Abstract
Antibody production is crucial for a successful vaccine response. Beyond the ability of vitamin A (VA) and its active metabolite, all-trans-retinoic acid (RA) to restore growth in VA-deficient animals, supplementation with VA and/or treatment with RA can augment antibody responses in both VA-deficient and VA-adequate animals. RA alone, and in combination with stimuli that are ligands for the Toll-like receptor family, can augment the adaptive immune response leading to a heightened primary antibody response, and a stronger recall response upon restimulation. Mechanisms may include regulation of cell populations, type 1/type 2 cytokines, and B-cell-related transcription factors, leading to accelerated B cell maturation.
Keywords: antibody, B cell, costimulation, differentiation, polyriboinosinic acid:polyribocytidylic acid, retinoic acid, T-cell dependent antigen, T-cell independent antigen, Toll-like receptor
1. Introduction
Adaptive immunity is characterized by antigen (Ag) specificity, diversity, and immunologic memory, and usually results in more effective immune protection than from innate immunity alone. Understanding the nutritional-hormonal factors that regulate adaptive immune responses is highly important to human health. It is now well demonstrated that provision of an adequate intake of vitamin A (VA) to children aged 6 months to 5 years can reduce all-cause mortality by 23%, measles-related mortality by 50% [1], and diarrhea-related mortality by 23% [2, 3]. The World Health Organization supports vitamin A supplementation as an important, cost-effective strategy to improve child health. For convenience and to reach more children, VA supplements are often delivered at vaccine clinics [1], and thus concurrent treatment with VA and vaccines has become a common practice.
Vitamin A (retinol) is an essential micronutrient required in the diet of all vertebrates [4]. VA is long been known as a requirement for the maintenance of normally differentiated epithelial cells, through the regulation of cell turnover, trafficking and mucin production. Thus, it is likely that some if not most of the clinical benefits attributed to VA supplementation are the result of its effects on the mucosal and systemic immune systems (see [5] and [6] for reviews of epidemiological studies). All-trans-retinoic acid (RA), one of two major metabolites of retinol, is a potent regulator of cell proliferation and differentiation [7], with pleiotropic effects, due to regulation of gene expression, in essentially all organ systems. The genomic actions of RA are mediated by its binding to transcription factors of the RA nuclear receptor family RAR (RARα, β and γ), while 9-cis-RA binds to RXR receptors [7, 8]. RA may also have nongenomic activities through epigenetic mechanisms and via crosstalk with other signaling pathways [9].
2. Could RA be a “fourth signal” in the regulation of the antibody response?
Signals generated by the binding of Ag (signal 1) to its receptors, and costimulatory/accessory molecules (signal 2) to their respective receptors, are well known to be crucial for the development of Ab responses. “Danger signals” (signal 3) [10, 11], now understood to be generated mainly by ligands for Toll-like receptors (TLR) [12], include biological agents such as lipopolysaccharide (LPS), a natural ligand for TLR4, and synthetic compounds such as polyriboinosinic:polyribocytidylic acid (PIC), a ligand for TLR3 [12], or by cytokines produced by antigen-presenting cells (APC) or Ag-activated T cells. The small lipophile, RA, can also have a profound effect on the development, differentiation and immune outcome of B cells. In combination with stimuli like LPS and PIC, RA has the potential to induce a heightened primary response as well as a robust memory response [13, 14]. RA may also accelerate the development of B cells, expanding or modifying the mature B-cell pool available for stimulation [15]. The idea that RA can induce changes that result in a permanent commitment of immune system cells, or “imprint” them, was expressed by Iwata et al. regarding gut-homing T cells [16], but may be more generally applicable. The notion of RA as potentiating a strong and long-lived immune response is consistent with the well established properties of RA as a powerful agent of cell differentiation [7]. Thus, RA could be an important “signal 4,” when RA is present at an adequate concentration during the period of initial B-cell and T-cell stimulation. A signal from RA would be expected to act principally at the nuclear level, through ligation of RAR-RXR receptors, resulting in the induction or repression of critical genes that code for intracellular, plasma membrane-associated, or secreted proteins that, in turn, directly or indirectly modulate the immune response. A fourth signal delivered by RA could provide a final “imprint” or imprimatur on the cells involved, setting them on a course of progression towards a stronger, longer acting, or potentially altered response as compared to that in the absence of RA.
In this review we first discuss studies demonstrating the significance of adequate VA and RA for normal immunity and the ability of RA to augment the Ab response in vivo. We then discuss research using isolated cells that has begun to reveal pathways and processes that are modulated in the presence RA, including class switch recombination (CSR), proliferation, cytokine production, signaling, and B-cell differentiation leading up to the formation of Ab-producing plasmacytes.
3. Vitamin A status, RA, and Ab titers in vivo
3.1 Vitamin A status as a factor in the in vivo antibody response
Studies published in the late 1980s and early 1990s revealed that Ab responses to Ag classified either as T-cell dependent (TD) and T-cell independent-type2 (TI-2) are markedly reduced by VA deficiency (reviewed in [17]). The poor responses could be restored to normal levels relatively rapidly by treatment with VA, indicating the defect was due to a specific lack of this nutrient, while the reversibility also suggested that VA therapy would be useful in VA-deficient populations. In support of this, Semba et al. [18] showed that Indonesian children with xerophthalmia who were supplemented with VA produced a stronger anti-tetanus toxoid (TT) response than children not supplemented with VA. Surprisingly, animal studies designed to compare the response to different types of Ag in VA-deficient rats showed that the IgM response to TI-type 1 (LPS as Ag, given at low immunogenic doses with the Ab response measured as O-saccharide-specific IgM] was not reduced by VA deficiency [19]. Thus, the defect in Ab production was characterized as a dysregulation, in response to some types of Ag, rather than a complete inability to form a strong Ab response, and the requirement for VA for Ab production could therefore be described as conditional, dependent on the antigenic challenge. Although these results were obtained prior to discovery of the TLR family of receptors and an understanding of the ligands that activate them, the idea of “danger signals” had already been proposed [10, 11], which suggested that microbial products like LPS might elicit cytokines or other factors that could have autocrine or paracrine effects on nearby cells. We therefore considered that coimmunization might effectively stimulate not only production of LPS-specific IgM, but also enhance the Ab response to a TD and TI-2 Ag, which otherwise was low in VA-deficient animals. Indeed, coimmunization Pseudomonas aeruginosa LPS (Pa-LPS) and with either pneumococcal polysaccharide, a TI-2 Ag, or TT, a TD Ag, resulted not only antibodies to Pa-LPS but a >3-fold stronger primary response to pneumococcal polysaccharide [19] or TT, both as IgM and IgG [20]. When TT (without LPS) was administered again to elicit a recall Ab response, the secondary anti-TT IgG response was 6-12 times higher compared to animals that had not been treated with LPS at priming [20]. In these studies LPS was not acting simply as a general B-cell mitogen because, despite induction of Ag-specific Ab production, the total plasma Ab concentrations did not rise. These results showed that the potentiation of Ab production by LPS required its presence at the time of priming with the TI-2 or TD Ag, and also illustrated augmentation of class-switched memory response to a TD Ag such as TT. Moreover, similar results were observed in VA-adequate animals [21], suggesting the possibility that VA or RA combined with other stimuli might be an effective means to augment antibody responses even in a well-nourished population. With the great advancement that has taken place in knowledge about the TLR family and its ligands, these results now can be explained as being the result of a danger signal (signal 3) provided by LPS through its engagement with TLR4 on APCs, T cells, or B cells during their activation in response to immunization with TT, that significantly augmented both the primary Ab response and the formation of memory.
