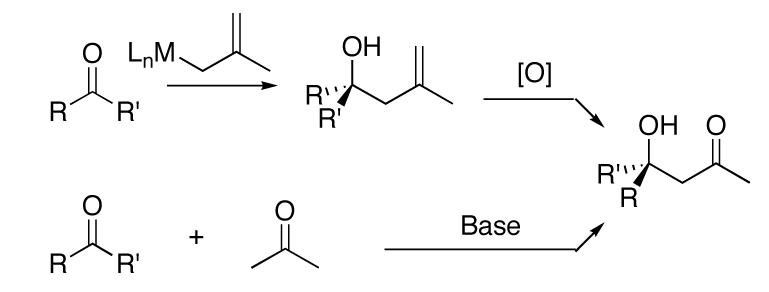
Our success in the catalytic asymmetric allylation of ketones led us to examine additions of related allyl groups. We were surprised to find, however, that our catalyst for the allylation of ketones gave low enantioselectivities in the methallylation reaction. It was thus necessary to develop a new protocol for this important addition reaction. Herein we report the first catalytic asymmetric methallylation of ketones.
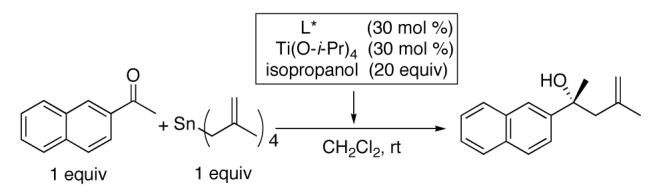
During mechanistic studies of allylation of ketones with the titanium BINOL-based catalyst developed by Tagliavini,26 we developed an improved catalyst for this reaction.13 Our catalyst for the asymmetric allylation of ketones employs titanium tetraisopropoxide, BINOL, isopropanol additive, and tetraallylstannane as the allyl source (Scheme 2).12,13 The key to high selectivity in this process is the isopropanol, although the role it plays in this reaction remains elusive.
Scheme 2.
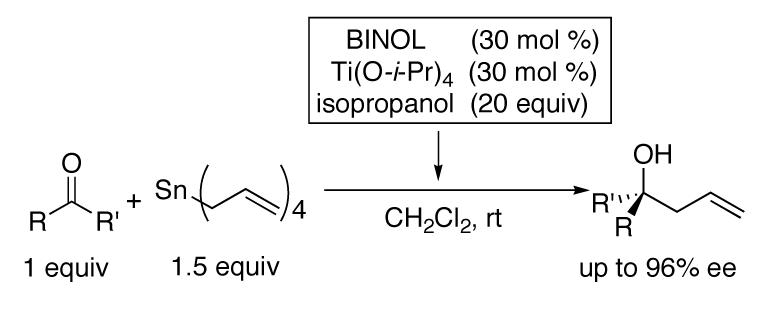
Catalytic Asymmetric Allylation of Ketones
The methallylation of ketones was initially examined with the same catalyst and procedure employed in the asymmetric allylation reaction. Thus, titanium tetraisopropoxide, BINOL, isopropanol, and tetramethallylstannane were combined and 2-acetonaphthone was added. Unfortunately the (BINOLate)Ti-based catalyst gave only 53% enantioselectivity in the methallylation reaction. This result can be contrasted to the allylation of the same substrate, which provided the product with 96% enantioselectivity. Similar erosion in enantioselectivity was observed when the aldehyde allylation conditions were applied to the methallyation of aldehydes.27
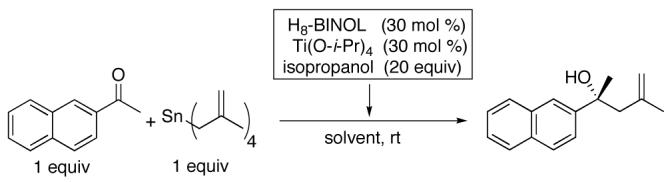
In an effort to improve enantioselectivity of the methallylation, several BINOL derivatives were examined with 2-acetonaphthone (Figure 1, Table 1). The impact of electron withdrawing bromo groups at the 6,6′-positions of the BINOL ligand was found to be very small. Substitution at the 3,3′-positions is known to dramatically alter the chiral environment of the substrate binding site. Unfortunately, use of 3,3′-diphenyl-BINOL (3,3′-Ph2-BINOL) and 3,3-dibromo-BINOL (3,3′-Br2-BINOL) led to significant decreases in enantioselectivity (entries 3 and 4).
