Abstract
Forced overexpression of TEAD1 in human uterine fibroblast (HUF) and human endometrial stromal cells markedly inhibited prolactin promoter activity in both cell types in a dose-dependent manner, with maximal inhibition of greater than 90%. Conversely, the knockdown of TEAD1 expression in HUF cells with a TEAD1 siRNA resulted in a 75–80% increase in prolactin mRNA levels (P<0.01) compared to control cells exposed to a scrambled nonsense RNA. Mutagenesis of the putative TEAD site inhibited basal promoter activity by about 80%. However, mutagenesis of the TEAD site did not prevent TEAD1-induced inhibition of promoter activity; and the transcription activity of a minimal promoter fragment lacking a putative TEAD binding site was repressed by overexpression of TEAD1. Taken together, these findings suggest that the TEAD binding site on the prolactin promoter is important for the maintenance of basal prolactin promoter activity and that overexpression of TEAD1 has a dominant-negative effect on prolactin promoter activity, probably by interacting directly with other transcription factors.
Keywords: transcription, gene regulation, siRNA, (human decidua)
Introduction
Prolactin in the endometrium is first expressed during the luteal phase of the menstrual cycle as the stromal cells undergo differentiation (decidualization) in response to progesterone (Daly et al., 1983; Casslen et al., 1990). If pregnancy ensues, the decidualized stromal cells that express prolactin become the predominant cell type that lines the uterus. Although the prolactin expressed by decidualized stromal cells and other extra-pituitary cells that express prolactin is identical to the prolactin expressed by the pituitary, the regulation of prolactin expression by decidual and other extra-pituitary cells is different than that of pituitary prolactin (Handwerger and Freemark, 1987). The expression of the prolactin gene by decidual stromal cells and other extra-pituitary cells is regulated by a promoter that is located 2.3 kb upstream of the transcription start site, while the expression of prolactin in pituitary cells is regulated by a promoter that is located immediately upstream of the transcription start site (Telgmann and Gellersen, 1998). As a result of these differences, factors such as dopamine and TRH, which affect the expression of pituitary prolactin, have no effect on the expression of decidual prolactin (Golander et al., 1979).
Since prolactin gene expression is markedly induced during the decidualization process, the induction of prolactin gene expression has been widely used as a marker to study the regulation of uterine stromal cell differentiation. While much is known about the transcription factors and other factors that induce decidualization and prolactin gene expression during the differentiation process, relatively little is known about the regulation of prolactin gene expression in endometrial stromal cells that have undergone decidualization. Since the human decidual prolactin promoter contains a putative TEAD DNA binding site at nt −586/−578, we have examined whether this site is important for regulation of decidual prolactin promoter activity and whether members of the TEAD (TEA/ATTS domain) family of DNA binding proteins regulated promoter activity. The TEA/ATTS domain was originally identified as a region of homology between the yeast TEC1, TEF-1, and the Aspergillus nidulans factor Aba (Burglin, 1991). The TEAD family in the human consists of four members, TEAD1 ((TEA domain factor 1 or SV40 transcriptional enhancer factor 1 (TEF-1)), TEAD3, TEAD4 and TEAD5, all of which are derived from different genes and bind to the same DNA sequence (Jacquemin et al., 1996). In addition, TEAD1 has three isoforms resulting from alternative splicing - TEAD1α (also called TEAD1), TEAD1β and TEAD1γ (Jiang et al., 2000). TEAD-1, the prototypic family member, is expressed in a wide variety of cell types and is involved in the expression of many genes, including those involved in mouse development (Jacquemin et al., 1996; Kaneko and DePamphilis, 1998) and in cardiac and skeletal muscle-specific gene expression (Doevendans and van Bilsen, 1996; Maeda et al., 2002; Chen et al., 2004). TEAD-3 and TEAD-4, which have more limited tissue expression, are implicated in muscle-specific gene expression and central nervous system differentiation, respectively. TEAD-5 is expressed in skeletal and cardiac muscle, but the strongest expression of this gene in the human is in the placenta where it is implicated in the regulation of placental lactogen gene expression.
Little is known about the expression of TEAD family members in the uterus. TEAD1 has been shown to be expressed in mouse uterus. However, TEAD family members have not been identified in human uterine tissue, and it is unknown whether the family members are important for the regulation of gene expression in the human uterus. In the present study, we examined whether the TEAD family is expressed in human decidual stromal cells and whether the TEAD binding site on the prolactin promoter is important for the regulation of prolactin gene expression in decidualized uterine stromal cells. We also examined whether the activity of the decidual prolactin promoter is regulated by TEAD1.
Materials And Methods
Materials
pXJ-hTEF-1, an expression plasmid for TEAD1, and the empty vector pSG5 were generously provided by Dr. Irwin Davidson (Strasbourg, France) (Xiao et al., 1991). A plasmid containing the 5′ flanking region of the decidual prolactin gene was constructed by ligating a DNA fragment of the decidual prolactin gene from nt −2927 to +66 (relative to the decidual prolactin initiation site) into pGL3-Basic containing a luciferase reporter gene (pGL3B-Luc, Promega, Madison, WI), as previously described (Watanabe et al., 2001). Plasmids containing shorter fragments of the prolactin promoter ligated into pGL3B-Luc were prepared using restriction enzymes (Watanabe et al., 2001). The lefty2 promoter (nt −2100/+1) was kindly provided by Dr. S. Tabibzadeh (Stonybrook University, NY); and the TFAP2A promoter (nt −1727/+287) was kindly provided by Dr. Timothy Williams (University of Colorado, CO). A TEAD1 siRNA (ATG GCC GAT TTG TAT ACC GAA) was purchased from Qiagen (Vista, CA).
Mutagenesis
Site-directed mutagenesis of the putative TEAD site on the decidual prolactin promoter was performed using a Gene Tailor Site-Directed Mutagenesis System (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Mutagenesis to the desired bases was confirmed by DNA sequencing (Sequenase Version 2.0, Pharmacia Biotech, Piscataway, NJ.).
Immunohistochemistry
Paraffin blocks of human endometrium from the proliferative and mid-secretory phases of the menstrual cycle were selected from specimens routinely received for diagnostic purposes by the Department of Pathology and Laboratory Medicine, University of Cincinnati, OH and processed as previously described by our laboratory (Shah et al., 1998). Approval of the protocol was obtained from the Institutional Review Board of Children’s Hospital Medical Center. Tissue sections (4µm) on positively charged slides were incubated at 60°C overnight, deparaffinized in xylene and rehydrated through decreasing concentrations of ethanol in water. Immunohistochemical staining was performed using an indirect biotin avidin method on a Ventana 320ES automated immunostainer (Ventana Medical Systems, Tucson, AZ) and counterstained with hematoxylin. A monoclonal antibody highly specific for human TEAD1 was purchased from BD Biosciences (San Jose, CA). According to the manufacturer, there is no cross-reactivity among the TEAD family members. Control experiments were performed in which sections were incubated with purified rabbit or mouse IgG (2µg/ml) in place of specific antibodies. The control sections were run concurrently with sections using specific antibodies and were similarly pre-treated. Non-specific staining was not detected with the non-immune antiserum.