3.2 Interactions of RA and TLR pathway ligands
TLR ligands are now of great interest for their potential as vaccine adjuvants (reviewed in [22]), including natural compounds like LPS, a ligand for TLR4; synthetic ligands such as polyriboinosinic:polyribocytidylic acid (PIC), a dsRNA that mimics some of the effects of retroviruses and a ligand for TLR3; flagellin, a ligand for TLR5; and CpG, a mimetic of bacterial DNA and a ligand for TLR9 [12]. Several studies, summarized in Table 1, have demonstrated that RA can synergize with these signals to augment Ab production.
Table 1.
Ab responses in adult rats and mice in vivo, or after stimulation of human peripheral blood mononuclear cells
| Animal or Cell Model | Stimulus/Ligand and TLR receptor | Retinoid | Effect on immune response | Concomitant effects | Reference |
|---|---|---|---|---|---|
| In vivo, VA-deficient or sufficient rat, primary and secondary immunization with TT | LPS; TLR4 | Dietary VA | Elevation in primary anti-TT IgG by LPS;
Elevation in recall anti-TT IgG by LPS, regardless of VA status |
Abrogation of 2/3 of the augmentation by LPS in animals treated with anti-TNFα; augmentation of antibody response by tumor necrosis factor-α alone | [20] |
| In vivo, VA-deficient rat, primary and secondary immunization with TT | PIC; TLR3 | RA | Synergistic elevation in primary anti-TT IgG;
Synergistic elevation in recall anti-TT IgG |
Increased interferon-related signaling molecules (Stat-1, IRF-1)
Reduced IL-12 |
[23] |
| In vivo, VA-adequate rat, primary immunization with TT | PIC; TLR3 | RA | Synergistic elevation in primary anti-TT IgG | Increased number of B cells, MHC class II+ cells, and NK cells; higher IL-10, IL-12, and STAT-1 mRNA | [21] |
| In vivo, VA-adequate mouse, primary and secondary immunization with TT | PIC; TLR3 | RA | Synergistic elevation in primary anti-TT total Ig and IgG1, IgG2a and IgG2b isotypes;
Synergistic elevation in recall anti-TT response for total Ig, IgG1, IgG2a and IgG2b isotypes |
Elevated cytokine production, increased Th1 (PIC) and Th2 (RA and RA+PIC in combination);
Increased costimulatory molecules (CD80 for RA, CD86 for PIC) |
[13] |
| In vitro, human peripheral blood cells | CpG; TLR9 | RA | Increased | Increased B-memory cell proliferation; IL-10 dependent cyclin D expression; increase by RA of the CpG-induced p38MAPK activation | [24] |
4. Possible mechanisms for augmentation of Ab responses in vivo
4.1 Altered cellularity
Normal VA status is required for maintaining the integrity of lymphoid organs and the balance of major lymphocyte populations. A lower proportion of CD4+ T cells and a lower CD4:CD8 T-cell ratio have been noted in studies of children with xerophthalmia [25] as well as in rats fed a VA-marginal diet [26]. VA deficiency also diminished the number of splenic germinal centers (GC), total spleen cells, and spleen and thymus mass [27]. Conversely, RA increased the CD4:CD8 T-cell ratio [14]. The effect of VA on APCs, and particularly dendritic cells (DC), has only recently begun to be elucidated, but evidence indicates that exposure to RA is critical for their functionality. Bone marrow cells cultured in low-VA medium with granulocyte-macrophage colony stimulating factor exhibited reduced DC differentiation, while granulocytes increased, while, conversely, repletion with RA significantly induced the formation of myeloid DC [28]. Furthermore, RA does-dependently increased the tumor necrosis factor-α-induced expression of major histocompatibility complex class II molecules and CD86 on immature Langerhans cell-type DC, suggesting enhanced DC maturation, and RA increased their Ag-presenting capability [29]; these effects were apparently mediated by both RXR-dependent and RARα/RXR-dependent pathways. Hence, Ab production may also be regulated by VA and RA through the promotion of DC maturation and functioning [see references [30], and [31] in this issue].
4.2 Expression of costimulatory molecules
Several studies have suggested that RA could regulate immunity by targeting APCs and costimulatory molecules [32, 33]. RA significantly induced expression of CD11b on murine spleen cells [14] and cultured human monocytic cells [34]. Moreover, in RA-treated TT-immunized mice RA upregulated the expression of CD80, mostly on CD11b+ cells, while PIC mainly increased CD86 on CD11b- cells, suggesting the interesting and unexpected finding that these costimulatory molecules can be modulated individually [13]. Notably, the ratio of CD80/CD86 was positively correlated with the ratio of IL-4:IFN-γ [13]. While the differential regulation of CD80 and CD86 by RA and PIC is not readily explained at this time it has been suggested that CD80 and CD86 are involved in the generation of Th1 and Th2 responses, respectively [35]. The early regulation of CD80 and CD86 molecules by RA combined and PIC, which was apparent within 3 days of priming with TT, might make a significant contribution to the ability of these treatments to rapidly modulate type 1/type2 cytokines [13].