Figure 1.
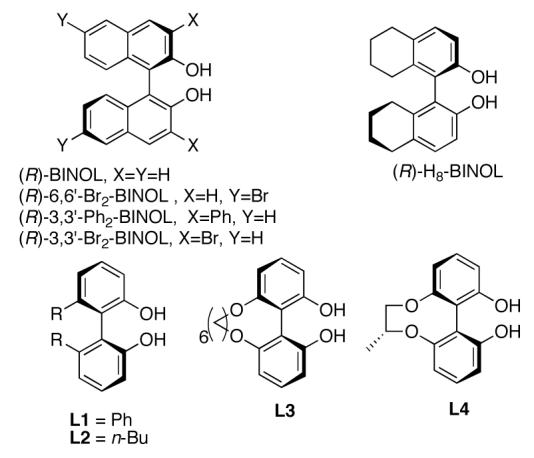
Additional Axially Chiral Ligands Tested
Table 1.
Asymmetric methallylation of 2-acetonaphthone with various BINOL derivatives.
 | ||
|---|---|---|
| entry | L* | ee (%) |
| 1 | (R)-BINOL | 53 |
| 2 | (R)-6,6’-Br2-BINOL | 55 |
| 3 | (R)-3,3’-Ph2-BINOL | 11 |
| 4 | (R)-3,3’-Br2-BINOL | 4 |
| 5 | (R)-H8-BINOL | 78 |
| 6 | L1 | 71 |
| 7 | L2 | 74 |
| 8 | L3 | 57 |
| 9 | L4 | 47 |
We next desired to explore the affect of the dihedral angle of BINOL-based ligands on the enantioselectivity of the methallylation reaction. In some cases, hydrogenated derivatives of BINOL, such as H8-BINOL (5,5′,6,6′,7,7′,8,8′-octahydro-1,1′-bi-2-naphthol, Figure 1) and H4-BINOL (5,6,7,8-tetrahydro-1,1′-bi-2-naphthol) have been shown to promote asymmetric reactions with better efficiency and enantioselectivity than reactions utilizing BINOL as a chiral ligand. The solid state structure and solution behavior of (BINOLate)Ti- and (H8-BINOLate)Ti-based complexes have been studied.28-30 Crystallographically characterized (BINOLate)Ti- and (H8-BINOLate)Ti-based complexes show a difference of about 7° in the dihedral angles between their naphthyl rings. The larger torsional angle of the H8-BINOLate ligand results in an increase in the O-Ti-O bite angle of just over 2°. The difference in dihedral angle may account for the enhanced performance of H8-BINOL over BINOL as a chiral ligand for certain reactions.
Substituting H8-BINOL for BINOL in our methallylation reaction gave a 25% increase in enantioselectivity (Table1). In view of these favorable results with H8-BINOL, we sought to test other axially chiral ligands having varied dihedral angles. In 1996, Harada used ligands L1-L3 (Figure 1) in an asymmetric Diels-Alder reaction.31 Harada speculated that the dihedral angle of L3 should be similar to that of BINOL while those of L1 and L2 would be larger than BINOL. In 2000, Harada reported a streamlined synthesis of this set of ligands in which L4 was an intermediate.32 Upon testing L1-L4 in our methallylation reaction, we found that only ligands L1 and L2 gave enantioselectivities rivaling that of H8-BINOL (Table 1).
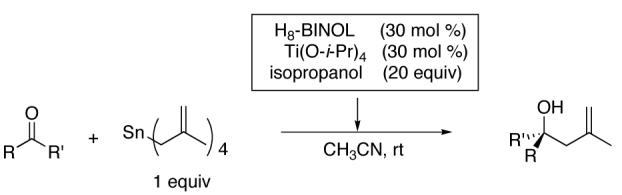
To maximize enantioselectivity, a variety of solvents were examined in the methallylation reactions with H8-BINOL (Table 2). Unlike the allylation of ketones with our BINOL based catalyst (Scheme 2), which exhibited virtually no solvent effect, a significant solvent effect was observed in the methallylation reaction. Reactions conducted in acetonitrile and propionitrile resulted in the highest enantioselectivities (entries 6 and 7) and acetonitrile was chosen for further optimization. Titanium(IV) alkoxide complexes with ligands closely related to BINOL have been shown to coordinate acetonitrile in the solid state.33
Table 2.