mRNA analyses
Semi-quantitative RT-PCR for the TEAD family members was performed as previously described in our laboratory for the determination of mRNA levels in HUF cells (Brar et al., 2002). Total RNA was extracted from decidual fibroblasts using Trizol reagent (Molecular Research Center, Inc., Carlsbad, CA.), according to the manufacturer’s directions. One to two micrograms of total RNA were reverse transcribed and polymerase chain reaction (PCR) amplification was performed after the addition of 0.14 µl 32P (3000 Ci/mmol) to the reaction mixture as described previously (Brar et al., 2002). The primer pairs used for amplification of TEAD1, TEAD3 and TEAD4 mRNAs were identical to those described by Jacquemin et al, 1996; and the primer pairs used for TEAD5 were identical to those used by Jacquemin et al, 1997. The PCR program for each primer pair consisted of 25 cycles, which was in the linear range of the amplification curve for each set of primers. Each cycle consisted of 1 min at 94°, 1 min at 55° and 1 min at 72°C. The radiolabeled PCR products were separated by electrophoresis on 6% polyacrylamide gels at 200 V for 3 h. The gels were transferred to 3M paper, dried, and quantified using a phosphorimager and Imagequant 1.2 software (Molecular Dynamics Inc, Sunnyvale, CA). In each instance, size of the PCR products for each mRNA was identical to the predicted size.
Electrophoretic Mobility Shift and Supershift Assays
Nuclear extracts of HUF cells were prepared and electrophoretic mobility shift (EMSA) and supershift assays were performed as previously described (Bohinski et al., 1994; Kelly et al., 1996). Briefly, 5 to 10 micrograms of nuclear extracts ((determined by the Bradford assay method (Bio-Rad) using bovine serum albumin as the standard)) were incubated for 10 min at RT in buffer containing 20 mM TRIS, pH 7.6, 50 mM KCl, 2 mM MgCl2, 10% glycerol and 40ng/ml poly(dI-dC). A double-stranded 32P-labeled oligonucleotide probe (100,000 dpm) was gel purified using the Bio 101 MERmaid Kit (Qiagen,Vista, CA). This fragment of the prolactin promoter containing the putative TEAD binding site (nt −597/−567) was added and incubation continued for an additional 10 min. Where indicated, 100-fold molar excess of non-labeled oligonucleotides containing mutations of the TEAD-1 consensus site were added along with the probe to determine specificity of binding. The sequences for the wild type consensus site probe and the competitor were −597 CAT CTG TTT AAT GCA TGC TTA ATA TTT TTGA −567. The sequence for the mutated TEAD-1 site was −597 CAT TTG TTT AAT TCT ACC TTA ATA TTT TTGA −567 (mutated bases are bolded). The reaction mixture was electrophoresed on a 5% polyacrylamide gel in 0.5 × TBE to separate bound and free probes. For supershift assays, nuclear extracts were incubated for 10 min at RT with a TEAD-1 antibody (BD Biosciences, San Jose, CA) prior to addition of the radiolabeled probe.
Cell culture
For the preparation of endometrial stromal cells, proliferative phase endometrium was removed by suction biopsy from women with normal menstrual cycles at the time of elective tubal ligation; and a highly purified stromal cell population was obtained as previously described (Brar et al., 1995). The study was approved by the Institutional Review Boards of Children's Hospital Medical Center and the University of Cincinnati and informed consent was obtained from the patients. The cells were cultured in DMEM medium containing 2% fetal bovine serum (FBS), 25 U/ml penicillin G, 25 µg/ml streptomycin and 2.5 µg/ml amphotericin B. For the preparation of HUF (human uterine fibroblast) cells, term human placentas were obtained from women undergoing cesarean section or vaginal delivery following uncomplicated pregnancies. Decidua parietalis tissue was dissected from the fetal membranes within 1–2 h of delivery, and the human uterine fibroblast (HUF) cells were prepared as previously described (Richards et al., 1995). After three sub-passages, the cells were plated and cultured in RPMI 1640 medium containing 2% FBS. BeWo and JEG3 choriocarcinoma cells were obtained from the American Tissue Culture Collection (Rockville, MD). The BeWo cells were cultured in Ham's F12 supplemented with 15% FBS, and the JEG3 cells were cultured in RPMI 1640 with 10% FBS.
Decidualization of the endometrial stromal cells was induced by treatment with medroxyprogesterone acetate (MPA; 1 µM) and estradiol (E2; 10 nM). Decidualization of the HUF cells was induced by treatment with medroxyprogesterone acetate (MPA; 1 µM), estradiol (E2; 10 nM) and dibutyryl-cAMP (db-cAMP; 50 mM). The media of the endometrial stromal and HUF cells were changed at 2–3 day intervals.
Transfection studies
Transient transfection assays of the endometrial stromal and HUF cells at 90–100% confluency were performed by the calcium phosphate precipitation method following days 9 and 6 of in vitro decidualization respectively as described previously (Kessler et al., 2006). JEG3 and BeWo cells were transfected at 60–70% confluency using the same method. In selected experiments, pXJ-hTEF-1 was co-transfected into the cells along with the LUC reporter plasmids containing decidual prolactin promoter fragments. The DNA plasmids used in the transient transfection studies were purified by QIAGEN plasmid Maxi kits (Qiagen, Vista, CA). The cells were incubated in 6-well plates for 4 h with 5 µg per well luciferase-prolactin promoter constructs. All transfection results were normalized to β-galactosidase activity resulting from co-transfection of 0.5 µg per well of pSV-βgal (Promega, Madison, WI). The values represent the mean ± SEM of triplicate wells. All transfections were performed in at least three separate experiments.
TEAD1 siRNA studies
HUF cells were plated in 6-well plates the day before transfection at a density of 2 × 106 cells/well and then incubated overnight at 37C in 5% CO2. The cells were transfected at 90% confluency with the siRNA using the RNAifect transfection reagents (Qiagen, Vista, CA). The transfection reaction mixture was changed after six hours and replaced with fresh growth media containing 1 µM MPA, 10 nM estradiol and 1 µM PGE2. Seventy-two hours later, the samples (triplicate wells for each time point) were analyzed for protein and RNA.
Statistics
Statistical differences between group means was determined by analysis of variance with Bonferroni adjustment or a Student’s t-test, depending on design. Differences were considered significant when P<0.01. The data are presented as the mean ± SEM.
Results
Initial studies were performed to determine whether the genes of the TEAD family members are expressed in HUF cells. As shown in Figure 1, RT-PCR analyses of RNA from decidualized HUF cells with highly specific primer pairs revealed that the cells contain the mRNAs for TEAD1, TEAD3, TEAD4 and TEAD5. The RT-PCR for TEAD 1 showed three distinct bands corresponding in size to the three TEAD1 isoforms. RT-PCR analyses of HUF cells prior to exposure to progesterone, estradiol and PGE2 revealed that undecidualized uterine stromal cells also express each of TEAD family members and isoforms.
Figure 1.