4.3 NK cell-NKT cell ratio
RA may also regulate the numbers and functions of natural killer (NK) cells and natural killer T (NKT cells). Marginal VA deficiency differentially regulated NK and NKT cell populations, significantly reducing NK-cell numbers and NK-cell-mediated cytotoxicity, while increasing the number of NKT cells in the blood of middle-aged and old rats [36]. In young adult mice, RA and PIC differentially regulated NK/NKT-cell populations, observed by 3 days after treatment and priming with Ag [13]. RA was a positive regulator for NKT cells, whereas PIC was a positive regulator for NK cells. The ratio of NKT cells to NK cells was positively correlated with the ratio of IL-4 to IFN-γ mRNA, suggesting that another means by which RA and PIC treatments may regulate type 1/type 2 cytokines occurs through modulation of NK/NKT cell populations [13].
4.4 T-cell responses and the balance of T-helper cells
Several studies have shown that VA deficiency, VA supplementation and treatment with RA alter the balance of Th1/Th2 cell differentiation. VA deficiency was shown to disrupt the balance of Th1/Th2 response, indicated by increased secretion of IFN-γ [37] and higher levels of IL-12 mRNA [38], with reduced secretion of IL-5 and IL-10 [37]. Conversely, RA as well as related synthetic retinoids repressed IFN-γ secretion and the Th1 response, while promoting IL-5 production and the Th2 response [39]. In a model of lupus nephritis, a single administration of RA helped to treat the disease partially while reducing the production of Th1 cytokines, such as IL-2, IL-12 and IFN-γ [40]. The retinoids 9-cis RA and LG69, agonistic ligands of RXR, inhibited IL-12 production by macrophages [33], while RA synergized with LPS in macrophage-like RAW 264.7 cells to augment the production of nitric oxide [41], an agent factor capable of restricting Th1 and promoting Th2 immunity [42]. These and other data [32] suggest that VA might influence Th1/Th2 differentiation through the regulation of cytokine production by APCs as well as T cells.
The combination of RA with other agents, such as PIC, could potentially alter the balance of type1/type 2 cytokine production, while stimulating the adaptive immune response in terms of Ab production. PIC, like dsRNA from retroviruses, activates TLR3-dependent MyD88-independent signaling, triggering NF-κB activation [43, 44], and selectively induces type 1 cytokines, such as IFN-γ and IL-12, while suppressing type 2 cytokines, such as IL-4 and IL-5 [45, 46]. PIC also increased the expression of costimulatory molecules, such as CD80 and CD86, as well as a maturation marker, CD83 in human DC [47]. RA and/or PIC administered to mice at priming with Ag modulated important immune regulators within a few days of immunization. By 3 days after priming with TT, RA and PIC differentially regulated mRNA levels of IFN-γ and IL-4, the signature cytokines of type 1 and type 2 immune responses, respectively [13]. PIC alone significantly induced IFN-γ mRNA, while RA was a positive, albeit modest, regulator for IL-4 but a negative regulator for IFN-γ (Table 2 and [13]). In combination, RA and PIC promoted a higher and more balanced type1/type 2 response, than either alone. Later, 10-12 days after priming, it was evident that RA and/or PIC robustly promoted the primary anti-TT IgG response, as mice treated with RA combined with PIC produced Ab levels that were up to 80-fold higher, while also differentially regulated anti-TT IgG isotypes. Whereas RA combined with PIC synergistically enhanced anti-TT IgG1, this combination attenuated anti-TT IgG2a, as compared to PIC alone. Therefore, RA combined with PIC not only potently stimulated total anti-TT IgG production, but also kept the balance of IgG1/ IgG2a antibodies similar to that in control mice.
Table 2. Differential effects of RA and PIC on T-helper cell factors and Ab production in adult mice 1.
| Factor/indicator | Direction of change by RA | Direction of change by PIC | P value for PIC | |
|---|---|---|---|---|
| Th1/type 1-related: | ||||
| IL-2 | ---- | Increase | ** | |
| IL-12 | Decrease | **2 | Increase | ** |
| IL-12Rβ2 | Decrease | * | Increase | * |
| t-bet | Decrease | * | Increase | |
| IFN-γ | Decrease | ** | Increase | ** |
| Th2/type 2-related: | ||||
| GATA-3 | Increase | * | ||
| IL-4 | Increase | ** | ||
| IL-10 | Increase | ** | ||
| Bcl-2 | Increase | * | ||
| Indicators of Th2 to Th1 balance: | ||||
| IL-4:IFNγ ratio | Increase | ** | ||
| IL-10:IL12 ratio | Increase | * | Increase | * |
| Antibody concentrations (in response to TT immunization) | ||||
| IgM | Increase | ** | ||
| IgG total | Increase | ** | ||
| IgG1 | Increase | ** | Increase | ** |
| IgG2a | Increase | ** | ||
| IgG2b | Increase | ** | ||
| Ratio IgG1 to sum of isotypes | Increase | ** | ||
| Ratio IgG2b to sum of isotypes | Decrease | ** |
Concomitantly, type 1/type 2 cytokine production was significantly regulated by RA and PIC. While PIC was expected to induce type 1 cytokines, it significantly induced both type 1 and type 2 cytokines as well as Th1/Th2-related genes. The enhancement of type 2 cytokines by PIC may be due to its ability to induce IFN-β [48], which has been shown to reduce type 1 cytokines (e.g., IL-12) but increase type 2/regulatory cytokines (e.g., IL-10), as observed in treatment of multiple sclerosis [49]. Thus, PIC was a relatively indiscriminant but potent inducer of immune responses, with very broad inducing effects on both type 1 and type 2 immunity, which correlated well with the increased production of all anti-TT IgG isotypes. Oppositely, RA inhibited type 1 cytokines, confirming previous reports [39, 50], and inhibited Th1-related genes (t-Bet, IRF-1) [13]. Interestingly, this inhibition by RA occurred in the presence of PIC and was strongly correlated with attenuation of the IgG2a response, suggesting that RA abolished part of PIC-induced IgG2a production by suppressing type 1 cytokine expression. Although RA did not significantly induce type 2 cytokines, it consistently suppressed type 1 cytokines and therefore skewed the balance in the type 2 direction [13]. Nevertheless, RA combined with PIC manipulated type 1/ type 2 cytokine expression, which in turn contributed to the enhancement of anti-TT Ab response and directed Ig isotype switching towards a nearly normal balance. Although RA and PIC were administered only at the time of priming, they synergistically increased both the primary and the secondary Ab responses. Thus, a requirement for RA at the time of Ag presentation and initial cell stimulation proved critical and sufficient for the long-term augmentation of the humoral Ab response.