Examination of the Effect of Solvents on Enantioselectivity
 | ||
|---|---|---|
| entry | solvent | ee(%) |
| 1 | CH2Cl2 | 78 |
| 2 | THF | 73 |
| 3 | Et2O | 79 |
| 4 | 1,4-Dioxane | 67 |
| 5 | Isopropanol | 81 |
| 6 | MeCN | 87 |
| 7 | EtCN | 87 |
While concurrently testing a variety of solvents, the affect of differing amounts of isopropanol on the enantioselectivity was examined. Consistent with our earlier investigations with the asymmetric allylation of ketones (Scheme 2),34 isopropanol proved necessary for high enantioselectivity. Methallylation reactions conducted in the absence of isopropanol led to racemic product. Enantioselectivities increased upon addition of isopropanol and reached a plateau at about 20 equiv of isopropanol (75% ee at 10 equiv isopropanol, 78% ee at 20 equiv isopropanol, and 79% ee at 30 equiv isopropanol in CH2Cl2 as solvent). However, there was a slight loss of ee with isopropanol as solvent (81% ee, Table 2, entry 5).
The results of our catalytic asymmetric methallylation of a range of ketones are presented in Table 3. Aryl methyl ketones were generally good substrates for our system with enantioselectivities reaching 90% (entries 1-4). Only marginal electronic influence was observed with substituted acetophenones (entries 1-3). Attempts to improve the enantioselectivity by decreasing the temperature gave mixed results. Acetophenone derivatives showed lower enantioselectivities at 0 °C than at room temperature (entries 1 and 4), although 4-phenyl-but-3-en-2-one gave higher enantioselectivity at 0 °C (entry 5). α,β-Unsaturated ketones gave mixed results under our methallylation conditions (compare entries 5, 8, and 9 versus 10 and 11).
Table 3.
Yields and Enantioselectivities for the Methallylation of Various Ketone Substrates
 | ||||
|---|---|---|---|---|
| entry | substrate | temp | ee (%) | yield (%) |
| 1 | 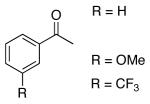 |
rt | 75 | 78 |
| 0 °C | 63 | 83 | ||
| 2 | rt | 72 | 99 | |
| 3 | rt | 67 | 95 | |
| 4 | 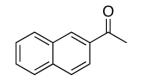 |
rt | 90 | 95 |
| 0 °C | 63 | 87 | ||
| 5 | 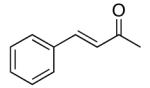 |
rt . | 72 | 93 |
| 0 °C | 76 | 87 | ||
| 6 | 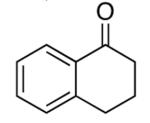 |
rt | 84 | 85 |
| 7 | 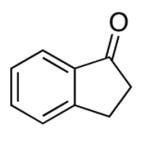 |
rt | 46 | 81 |
| 8 | 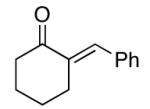 |
rt | 80 | 90 |
| 9 | 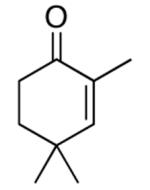 |
rt | 77 | 99 |
| 10 | 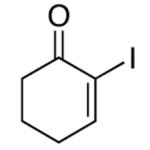 |
rt | 49 | 55 |
| 11 | 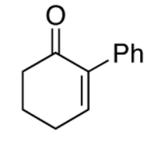 |
rt | 46 | 91 |
The homoallylic alcohol products that result from the methallylation of ketones can be converted into chiral tertiary β-hydroxy ketones via ozonolysis. These β-hydroxy ketones are known precursors to several natural products and are currently only accessible through kinetic resolution of racemic products.25 Subjecting 3′-(trifluoromethyl) acetophenone to methallylation conditions resulted in the formation of the product with 67% ee and 95% yield (Scheme 3). Subsequent ozonolysis generated the tertiary βhydroxy ketone with no loss of enantioselectivity.