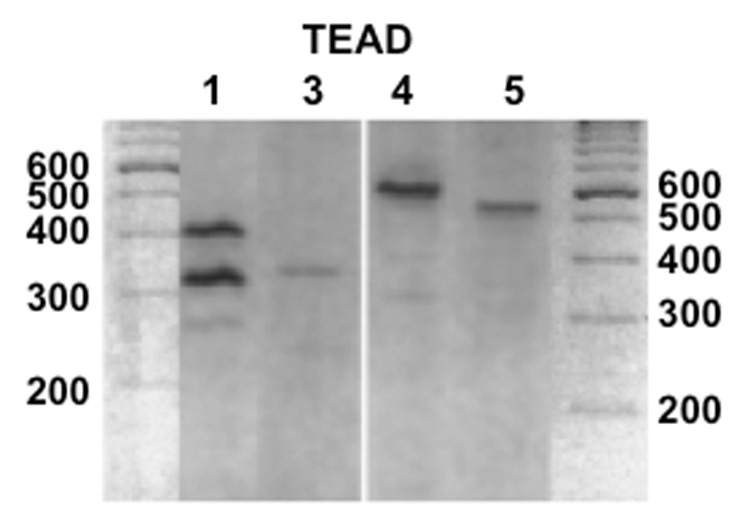
RT-PCR analysis of HUF cell RNA for TEAD1, TEAD3, TEAD4 and TEAD5. Total RNA was prepared from HUF cells and analyzed for the mRNAs of the TEAD family members by RT-PCR using highly specific primer pairs as described in Methods.
Subsequent investigations focused on TEAD1, the prototypic member of the family. Immunohistochemical studies using a specific TEAD1 antiserum were performed to localize TEAD1 in the uterus during the proliferative phase of the menstrual cycle, prior to decidualization and the induction of prolactin gene expression, and during the secretory phase of the menstrual cycle, when the cells are undergoing decidualization and express prolactin. As shown in Figure 2, immunostaining for TEAD1 was detected in stromal cells and glandular epithelium at both phases of the cycle. In contrast, control immunohistochemical studies using a non-immune serum revealed no staining.
Figure 2.
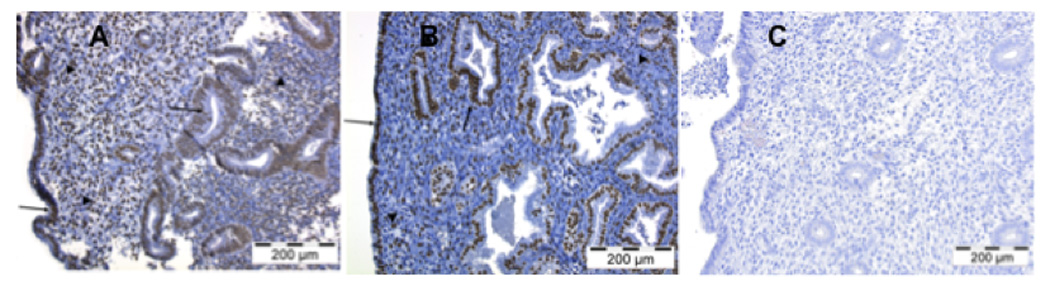
The localization of TEAD1 in the human uterus during the proliferative (panel A) and mid-secretory (panel B) phases of the human menstrual cycle. Panels A and B show staining with an anti-TEAD1 serum; and the panel shows staining of mid-secretory endometium with a non-immune serum. Positive staining was observed in the endometrial stromal (arrowheads) and epithelial glandular cells (arrows) in both the proliferative and secretory phases. No immunostaining was noted with the non-immune serum. Magnification is 200x, and the length of each scale is 200 µM.
Gel mobility and supershift assays revealed that TEAD1 binds to the putative TEAD binding site identified on the decidual prolactin promoter. Incubation of a 32P-labeled oligonucleotide probe (nt −597/−567) containing the TEAD binding site on the prolactin promoter and a nuclear extract of human decidual tissue revealed three retarded complexes (Figure 3, lane A). The addition of a 100-fold excess of unlabeled TEAD1 oligonucleotide completely competed with the labeled probe for binding to the nuclear extract (lane C), but an oligonucleotide containing a mutation in the TEAD1 binding site did not compete with the wild-type binding site for the binding of nuclear proteins (lane D). Incubation of the labeled oligonucleotide-nuclear extract complexes with a specific TEAD1 antiserum caused a diminution of band 2 and a supershift of band 1 (lane B). The TEAD1 antiserum did not supershift or diminish the intensity of band 3, suggesting that this band is due to a complex formed by the binding of another TEAD family member to the oligonucleotide probe.
Figure 3.
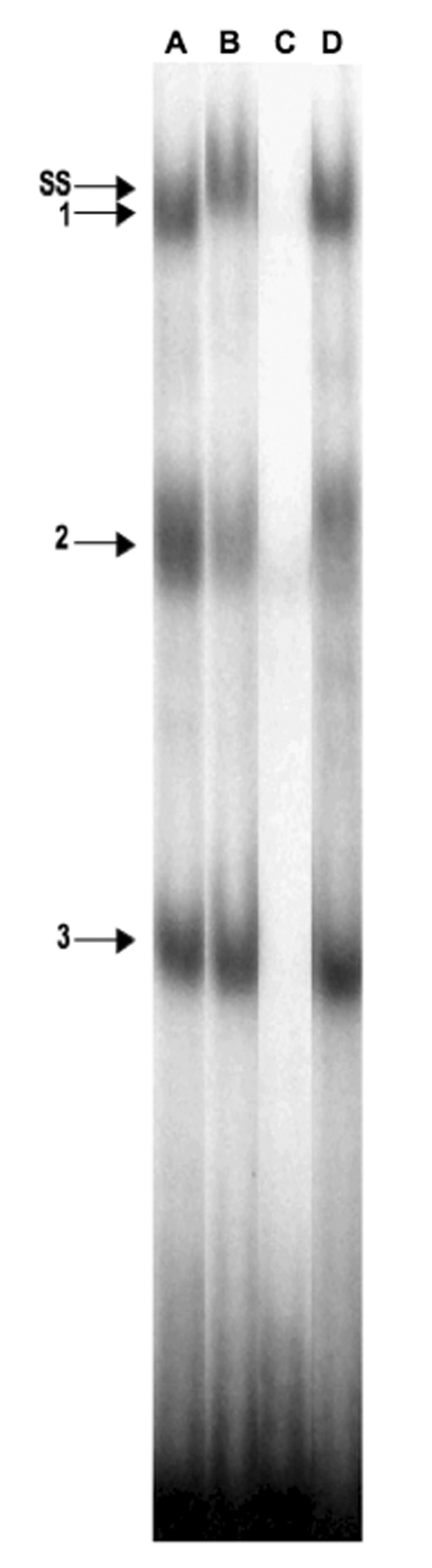
EMSA and supershift analyses of the consensus TEAD1 binding site on the decidual prolactin promoter. EMSA analysis was performed using an oligonucleotide probe corresponding to the TEAD1 binding site and nuclear extracts of HUF cells. A: TEAD1 oligonucleotide and HUF cell extract alone; B: supershift analysis using a specific antiserum to TEAD1; C: the effect of a 100-fold excess of unlabeled TEAD1 oligonucleotide; D: the effect of a 100-fold excess of the mutated oligonucleotide as competitor. The numbers indicate the locations of the retarded bands; and SS indicates the supershifted band.