Besides these results for IgG production, the activation of gut-associated DC and T-cells, leading to IgA production, is also augmented by RA; these topics are reviewed by [31] and [51] in this series.
4.5. Augmentation of the TD antibody response in vivo in a neonatal model
Neonates represent a particular challenge regarding Ab production. Neonates are highly susceptible to infectious diseases and, in general, respond poorly to conventional vaccines [52]. Moreover, infants and young children are most susceptible to developing VA deficiency due to the low reserves of VA that are present at birth [53]. The high susceptibility of infants and neonates to infections, and their generally weaker response to vaccination as compared to older children and adults, is mostly attributed to the relative immaturity of the immune system [52, 54, 55].
Based on observing augmented Ab responses in adult mice treated with the combination of RA and PIC, it was hypothesized that the anti-tetanus Ab response of neonatal mice could be enhanced by this combination [14]. Early-life treatments of neonatal mice with RA and/ or PIC were well tolerated, resulting in no reduction in growth rate. TT-specific lymphocyte proliferation and type 1/ type 2 cytokine production were significantly augmented [14]. In addition, RA and PIC modulated the maturation and/ or differentiation of neonatal B cells, NK and NKT cells, and APCs. Whereas RA alone increased neonatal anti-TT Ab response, it selectively increased anti-TT IgG1 and IL-5, resulting in a skewed type 2 response, similar to that observed for IL-4 in adult mice [13]. PIC elevated neonatal anti-TT IgG as well as all IgG isotypes (IgG1, IgG2a, IgG2b). While PIC alone increased the production of TT-specific IFN-γ; it failed to benefit the memory response to TT. The combination of RA and PIC induced a significantly higher level of IFN-γ compared to that in the control group, and resulted in a significantly higher recall anti-TT Ab response when the neonates were reimmunized at 6 weeks of age [14]. Moreover, RA and PIC in combination increased both TT-elicited IFN-γ and IL-5 production by spleen cells ex vivo, suggesting that RA and PIC combined effectively promoted both type 1 and type 2 responses in neonatal mice [14].
Lymphoid organ cellularity, known to be a factor in the low Ab response of neonates, was significantly altered by RA alone or RA combined with PIC. While the B-cell population (B220+ cells) was only slightly reduced in neonatal spleen, neonatal B cells expressed much less CD19 [14], a 95-kDa transmembrane protein known to be as an essential downstream element of B-cell receptor (BCR) signaling required for B-cell maturation, TD Ag-specific Ab responses, and GC formation [56, 57]. Notably, RA and RA combined with PIC, but not PIC alone, up-regulated the percentage and number of CD19+ cells. Treatment of neonatal mice with RA, or RA and PIC in combination, also increased the ratio of CD4 to CD8 T-cells, the percentage of NK cells, and number of NK and NKT cells, while the combination of RA and PIC significantly increased CD11b+CD80+ cells [14]. The defective Ag-presenting ability of neonatal APCs has been strongly associated with the absence of costimulatory molecules, such as CD80 and CD86 [55]. Hence, the up-regulation of CD80 on macrophages suggests that RA and/or PIC may enhance the Ag-presenting capacity of neonatal macrophages, thereby contributing to augmentation of anti-TT lymphocyte responses and Ab production.
Because the lack or immaturity of type 1 responses is thought to be a major contributor to the increased susceptibility of neonates to intracellular pathogens [52], a selective induction of type 2 immunity by RA could be disadvantageous for the development of type 1 responses and cell-mediated immunity in neonates. But compared with either RA or PIC alone, the combination of RA and PIC was more potent in augmenting both primary and secondary anti-TT IgG responses as well as all IgG isotypes [14]. Moreover, the balance of anti-TT IgG1/IgG2a was maintained close to that of vehicle-treated neonates. Furthermore, the combination of RA and PIC significantly increased the production of TT-specific IFN-γ and IL-5, thereby effectively promoting both type 1 and type 2 cytokine responses. Overall, treatment with RA in combination with PIC at the neonatal stage was more effective than either of these agents alone in promoting anti-TT Ab production in infancy, resulting in stimulation of both type 1 and type 2 cytokines, and in a heightened recall response to Ag at the young adult stage. These results suggest that this nutritional-immunological intervention could act as a powerful adjuvant for neonatal vaccination.
5. In vitro studies of B cell activation leading to Ab production
While in vivo models, as discussed above, present an integrated picture, in vitro studies are useful for dissecting the mechanisms involved in B cell proliferation and differentiation in well defined systems (see also [58] in this series). As illustrated in Fig. 1, stimulation of mature B cells by cross-linking of the BCR, mimicking the Ag binding event (signal 1), results in a marked increase in B cell proliferation, represented by increased DNA synthesis and cell division rate [59]. Additionally, significant B cell proliferation is induced by ligation of accessory molecules (signal 2). One such molecules is CD40, a transmembrane protein on the B-cell surface that is activated by the binding of CD40L (CD154) expressed on activated T cells [60]. Another molecule is CD38, a surface-bound protein that functions as an accessory receptor of the BCR, as an ectoenzyme involved in pyridine nucleotide (NAD) metabolism, and as a signal-transducing molecule [61], which, in addition, is inducible by RA in a number of types of cells [62, 63]. Moreover, several cytokines such as IL-4, IL-5, IL-21, and transforming growth factor-β play important roles in the regulation of B-cell proliferation and plasmacyte differentiation [64-67].
Figure 1.