Scheme 3.
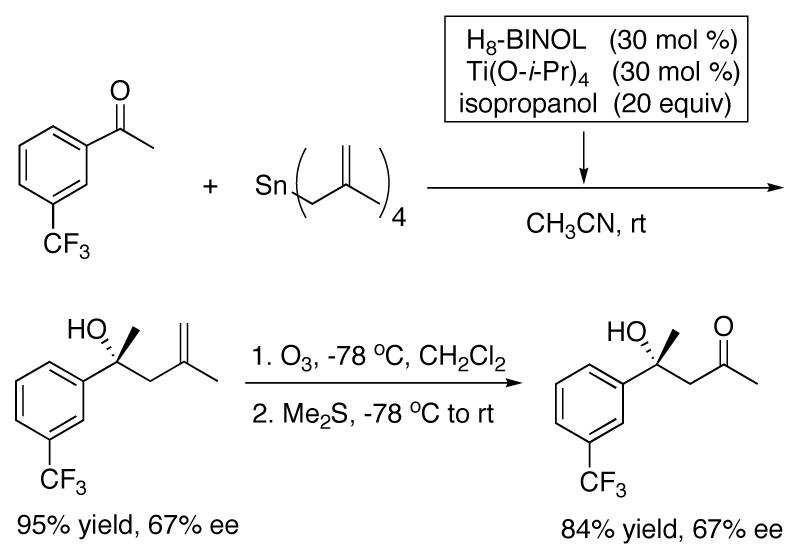
In conclusion, we have developed an (H8-BINOLate)Ti-based catalyst for the asymmetric methallylation of ketones. Good to high levels of enantioselectivity were observed across a range of substrates. These enantioenriched tertiary substituted homoallylic alcohols can be converted to a variety of structures such as β-hydroxy ketones. We are currently examining the possibility of significantly reducing the catalyst loading below the 30 mol % currently required to obtain maximum levels of enantioselectivity. Furthermore, we are examining other allyl transfer reagents to circumvent the known toxicity of tin.
Supplementary Material
Acknowledgment
This work was supported by the NIH (National Institute of General Medical Sciences, GM58101).
Footnotes
Supporting Information Available. Procedures and full characterization of new compounds (PDF) is available free of charge via the internet at http://pubs.acs.org.
References
- (1).Yanagisawa A. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 2. Springer; Berlin: 1999. pp. 965–979. [Google Scholar]
- (2).Pu L. Tetrahedron. 2003;59:9873–9886. [Google Scholar]
- (3).Pu L, Yu H-B. Chem. Rev. 2001;101:757–824. doi: 10.1021/cr000411y. [DOI] [PubMed] [Google Scholar]
- (4).Soai K, Niwa S. Chem. Rev. 1992;92:833–856. [Google Scholar]
- (5).Denmark SE, Fu J. Chem. Rev. 2003;103:2763–2794. doi: 10.1021/cr020050h. [DOI] [PubMed] [Google Scholar]
- (6).Garcia C, LaRochelle LK, Walsh PJ. J. Am. Chem. Soc. 2002;124:10970–10971. doi: 10.1021/ja026568k. [DOI] [PubMed] [Google Scholar]
- (7).Jeon S-J, Walsh PJ. J. Am. Chem. Soc. 2003;125:9544–9545. doi: 10.1021/ja036302t. [DOI] [PubMed] [Google Scholar]
- (8).Betancort JM, Garcia C, Walsh PJ. Synlett. 2004:749–760. [Google Scholar]
- (9).Garcia C, Walsh PJ. Org. Lett. 2003;5:3641–3644. doi: 10.1021/ol0352963. [DOI] [PubMed] [Google Scholar]
- (10).Li H, Garcia C, Walsh PJ. Proc. Nat. Acad. Sciences. 2004;101:5425–5427. doi: 10.1073/pnas.0307119101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Li H, Walsh PJ. J. Am. Chem. Soc. 2004;126:6538–6539. doi: 10.1021/ja049206g. [DOI] [PubMed] [Google Scholar]
- (12).Kim JG, Garcia IF, Kwiatokowski D, Walsh PJ. J. Am. Chem. Soc. 2004;126:12580–12585. doi: 10.1021/ja047758t. [DOI] [PubMed] [Google Scholar]
- (13).Waltz KM, Gavenonis J, Walsh PJ. Angew. Chem., Int. Ed. 2002;41:3697–3699. doi: 10.1002/1521-3773(20021004)41:19<3697::AID-ANIE3697>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- (14).Wadamoto M, Yamamoto H. J. Am. Chem. Soc. 2005;127:14556–14557. doi: 10.1021/ja0553351. [DOI] [PubMed] [Google Scholar]