Since TEAD1 binds to the prolactin promoter, we next examined whether TEAD1 activates the decidual prolactin promoter (Figure 4). Transient transfection of decidualized HUF cells with p2000/+66 dPRL-Luc and the TEAD1expression plasmid pXJ-TEF-1 (0.5 to 3.0 ug) resulted in a marked dose-dependent decrease in luciferase activity, with maximal inhibition that was >90% less than that noted in control cells co-transfected with the control vector alone (p<0.001). Transient transfection of decidualized endometrial stromal cells with p-2000/+66dPRL-Luc and pXJ-TEF-1 (0.1 ug) resulted in 85% decrease in luciferase activity (p<0.001). Conversely, silencing of the TEAD1 gene with a siRNA to TEAD1 markedly induced prolactin mRNA levels in decidualized HUF cells (Figure 5). TEAD1 mRNA levels after 2 and 4 days of exposure to the TEAD1 siRNA were 64.5 ± 15.0 and 92.7 ± 12.1% less, respectively, than that of cells exposed to a scrambled RNA; and prolactin mRNA levels were 39.5 ± 6.0 and 312.0 ± 21.2% greater than that of the cells exposed to the scrambled RNA.
Figure 4.

Overexpression of TEAD1 inhibits decidual prolactin promoter activity. HUF cells (left panel) and endometrial stromal cells (right panel) were transiently co-transfected with a TEAD1 expression plasmid (pXJJ-TEF-1) and a plasmid that contains the prolactin promoter coupled to a luciferase reporter gene (p-2000/+66GL3B. Each bar represents the mean of triplicate wells; and the brackets enclose +/−1 SEM. ***=p<0.001.
Figure 5.
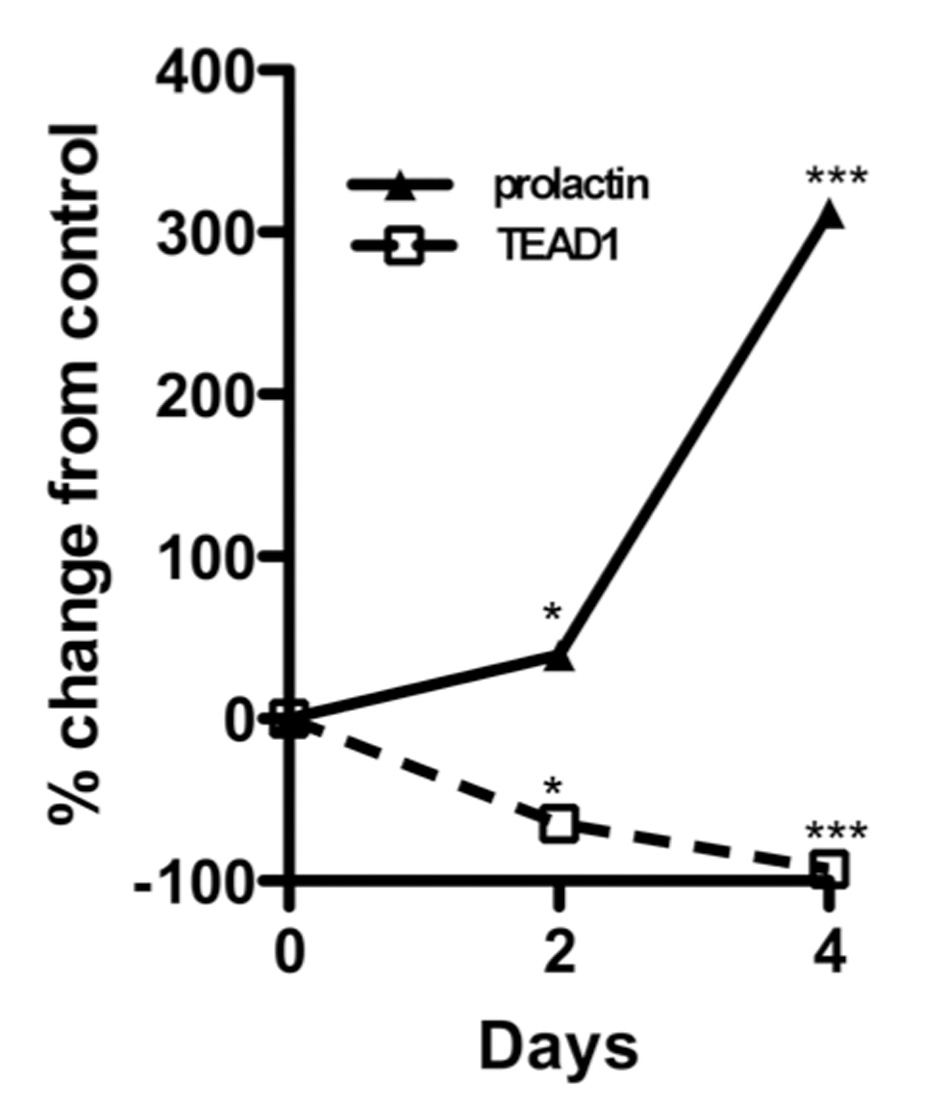
The effect of TEAD1 gene silencing on prolactin mRNA levels in HUF cells. HUF cells were decidualized for four days in the presence or absence of a siRNA to TEAD1 or a scrambled nonsense RNA. The medium was changed every 48 hours, and RNA was isolated from the cells at the end of 48 and 96 hours for the determination of prolactin, TEAD1 and GAPDH mRNA levels. Each point represents the mean ± SEM of triplicate wells. The amount of prolactin or TEAD1 mRNA was normalized to the amount of GAPDH mRNA in the same sample. The final results are expressed as the percent change of mRNA levels of the TEAD1 siRNA exposed cells relative to levels in the cells that had been exposed to the scrambled RNA. *=p<0.01, ***=p<0.001
The inhibitory effect of TEAD1 was not due to a non-specific effect on promoter activity. In the experiment depicted in Figure 6 (left panel), TEAD1 overexpression inhibited transactivation of the prolactin by 97.2 ± 2.9%, but induced lefty2 promoter activity by 18.2 ± 2.1-fold. In the experiment shown in the right panel, TEAD1 overexpression inhibited prolactin promoter activity by 87.5 ± 6.2% but had no effect on the activity of the TFAP2A promoter (13.1 ± 1.2 vs 11.5 ± 1.1). Furthermore, the effect of TEAD1 on prolactin promoter activity was relatively cell-specific. In contrast to its effect on prolactin promoter activity in decidual cells, TEAD1 overexpression had no effect on prolactin promoter activity in JEG3 and BeWo human choriocarcinoma cells (Figure 7). Although TEAD1 significantly induced the lefty2 promoter in HUF cells, TEAD1 had no effect on transactivation of the lefty2 promoter in JEG3 cells (data not shown).
Figure 6.