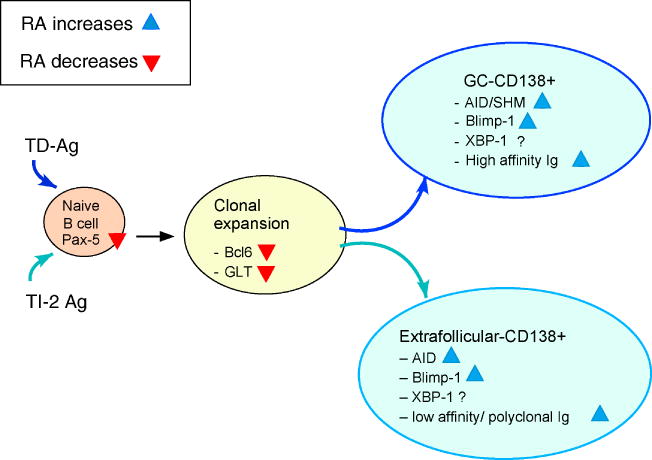
Model of B cell activation by TD and TI/type2 Ag, showing processes in B-cell maturation and differentiation that have been shown to be positively (blue triangles) and negatively (red inverted triangles) by RA. Additional abbreviations: SHM, somatic hypermutation.
5.1 RA and early B cell development
The regulatory effect of RA on B cells starts at the beginning of B cell development. Experiments in C57BL/6 mice showed that RA substantially increased the number of total CD19+ B cells in bone marrow and spleen, while lymphoid progenitors were reduced [15]. In vitro experiments confirmed that the increase of CD19+ cells was accompanied by a shortening of the B cell maturation time, and that the reduced overall yield of B lymphoid progenitors is due to the accelerated maturation of B cells, rather than to a reduction in progenitor proliferation [15]. Associated with the increased number of CD19+ B cells, expression of the transcription factor Pax-5 increased (section 6.5). This agrees with the findings by others that Pax-5 plays a critical role in promoting early B cell development and expansion [68].
5.2 RA and proliferation of mature B cells
Several groups have reported that RA is a strong suppressor of mature B cell proliferation, induced by a variety of stimuli. A reduced rate of cell proliferation does not seem surprising from the viewpoint that RA often slows cell growth, especially of transformed cells [7], but it is somewhat surprising for normal B cells which, after activation by Ag, must undergo clonal expansion and differentiation in the course of becoming plasma cells, and it is especially surprising given the in vivo evidence that RA augments Ab responses, including Ab titers [13, 14, 21] and the number of Ab-secreting cells [13]. Moreover, the reduced rate of proliferation in RA-treated B cells contrasts to the increase in proliferation and cell survival in RA-treated T lymphocytes [69]. The growth inhibitory effect of RA on B cells appears to be mediated through the up-regulation of p27(Kip1), a cyclin inhibitory protein, resulting in arrest of B cells in G0/G1 phase of the cell cycle [70, 71]. In a human tonsillar B cell model, RA decreased anti-CD40 and IL-4-induced B cell proliferation [63]. At the same time, RA increased B cell surface expression of CD38, indicative of B cell differentiation towards the plasma cell phenotype (CD38+/CD20-/IgD-) [63]. These findings agree with experiments in mouse splenic B cells stimulated through the BCR, and/or with anti-CD38, in which RA inhibited B cell proliferation, while at the same time it increased the pool of B-cells expressing IgG1 [72]. These results suggest that although B cell proliferation is a required step for a sufficient immune response, further differentiation to the Ab-producing stage may require the cessation of proliferation, which is promoted by RA.
Nonetheless, not all B cell proliferation is inhibited by RA. Studies of B cells isolated from human peripheral blood and characterized as CD27+ (naïve phenotype) and CD27 negative (memory phenotype) showed that the memory B-cell pool, when stimulated with CpG, a TLR9 ligand, and treated with RA were driven to a higher level of proliferation and were induced to secrete 3-fold more Ig than cells treated with CpG alone [24] (see also [58] in this issue).
5.3 RA and the regulation of germline heavy chain gene transcription and Ig isotype switching
Ig isotype switching is in general a T-cell driven process, regulated by cytokines produced by T-helper cells [73]. Whereas RA alone did not affect Ig heavy chain isotype switching, it enhanced LPS-induced IgA production by splenic B cells when combined with IL-5 [74]. Furthermore, RA strongly inhibited IL-4-dependent Sμ-Sγ1 switch rearrangement necessary for CSR and IgG1 production [74, 75]. Although RA inhibited the response of B cells to BCR ligation and/or to CD38 ligation-triggered γ1-germ line transcript (GLT) expression, it increased the percentage of B cells that expressed surface (s)IgG1, implying differential regulation of proliferation and GLT, versus class switching and productive expression of IgG1 [72]. Whereas the process of chromosomal remodeling and GLT formation is known as a prerequisite event prior to CSR and somatic hypermutation [76] (see also Fig. 1), data from RA-treated B cells suggests that, quantitatively, the level of GLT transcripts that are formed do not necessarily correlate with, or predict, the level of sIgG that will be expressed a few days later. Indeed, the addition of a physiological concentration of RA (10 nM) at the time of stimulation decreased γ1-GLT levels within 1.5 h hours of stimulation, while the same RA-treated cultures expressed more sIgG 5 days later [72, 77]. The marked reduction of germline transcript levels by RA may go along with the decreased proliferation rate of the B cells, which facilitates B cells to move forward for differentiation.
Cloning and analysis of the promoters of the germline transcripts revealed an IL-4 responsive region in murine C γ1 and ε genes, which mediated a strong positive response upon IL-4 stimulation [78]. A detailed study uncovered a specific NF-kB/Rel binding site and STAT6 binding site, which mediated the regulatory effects of anti-CD40 and IL-4 respectively to stimulate germline transcription and CSR of the murine γ1 gene [79, 80]. Furthermore, it was found that NF-IL4, C/EBP family of transcription factors, and NF-kB/p50 complex were also critical for the germline C-ε gene expression [81]. While these studies did not investigate RA, it is known that RA often inhibits NF-kB [32]. It is interesting to note that RA significantly suppressed germline transcript expression induced by several different stimuli [72, 77], suggesting that RA acts on a common pathway.
5.4 RA in the regulation of AID expression
AID is an essential factor for both CSR and somatic hypermutation [82]. AID is thus considered as the Ab diversification enzyme that is crucial for higher organisms to produce a highly specific and potent Ab response. AID expression is induced in mouse splenic B cells activated to undergo class switching in culture, and also in GC B cells in vivo, where B cells undergo somatic hypermutation and probably CSR [83]. The stage-specific expression of AID is controlled transcriptionally and is regulated at least partially by binding of E-protein and Pax5 transcription factors to the promoter region of the AID gene [84, 85]. RA significantly increased AID expression in murine splenic B cells stimulated with a combination of anti-μ, anti-CD40 and IL-4 [77]. Moreover, AID expression occurred nearly exclusively in a B-cell population that is also characterized as the locus of GLT expression, and as having undergone fewer division cycles as indicated by reduced 3H-thymidine incorporation and less dilution of the intracellular vital dye carboxyfluorescein succinimidyl diester. Increased AID expression may be one mechanism by which RA promotes B cell differentiation [77].