- (15).Wada R, Oisaki K, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2004;126:8910–8911. doi: 10.1021/ja047200l. [DOI] [PubMed] [Google Scholar]
- (16).Teo Y, Goh J-D, Loh T-P. Org. Lett. 2005;7:2743–2745. doi: 10.1021/ol051018n. [DOI] [PubMed] [Google Scholar]
- (17).Cunningham A, Mokal-Parekh V, Wilson C, Woodward S. Organic & Biomolecular Chemistry. 2004;2:741–748. doi: 10.1039/b313384b. [DOI] [PubMed] [Google Scholar]
- (18).Cunningham A, Woodward S. Synlett. 2002:43–44. [Google Scholar]
- (19).Hanawa H, Kii S, Maruoka K. Adv. Synth. Cat. 2001;1:57–60. [Google Scholar]
- (20).Loh T-P, Zhou JR, Li XR. Tetrahedron Lett. 1999;40:9333–9336. [Google Scholar]
- (21).Prieto O, Ramon DJ, Yus M. Tetrahedron: Asymmetry. 2003;14:1955–1957. [Google Scholar]
- (22).Jiang B, Chen Z, Tang X. Org. Lett. 2002;4:5489–5492. doi: 10.1021/ol026544i. [DOI] [PubMed] [Google Scholar]
- (23).Funabashi K, Jachmann M, Kanai M, Shibasaki M. Angew. Chem., Int. Ed. 2003;42:5489–5492. doi: 10.1002/anie.200351650. [DOI] [PubMed] [Google Scholar]
- (24).Lu G, Li X, Jia X, Chan WL, Chan ASC. Angew. Chem., Int. Ed. 2003;42:5057–5058. doi: 10.1002/anie.200352013. [DOI] [PubMed] [Google Scholar]
- (25).List B, Shabat D, Zhang G, Turner JM, Li A, Bui T, Anderson J, Barbas CFI. J. Am. Chem. Soc. 1999;121:7283–7291. [Google Scholar]
- (26).Casolari S, D’Addario D, Tagliavini E. Org. Lett. 1999;1:1061–1063. [Google Scholar]
- (27).Keck GE, Krishnamurthy D, Grier MC. J. Org. Chem. 1993;58:6543–6544. [Google Scholar]
- (28).Balsells J, Davis TJ, Carroll PJ, Walsh PJ. J. Am. Chem. Soc. 2002;124:10336–10348. doi: 10.1021/ja0171658. [DOI] [PubMed] [Google Scholar]
- (29).Davis TJ, Balsells J, Carroll PJ, Walsh PJ. Org. Lett. 2001;3:699–702. doi: 10.1021/ol0070381. [DOI] [PubMed] [Google Scholar]
- (30).Waltz KM, Carroll PJ, Walsh PJ. Organometallics. 2004;23:127–134. [Google Scholar]
- (31).Harada T, Takeuchi M, Hatsuda M, Ueda S, Oku A. Tetrahedron: Asymmetry. 1996;7:2479–2482. [Google Scholar]
- (32).Harada T, Tuyet TMT, Oku A. Org. Lett. 2000;2:1319–1322. doi: 10.1021/ol0057840. [DOI] [PubMed] [Google Scholar]
- (33).Davis TJ, Carroll PJ, Walsh PJ. J. Organomet. Chem. 2002;63:70–77. [Google Scholar]
- (34).Waltz KM, Gavenonis J, Walsh PJ. Angew. Chem. Int. Ed. Engl. 2002;41:3697–3699. doi: 10.1002/1521-3773(20021004)41:19<3697::AID-ANIE3697>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