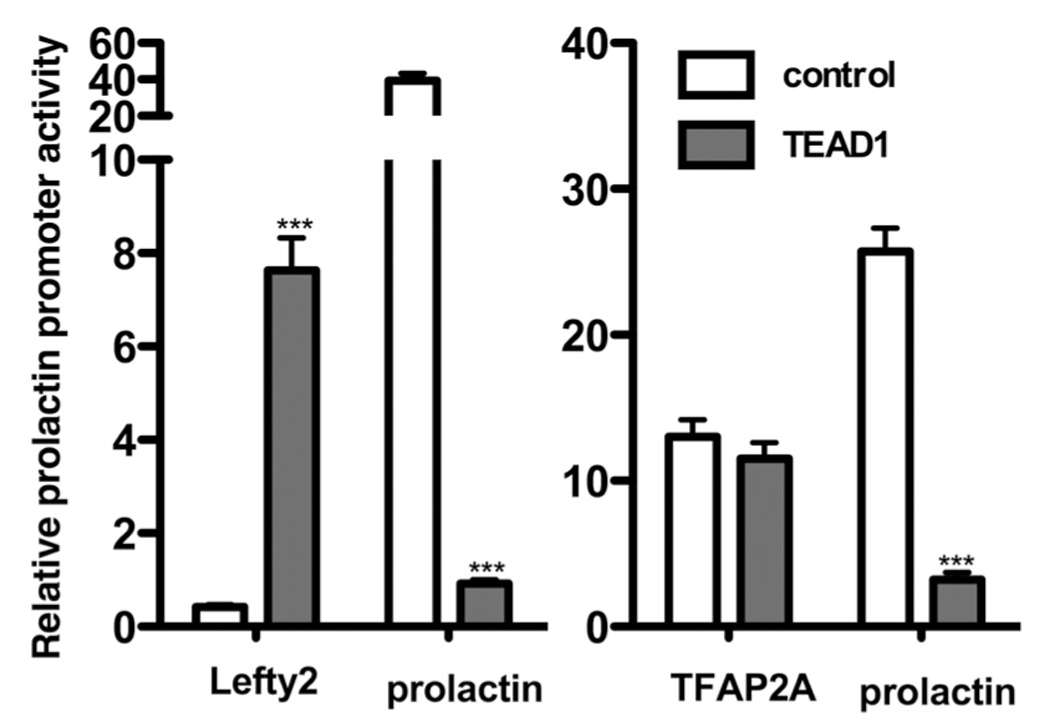
The effects of TEAD1 overexpression on the activities of the lefty2 (left panel) and TFAP2 (right panel) promoters. In the experiment depicted on the left, decidualized HUF cells were transiently transfected with luciferase expression vectors driven by either the prolactin promoter or the lefty2 promoter and pXJ-TEF-1 or the empty vector pSG5 as described in Methods. In the experiment depicted on the right, HUF cells were transiently transfected with the prolactin promoter or the promoter for TFAP2A. Each bar represents the mean of triplicate wells, and the brackets enclose ± 1 SEM. ***=p<0.001. Similar results were observed in two other experiments.
Figure 7.
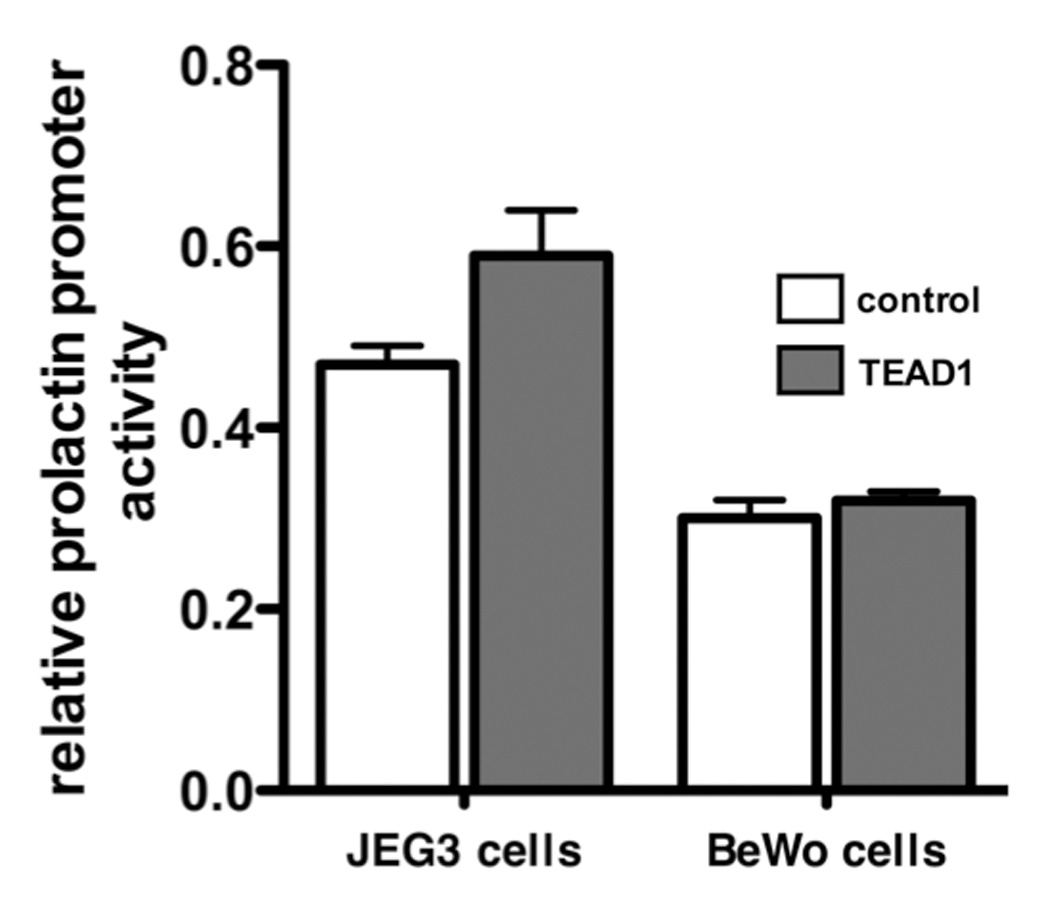
The effect of TEAD1 overexpression on prolactin promoter activity in JEG3 and BeWo cells. JEG3 and BeWo cells were transiently transfected with pXJ-TEF-1 or the empty vector pSG5. Each bar represents the mean of triplicate wells; and each bracket encloses ± 1 SEM. The luciferase activities of the TEAD1-expressing and control cells were not significantly different (p<0.05).
To examine whether the TEAD binding site on the decidual prolactin promoter is critical for basal transcriptional activity and the inhibitory effect of TEAD1 overexpression on transcriptional activity, we next compared the basal transcriptional activities of the wild-type promoter and a promoter fragment of the same size in which the TEAD binding site was mutated by site-directed mutagenesis (Figure 8). As shown in the panel on the left, TEAD1 overexpression repressed the basal transcriptional activity of the wild-type prolactin promoter fragment by 91.5%. Nevertheless, TEAD1 overexpression repressed the transcriptional activity of the mutated prolactin promoter fragment by an additional 89.5%. Similar results were observed in 2 other experiments in which TEAD overexpression inhibited transactivation of the mutated prolactin promoter by 88.2 ± 4.4 and 91.0 ± 5.1% (n=3 and p<0.001 in each experiment. Taken together, these findings strongly suggest that the repression of prolactin promoter activity by TEAD1 is not mediated by the binding of the transcription factor to the TEAD site. This point is further supported by transfection experiments in HUF and endometrial stromal cells using different size fragments of the decidual prolactin promoter. As shown in Figure 9, the overexpression of TEAD1 in both of these cell types inhibited the transcriptional activity of a decidual prolactin promoter fragment lacking the TEAD binding site (nt −345/+66). The fragment lacking the TEAD binding site was inhibited in HUF cell by 74.4 ± 2.2% and in endometrial stromal cells by 67.3 ± 3.8% (P<0.001 in each instance.