5.5 Transcription factors in the regulation of B cell proliferation and differentiation
Along the route of B cell development to plasma cells, many cell signaling events occur which are coordinated together to decide the fate of the cell. Two main groups of factors have been classified based on their activity in promoting B cell proliferation versus plasma cell differentiation, the Pax-5/Bcl-6 group, and the Blimp-1/Xbp-1 group (Fig. 1). Pax-5 is known as a transcription factor that determines B cell lineage differentiation, maintains B cell identity and increases B cell expansion [86]. While Pax-5 is exclusively expressed in the B lineage from the pro-B to the mature B cell stage, its expression is suppressed in plasma cells. Over-expression of Pax-5 can increase the level of AID expression, suggesting a role for Pax-5 in early B cell activation [85]. Bcl-6 is a repressor of transcription that is expressed when B cells are activated [87]. Bcl-6 is abundant in GC B-cells and is required for GC formation, affinity maturation, and CSR [88]. By maintaining adequate levels of Bcl-6, Pax-5 can suppress the plasma cells differentiation [89]. Conversely, Blimp-1 is a transcription factor whose expression is suppressed in early B cell differentiation by several transcription factors such as Pax5 and Bcl-6 [90]. However, Blimp-1 is induced in Ag-stimulated B cells and is required for Ag-dependent terminal differentiation of small groups of cells, such as marginal zone B cells, follicular B cells, and GC B-cells into Ig-secreting plasmablasts and mature plasma cells [91]. Blimp-1 works together with XBP-1, a transcription factor that promotes Ig secretion by coordinating various cellular changes that are needed for the secretory phenotype of plasma cells [92], thereby controlling efficient antibody production. Adequate expression of Pax-5/Bcl-6 and suppression of Blimp-1 level at the early stage of B cell activation ensure efficient B cell proliferation, while the proper shutting off of Pax-5/Bcl-6 and turning on of Blimp-1 expression promote XBP-1 activity that guarantee adequate terminal plasma cell differentiation.
The accelerated development of RA-treated CD19+ B cells was associated with a reduction of Pax-5 expression [15]. In cultured murine splenic B cells stimulated with anti-μ and anti-CD38, RA markedly reduced Pax-5 expression, while at same time RA increased AID and Blimp-1 expression [72]. Among the heterogeneous population of stimulated mouse splenic B cells, RA enriched a B cell subset which was less proliferative, expressed AID and Blimp-1, and had higher level of sIgG1 and CD138 expression, further suggesting that RA plays role in promoting plasma cell differentiation [77]. These findings agree with the theory that some of the characteristics of B cells are division-linked. Overall, B cells in the early phase of their proliferating life are more responsible for the expansion of the B cell pool, which is inhibited by RA, while B cells that have already passed several division cycles and are ready to leave the proliferating pool and proceed to more differentiation stage are promoted by RA via the regulation of transcription factors (Fig. 1).
5.6 RA in the regulation of IgE expression
Oppositely to the augmenting effect on RA on the production of IgG and IgA, RA has been reported to inhibit CD40 and interleukin-4-mediated IgE production in vitro [93]. IgE synthesis was markedly inhibited by RA [93]. Mechanistic studies showed that RA decreases IgE production by regulating the epsilon (ε) GLT expression. These data indicate the importance of B cells in type I allergy and suggest a potential therapeutic role of RA in the treatment of allergy [94, 95].
6. Concluding summary and future challenges
The evidence to date outlines a consistent picture in which physiological concentrations of RA act as part of the regulatory mechanism that controls the development of B cells, and promotes their differentiation in response to various stimuli. These results raise the possibility that RA, as an “imprinting factor,” or signal 4, when combined with either known stimuli, such as TLR ligands, or novel stimuli, in the presence of Ag and accessory molecules, could be very effective in augmenting Ab production, and thus could improve vaccine responses in vivo. Several lines of evidence suggest that RA shifts the balance of certain populations of lymphocytes, including Th1, Th2 and regulatory T cells, NK and NKT cells, and Ag-activated B cells. For B cells, the presence of a physiological concentration of RA at the time of Ag stimulation consistently expanded a population of B cells exhibiting reduced cell proliferation, larger size, enrichment of AID, and, later in the differentiation program, higher expression of surface CD138 and sIg [72, 77]. Future challenges include examining the role of RA in APC activation, the role of RA in GC formation and B-memory cell formation in vivo, and in better understanding the mechanisms by which RA modulates B-cell transcription factors and enzymes, like Pax5 and AID, that are likely to be critical determinants of the Ab response in vivo.