Figure 8.
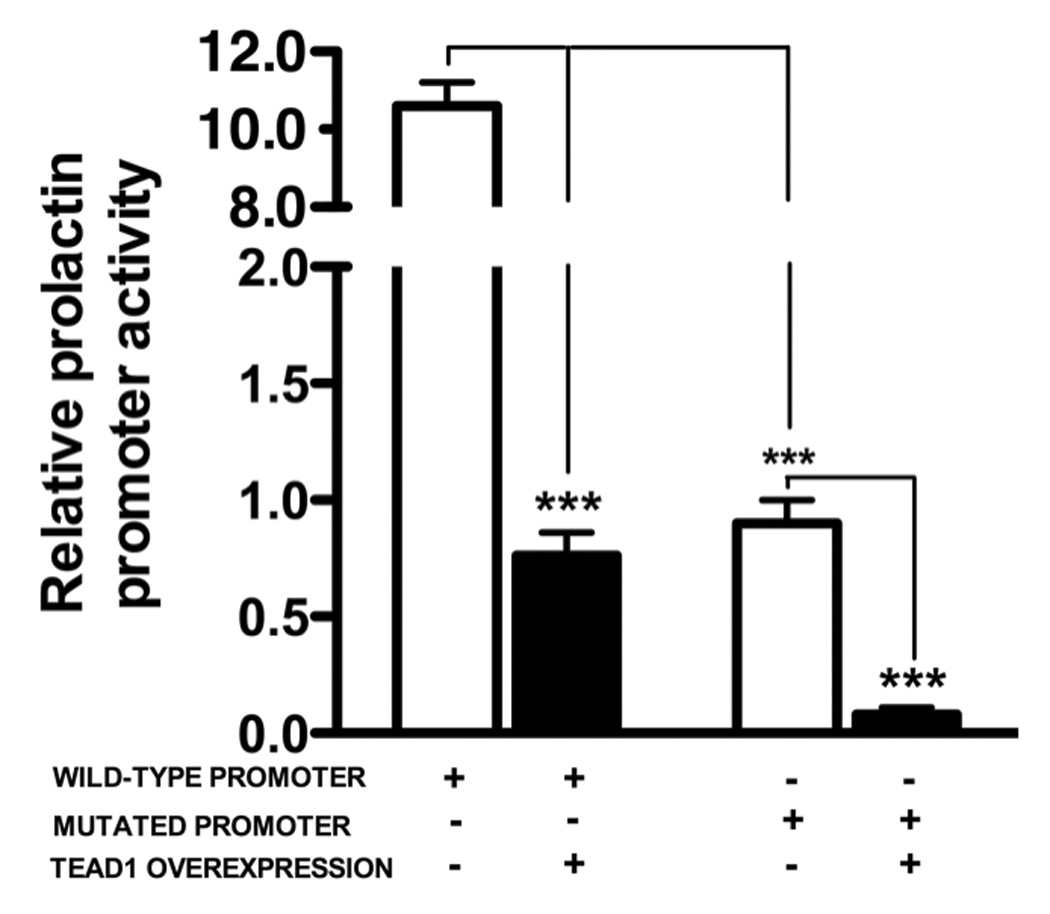
Mutagenesis of the putative TEAD1 binding site on the decidual prolactin promoter does not prevent the inhibitory effect of TEAD1 overexpression in decidualized HUF cells. Site-directed mutagenesis was performed as described in Methods. The left half of figure shows the effect of TEAD1 overexpression on the wild-type promoter, and the right half shows the effect of TEAD1 on the promoter fragment with a mutated TEAD binding site. In each instance, the bars represent the mean of triplicate wells, and the brackets enclose ± SEM. ***=p<0.001. Similar results were observed in 2 other experiments in which TEAD overexpression inhibited transactivation of the mutated prolactin promoter by 88.2 ± 4.4 and 91.0 ± 5.1% (n=3 and p<0.001 in each experiment.
Figure 9.
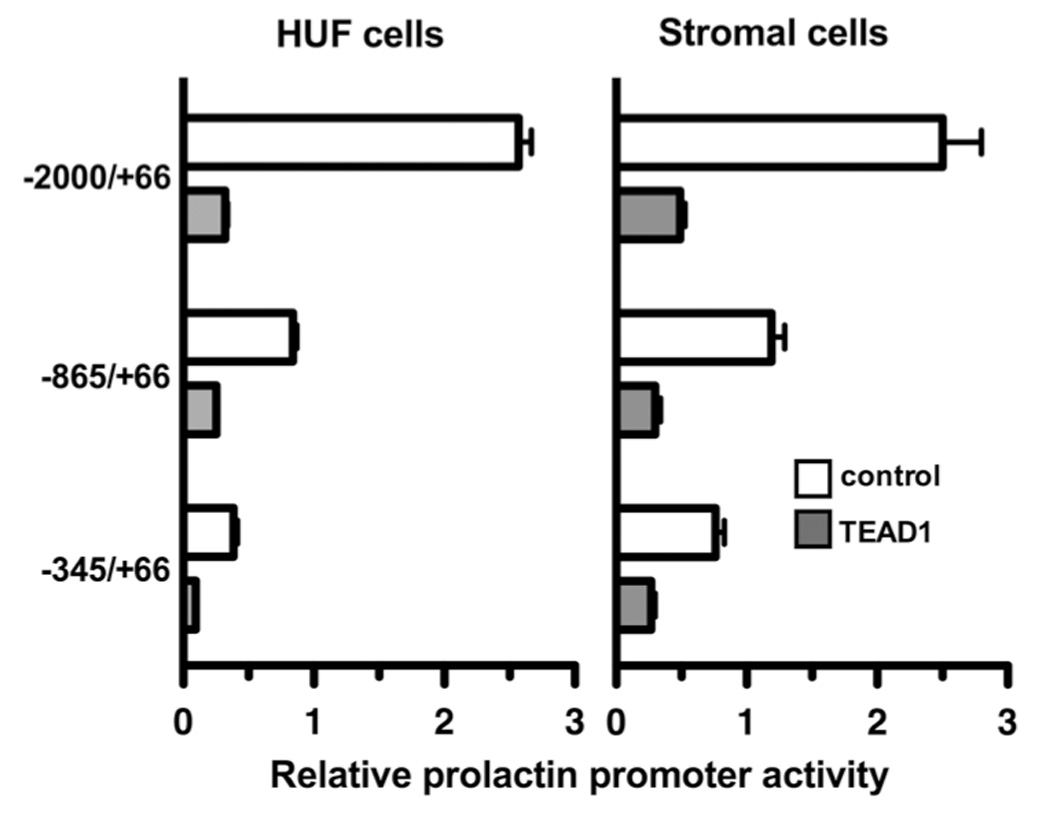
The effect of TEAD1 overexpression on the transcriptional activities of different size fragments of the decidual prolactin promoter. Decidualized HUF cells (left panel) and endometrial stromal cells (right panel) were transiently transfected with fragments of the decidual prolactin promoter and pXJ-TEF-1 or pSG5. Each bar represents the mean of triplicate wells, and the brackets enclose ± 1 SEM. In each instance, the luciferase activity of the TEAD1-exposed cells was significantly less than that of the control cells (**=p<0.005).