Abbreviations
- Ab
antibody
- Ag
antigen
- AID
activation-induced cytidine deaminase
- APC
antigen-presenting cell
- BCR
B cell antigen receptor
- CSR
class switch recombination
- DC
dendritic cell
- GC
germinal center
- GLT
germ line transcript(ion)
- Ig
immunoglobulin
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- NK
natural killer
- NKT
natural killer T (cells)
- PMBC
peripheral blood mononuclear cells
- PIC
polyriboinosinic:polyribocytidylic acid
- RA
retinoic acid
- RAR
retinoic acid receptor
- RXR
retinoic X receptor
- TD
T-cell dependent
- Th
T helper cell
- TI
T-cell independent
- TLR
Toll-like receptor(s)
- TT
tetanus toxoid
- VA
vitamin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO/UNICEF. Report of a meeting, 12-13 January 1998. UNICEF; New York: 1998. Integration of vitamin A supplementation with immunization: policy and programme implication; pp. 1–7. [Google Scholar]
- 2.Beaton GH, Martorell R, Aronson KA, Edmonston B, McCabe G, Ross AC, et al. Vitamin A supplementation and child morbidity and mortality in developing countries. Food Nutr Bull. 1994;15:282–9. [Google Scholar]
- 3.Fischer Walker CL, Black RE. Micronutrients and diarrheal disease. Clin Inf Dis. 2007;45:S73–7. doi: 10.1086/518152. [DOI] [PubMed] [Google Scholar]
- 4.Ross AC, Harrison EH, Vitamin A. Nutritional Aspects of Retinoids and Carotenoids. In: Zempleni J, Rucker RB, McCormick DB, Suttie JW, editors. Handbook of Vitamins. Taylor & Francis Group; Boca Raton: 2007. pp. 1–40. [Google Scholar]
- 5.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–92. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 6.Villamor E, Fawzi WW. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–64. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 8.Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Bour G, Lalevee S, Rochette-Egly C. Protein kinases and the proteasome join in the combinatorial control of transcription by nuclear retinoic acid receptors. Trends Cell Biol. 2007;17:302–9. doi: 10.1016/j.tcb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 11.Janeway CA, Jr, Goodnow CC, Medzhitov R. Danger - pathogen on the premises! Immunological tolerance. Curr Biol. 1996;6:519–22. doi: 10.1016/s0960-9822(02)00531-6. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 13.Ma Y, Chen Q, Ross AC. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol. 2005;174:7961–9. doi: 10.4049/jimmunol.174.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y, Ross AC. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc Natl Acad Sci U S A. 2005;102:13556–61. doi: 10.1073/pnas.0506438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Esplin BL, Garrett KP, Welner RS, Webb CF, Kincade PW. Retinoids accelerate B lineage lymphoid differentiation. J Immunol. 2008;180:138–45. doi: 10.4049/jimmunol.180.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Ross AC. Vitamin A deficiency and retinoid repletion regulate the antibody response to bacterial antigens and the maintenance of natural killer cells. Clin Immunol Immunopathol. 1996;80:S63–S72. doi: 10.1006/clin.1996.0143. [DOI] [PubMed] [Google Scholar]
- 18.Semba RD, Muhilal, Scott AL, Natadisastra G, West KP, Jr, Sommer A. Effect of vitamin A supplementation on immunoglobulin G subclass responses to tetanus toxoid in children. Clin Diagn Lab Immunol. 1994;1:172–5. doi: 10.1128/cdli.1.2.172-175.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasatiempo AM, Kinoshita M, Foulke DT, Ross AC. The antibody response of vitamin A-deficient rats to pneumococcal polysaccharide is enhanced through coimmunization with lipopolysaccharide. J Infect Dis. 1994;169:441–4. doi: 10.1093/infdis/169.2.441. [DOI] [PubMed] [Google Scholar]
- 20.Arora D, Ross AC. Antibody response against tetanus toxoid is enhanced by lipopolysaccharide or tumor necrosis factor-alpha in vitamin A-sufficient and -deficient rats. Am J Clin Nutr. 1994;59:922–8. doi: 10.1093/ajcn/59.4.922. [DOI] [PubMed] [Google Scholar]
- 21.DeCicco KL, Youngdahl JD, Ross AC. All-trans-retinoic acid and polyriboinosinic:polyribocytidylic acid in combination potentiate specific antibody production and cell-mediated immunity. Immunology. 2001;104:341–8. doi: 10.1046/j.1365-2567.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov Today. 2003;8:934–43. doi: 10.1016/s1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]
- 23.DeCicco KL, Zolfaghari R, Li N, Ross AC. Retinoic acid and polyriboinosinic acid act synergistically to enhance the antibody response to tetanus toxoid during vitamin A deficiency: possible involvement of interleukin-2 receptor-beta, signal transducer and activator of transcription-1, and interferon regulatory factor-1. J Infect Dis. 2000;182 1:S29–36. doi: 10.1086/315908. [DOI] [PubMed] [Google Scholar]
- 24.Ertesvag A, Aasheim HC, Naderi S, Blomhoff HK. Vitamin A potentiates CpG-mediated memory B-cell proliferation and differentiation: involvement of early activation of p38MAPK. Blood. 2007;109:3865–72. doi: 10.1182/blood-2006-09-046748. [DOI] [PubMed] [Google Scholar]
- 25.Semba RD, Muhilal, Ward BJ, Griffin DE, Scott AL, Natadisastra G, et al. Abnormal T-cell subset proportions in vitamin-A-deficient children. Lancet. 1993;341:5–8. doi: 10.1016/0140-6736(93)92478-c. [DOI] [PubMed] [Google Scholar]
- 26.Dawson HD, Ross AC. Chronic marginal vitamin A status affects the distribution and function of T cells and natural T cells in aging Lewis rats. J Nutr. 1999;129:1782–90. doi: 10.1093/jn/129.10.1782. [DOI] [PubMed] [Google Scholar]
- 27.Ross AC. Retinoids and the immune system. In: Sporn MB, Roberts AB, Goodman DS, editors. THE RETINOIDS: Biology, Chemistry, and Medicine. 2nd. 1994. pp. 521–43. [Google Scholar]
- 28.Hengesbach LM, Hoag KA. Physiological concentrations of retinoic acid favor myeloid dendritic cell development over granulocyte development in cultures of bone marrow cells from mice. J Nutr. 2004;134:2653–9. doi: 10.1093/jn/134.10.2653. [DOI] [PubMed] [Google Scholar]
- 29.Geissmann F, Revy P, Brousse N, Lepelletier Y, Folli C, Durandy A, et al. Retinoids regulate survival and antigen presentation by immature dendritic cells. J Exp Med. 2003;198:623–34. doi: 10.1084/jem.20030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulendran B. Sem Immunol. 2009 [Google Scholar]
- 31.Iwata M. Sem Immunol. 2009 [Google Scholar]