Discussion
The results of this study indicate that human uterine stromal and glandular cells express the mRNAs for each of the members of the TEAD family of DNA binding proteins. Several lines of evidence suggest a role for the prototypic TEAD family member TEAD1 in the regulation of prolactin gene expression in decidualized human uterine stromal cells. Both TEAD1 and prolactin mRNA and protein are localized in the stromal cells of the endometrium; and EMSA and supershift assays demonstrated that TEAD1 can bind to the TEAD binding site on the decidual prolactin promoter. Furthermore, mutation of this binding site inhibited basal promoter activity by greater than 90%. Since each of the members of the TEAD family can bind to the same consensus DNA binding site, it is also possible that other members of the family besides TEAD1 may be involved in the regulation of the decidual prolactin promoter. This possibility, in fact, is suggested by the observation that the TEAD1 antiserum failed to supershift or diminish the intensity of one of the complexes (band 3) formed by the interaction of an extract of HUF cells with an oligonucleotide corresponding to the TEAD binding site.
Although the TEAD site on the decidual prolactin promoter is important for basal promoter activity, overexpression of TEAD1 markedly inhibits promoter activity. This inhibitory effect of TEAD1 overexpression, however, is not due to the direct binding of TEAD1 to the TEAD site since site-directed muatagenesis of the binding site or deletion of the site did not prevent the inhibitory effect of TEAD1. Although mutagenesis of the TEAD site markedly inhibited basal expression of the decidual prolactin promoter, overexpression of TEAD1 caused a further inhibition of promoter activity. Furthermore, the overexpression of TEAD1 inhibited the transcriptional activity of a decidual prolactin promoter fragment that did not contain the TEAD binding site. Since the inhibitory effect of TEAD1 overexpression on the decidual prolactin promoter is not affected by mutagenesis or deletion of the TEAD binding site on the promoter, the repression of prolactin promoter activity by TEAD1 does not appear to be mediated by the direct binding of TEAD1 to the promoter but rather to another factor(s) that interacts with the transcription machinery essential for prolactin expression. While the factor that interacts with TEAD1 to repress prolactin gene expression in decidual stromal cells is unknown, the factor does not appear to be in JEG3 or BeWo cells since TEAD1 overexpression has no effect on the transcriptional activity of the prolactin promoter in these cells. Furthermore, the repression of promoter activity is not due to a generalized inhibitory effect on transcription since overexpression of TEAD1 in HUF cells induces lefty2 promoter activity and has no effect on TFAP2 activity. Taken together, these observations suggest that overexpression of TEAD1 has a dominant/negative effect on transactivation of the prolactin promoter, most likely resulting from the quenching of an activator of the prolactin promoter activity that is present in decidua but not placenta-derived cells.
There are several other examples in the literature for repression of gene expression by TEAD1 that is most likely due to the binding to and subsequent quenching of another transcription factor that regulates basal promoter activity (Xiao et al., 1991; Chaudhary et al., 1995). In HeLa cells, the expression of recombinant TEAD1 does not activate transcription of a cognate reporter gene to a level above that generated by the endogenous HeLa TEAD1, but rather represses endogenous TEAD1 activity (Xiao et al., 1991). No transactivation was seen in cells that do not contain TEAD1, leading to the hypothesis that TEAD1 requires cell-specific co-factors for activity. The dominant negative phenotype in HeLa cells does not require site-specific DNA binding but does require the presence of the TEAD1 regions involved in transactivation. These results suggest that the dominant/negative phenotype observed upon overexpression of TEAD1 in HeLa and HUF cells corresponds to a transcriptional interference/squelching effect that results from the titration of a transcriptional intermediary factor(s).
Jiang and co-workers showed that TEAD1 overexpression inhibits placental lactogen and a variety of other promoters in BeWo cells and that the inhibition does not depend on the presence of TEAD1 binding sites (Jiang and Eberhardt, 1996). They observed that TEAD1 can interact with TATA binding protein (TBP), a component of the basal transcriptional apparatus, to inhibit TBP binding to the TATA box. Proline-rich and zinc finger domains on TEAD1 are required for the binding to TBP (Jiang and Eberhardt, 1996). Earlier studies in which TEAD1 induces gene expression also suggested that TEAD1 activity requires a co-activator since ectopic expression of TEAD1 in non-TEAD1-expressing cells does not elicit enhancer function from appropriate DNA sequences (Mahoney et al., 2005). In addition, overexpression of TEAD1 in TEAD1-expressing cultured cells results in down-regulation of enhancer activity, consistent with titrating out a co-activator activity. The fact that TEAD1 has no effect on prolactin promoter activity in BeWo cells but affects the activities of other promoters suggests that the co-factor is unique for the prolactin promoter. Since TBP is expressed ubiquitously and binds to TEAD1 in BeWo cells, the inability of TEAD1 overexpression to inhibit decidual prolactin promoter activity in BeWo cells can not be due solely to the titration of TBP.
Several studies have shown that TEAD1 can either activate or repress genes through association with different co-factors. For example, TEAD1 can repress the SV40 late gene promoter, and SV40 T-antigen can relieve this repression by binding TEAD1 (Kaneko and DePamphilis, 1998). VITO1, a SID (Scalloped Interaction Domain)–containing protein, has been shown to be an essential co-factor of TEAD1-dependent muscle-specific gene regulation (Gunther et al., 2004). TEAD1 by itself is unable to activate reporter plasmids bearing TEAD1 binding sites, suggesting that additional bridging or co-activating factors are necessary to allow interaction of TEAD1 with the transcriptional machinery. Maeda and co-workers (Maeda et al., 2002) identified three mammalian vestigial-like genes (Vgl11-3) that share homology and a TEAD1 interaction domain; and Mahoney and co-workers (Mahoney et al., 2005) showed that the transcriptional co-activator TAZ interacts differentially with TEAD1 family members. TEAD1 activity is regulated by interactions with transcriptional co-factors p160, TONDU (Vgl1, Vestigial-like protein1), Vgl-2 and YAP 65 (YES-associated protein 65 kDa). At present, the factors in decidualized uterine stromal cells that interact with TEAD1 to modulate promoter activity are unknown.
In summary, the findings of this study indicate that the TEAD binding site on the decidual prolactin promoter is essential for maximal basal activity in decidual stromal cells. However, overexpression of TEAD1 inhibits promoter activity independent of the TEAD binding site, likely by titration of another limiting factor in these cells that is required for transcriptional activation. This inhibitory effect of TEAD1 is relatively cell- and gene-specific.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bohinski RJ, Di Lauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol. 1994;14:5671–5681. doi: 10.1128/mcb.14.9.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar AK, Frank GR, Richards RG, Meyer AJ, Kessler CA, Cedars MI, Klein DJ, Handwerger S. Laminin decreases PRL and IGFBP-1 expression during in vitro decidualization of human endometrial stromal cells. Journal of Cellular Physiology. 1995;163:30–37. doi: 10.1002/jcp.1041630105. [DOI] [PubMed] [Google Scholar]
- Brar A, Kessler CA, Handwerger S. An Ets motif in the proximal decidual prolactin promoter is essential for basal gene expression. J Mol Endocrinol. 2002;29:99–112. doi: 10.1677/jme.0.0290099. [DOI] [PubMed] [Google Scholar]
- Burglin TR. The TEA domain: a novel, highly conserved DNA-binding motif. Cell. 1991;66:11–12. doi: 10.1016/0092-8674(91)90132-i. [DOI] [PubMed] [Google Scholar]
- Casslen BG, Siler-Khodr TM, Harper MJ. Progesterone regulation of prolactin release from human endometrial stromal cells in culture: potential bioassay for progestational activity. Acta Endocrinologica. 1990;122:137–144. doi: 10.1530/acta.0.1220137. [DOI] [PubMed] [Google Scholar]
- Chaudhary S, Tora L, Davidson I. Characterization of a HeLa cell factor which negatively regulates transcriptional activation in vitro by transcriptional enhancer factor-1 (TEF-1) J Biol Chem. 1995;270:3631–3637. doi: 10.1074/jbc.270.8.3631. [DOI] [PubMed] [Google Scholar]
- Chen HH, Maeda T, Mullett SJ, Stewart AF. Transcription cofactor Vgl-2 is required for skeletal muscle differentiation. Genesis. 2004;39:273–279. doi: 10.1002/gene.20055. [DOI] [PubMed] [Google Scholar]
- Daly DC, Maslar IA, Riddick DH. Prolactin production during in vitro decidualization of proliferative endometrium. Am J Obstet Gynecol. 1983;145:672–678. doi: 10.1016/0002-9378(83)90572-0. [DOI] [PubMed] [Google Scholar]
- Doevendans PA, van Bilsen M. Transcription factors and the cardiac gene programme. Int J Biochem Cell Biol. 1996;28:387–403. doi: 10.1016/1357-2725(95)00145-x. [DOI] [PubMed] [Google Scholar]
- Golander A, Barrett J, Hurley T, Barry S, Handwerger S. Failure of bromocriptine, dopamine, and thyrotropin-releasing hormone to affect prolactin secretion by human decidual tissue in vitro. J Clin Endocrinol Metab. 1979;49:787–789. doi: 10.1210/jcem-49-5-787. [DOI] [PubMed] [Google Scholar]
- Gunther S, Mielcarek M, Kruger M, Braun T. VITO-1 is an essential cofactor of TEF1-dependent muscle-specific gene regulation. Nucleic Acids Res. 2004;32:791–802. doi: 10.1093/nar/gkh248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger S, Freemark M. Role of placental lactogen and prolactin in human pregnancy. Adv Exp Med Biol. 1987;219:399–404. doi: 10.1007/978-1-4684-5395-9_19. [DOI] [PubMed] [Google Scholar]
- Jiang SW, Eberhardt NL. TEF-1 transrepression in BeWo cells is mediated through interactions with the TATA-binding protein, TBP. J Biol Chem. 1996;271:9510–9518. doi: 10.1074/jbc.271.16.9510. [DOI] [PubMed] [Google Scholar]
- Jiang SW, Trujillo MA, Sakagashira M, Wilke RA, Eberhardt NL. Novel human TEF-1 isoforms exhibit altered DNA binding and functional properties. Biochemistry. 2000;39:3505–3513. doi: 10.1021/bi991048w. [DOI] [PubMed] [Google Scholar]
- Kaneko KJ, DePamphilis ML. Regulation of gene expression at the beginning of mammalian development and the TEAD family of transcription factors. Dev Genet. 1998;22:43–55. doi: 10.1002/(SICI)1520-6408(1998)22:1<43::AID-DVG5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kelly SE, Bachurski CJ, Burhans MS, Glasser SW. Transcription of the lung-specific surfactant protein C gene is mediated by thyroid transcription factor 1. J Biol Chem. 1996;271:6881–6888. doi: 10.1074/jbc.271.12.6881. [DOI] [PubMed] [Google Scholar]
- Kessler CA, Schroeder JK, Brar AK, Handwerger S. Transcription factor ETS1 is critical for human uterine decidualization. Mol Hum Reprod. 2006;12:71–76. doi: 10.1093/molehr/gal008. [DOI] [PubMed] [Google Scholar]
- Maeda T, Chapman DL, Stewart AF. Mammalian vestigial-like 2, a cofactor of TEF-1 and MEF2 transcription factors that promotes skeletal muscle differentiation. J Biol Chem. 2002;277:48889–48898. doi: 10.1074/jbc.M206858200. [DOI] [PubMed] [Google Scholar]
- Maeda T, Gupta MP, Stewart AF. TEF-1 and MEF2 transcription factors interact to regulate muscle-specific promoters. Biochem Biophys Res Commun. 2002;294:791–797. doi: 10.1016/S0006-291X(02)00556-9. [DOI] [PubMed] [Google Scholar]
- Mahoney WM, Jr, Hong JH, Yaffe MB, Farrance IK. The transcriptional co-activator TAZ interacts differentially with transcriptional enhancer factor-1 (TEF-1) family members. Biochem J. 2005;388:217–225. doi: 10.1042/BJ20041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RG, Brar AK, Frank GR, Hartman SM, Jikihara H. Fibroblast cells from term human deciduas closely resemble endometrial stromal cells: induction of prolactin and insulin- like growth factor binding protein-1 expression. Biology of Reproduction. 1995;52:609–615. doi: 10.1095/biolreprod52.3.609. [DOI] [PubMed] [Google Scholar]
- Shah M, Stanek J, Handwerger S. Differential localization of heat shock proteins 90, 70, 60 and 27 in human decidua and placenta during pregnancy. Histochem J. 1998;30:509–518. doi: 10.1023/a:1003259907014. [DOI] [PubMed] [Google Scholar]
- Telgmann R, Gellersen B. Marker genes of decidualization: activation of the decidual prolactin gene. Hum Reprod Update. 1998;4:472–479. doi: 10.1093/humupd/4.5.472. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kessler CA, Bachurski CJ, Kanda Y, Richardson BD, Stanek J, Handwerger S, Brar AK. Identification of a decidua-specific enhancer on the human prolactin gene with two critical activator protein 1 (AP-1) binding sites. Mol Endocrinol. 2001;15:638–653. doi: 10.1210/mend.15.4.0623. [DOI] [PubMed] [Google Scholar]
- Xiao JH, Davidson I, Matthes H, Garnier JM, Chambon P. Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell. 1991;65:551–568. doi: 10.1016/0092-8674(91)90088-g. [DOI] [PubMed] [Google Scholar]