- 32.Na SY, Kang BY, Chung SW, Han SJ, Ma X, Trinchieri G, et al. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB. J Biol Chem. 1999;274:7674–80. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- 33.Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J Nutr. 2002;132:3736–9. doi: 10.1093/jn/132.12.3736. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q, Ma Y, Ross AC. Opposing cytokine-specific effects of all trans-retinoic acid on the activation and expression of signal transducer and activator of transcription (STAT)-1 in THP-1 cells. Immunology. 2002;107:199–208. doi: 10.1046/j.1365-2567.2002.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 36.Dawson HD, Ross AC. Chronic marginal vitamin A status affects the distribution and function of T cells and natural T calls in aging Lewis rats. J Nutr. 1999;129:1782–90. doi: 10.1093/jn/129.10.1782. [DOI] [PubMed] [Google Scholar]
- 37.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152:1515–22. [PubMed] [Google Scholar]
- 38.DeCicco KL, Ross AC. All-trans-retinoic acid and polyriboinosinoic:polyribocytidylic acid cooperate to elevate anti-tetanus immunoglobulin G and immunoglobulin M responses in vitamin A-deficient Lewis rats and Balb/c mice. Proc Nutr Soc. 2000;59:519–29. doi: 10.1017/s0029665100000756. [DOI] [PubMed] [Google Scholar]
- 39.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15:1017–25. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 40.Nozaki Y, Yamagata T, Yoo BS, Sugiyama M, Ikoma S, Kinoshita K, et al. The beneficial effects of treatment with all-trans-retinoic acid plus corticosteroid on autoimmune nephritis in NZB/WF mice. Clin Exp Immunol. 2005;139:74–83. doi: 10.1111/j.1365-2249.2005.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Austenaa LM, Ross AC. Potentiation of interferon-gamma-stimulated nitric oxide production by retinoic acid in RAW 264.7 cells. J Leukoc Biol. 2001;70:121–9. [PubMed] [Google Scholar]
- 42.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 43.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 44.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 45.Avramidis N, Victoratos P, Yiangou M, Hadjipetrou-Kourounakis L. Adjuvant regulation of cytokine profile and antibody isotype of immune responses to Mycoplasma agalactiae in mice. Vet Microbiol. 2002;88:325–38. doi: 10.1016/s0378-1135(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 46.Manetti R, Annunziato F, Tomasevic L, Gianno V, Parronchi P, Romagnani S, et al. Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-alpha and interleukin-12. Eur J Immunol. 1995;25:2656–60. doi: 10.1002/eji.1830250938. [DOI] [PubMed] [Google Scholar]
- 47.Verdijk RM, Mutis T, Esendam B, Kamp J, Melief CJ, Brand A, et al. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163:57–61. [PubMed] [Google Scholar]
- 48.Lepe-Zuniga JL, Rotbein J, Gutterman JU. Production of interferon-alpha induced by dsRNA in human peripheral blood mononuclear cell cultures: role of priming by dsRNA-induced interferons-gamma and -beta. J Interferon Res. 1989;9:445–56. doi: 10.1089/jir.1989.9.445. [DOI] [PubMed] [Google Scholar]
- 49.Huang YM, Adikari S, Bave U, Sanna A, Alm G. Multiple sclerosis: interferon-beta induces CD123(+)BDCA2- dendritic cells that produce IL-6 and IL-10 and have no enhanced type I interferon production. J Neuroimmunol. 2005;158:204–12. doi: 10.1016/j.jneuroim.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Cantorna MT, Nashold FE, Chun TY, Hayes CE. Vitamin A down-regulation of IFN-gamma synthesis in cloned mouse Th1 lymphocytes depends on the CD28 costimulatory pathway. J Immunol. 1996;156:2674–9. [PubMed] [Google Scholar]
- 51.Andrian UHv. Sem Immunol. 2009 [Google Scholar]
- 52.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 53.Ross AC. Introduction to vitamin A: a nutritional and life cycle perspective. In: Packer L, Obermüller-Jevic U, Kraemer K, Sies H, editors. Carotenoids and Retinoids. Molecular Aspects and Health Issues. AOCS Press; Champaign, IL: 2005. pp. 23–41. [Google Scholar]
- 54.Levin D, Gershon H. Antigen presentation by neonatal murine spleen cells. Cell Immunol. 1989;120:132–44. doi: 10.1016/0008-8749(89)90181-0. [DOI] [PubMed] [Google Scholar]
- 55.Muthukkumar S, Goldstein J, Stein KE. The ability of B cells and dendritic cells to present antigen increases during ontogeny. J Immunol. 2000;165:4803–13. doi: 10.4049/jimmunol.165.9.4803. [DOI] [PubMed] [Google Scholar]
- 56.Otero DC, Anzelon AN, Rickert RC. CD19 function in early and late B cell development: I. Maintenance of follicular and marginal zone B cells requires CD19-dependent survival signals. J Immunol. 2003;170:73–83. doi: 10.4049/jimmunol.170.1.73. [DOI] [PubMed] [Google Scholar]
- 57.Otero DC, Rickert RC. CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition. J Immunol. 2003;171:5921–30. doi: 10.4049/jimmunol.171.11.5921. [DOI] [PubMed] [Google Scholar]
- 58.Blomhoff HK. Sem Immunol. 2009 [Google Scholar]
- 59.DeFranco AL. B-cell activation 2000. Immunol Rev. 2000;176:5–9. [PubMed] [Google Scholar]
- 60.Bishop GA, Hostager BS. Signaling by CD40 and its mimics in B cell activation. Immunol Res. 2001;24:97–109. doi: 10.1385/IR:24:2:097. [DOI] [PubMed] [Google Scholar]
- 61.Lund FE. Signaling properties of CD38 in the mouse immune system: enzyme-dependent and -independent roles in immunity. Mol Med. 2006;12:328–33. doi: 10.2119/2006-00099.Lund. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao Y, Camacho LH, Mehta K. Retinoic acid-induced CD38 antigen promotes leukemia cells attachment and interferon-gamma/interleukin-1beta-dependent apoptosis of endothelial cells: implications in the etiology of retinoic acid syndrome. Leuk Res. 2007;31:455–63. doi: 10.1016/j.leukres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Morikawa K, Nonaka M. All-trans-retinoic acid accelerates the differentiation of human B lymphocytes maturing into plasma cells. Int Immunopharmacol. 2005;5:1830–8. doi: 10.1016/j.intimp.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Ohtsuka Y, Sanderson IR. Transforming growth factor-beta: an important cytokine in the mucosal immune response. Curr Opin Gastroenterol. 2000;16:541–5. doi: 10.1097/00001574-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Park JY, Yoon SH, Kim EK, Yun SO, Park MY, Sohn HJ, et al. A membrane-bound form of IL-4 enhances proliferation and antigen presentation of CD40-activated human B cells. Immunol Lett. 2008;116:33–40. doi: 10.1016/j.imlet.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 66.Moon BG, Yoshida T, Shiiba M, Nakao K, Katsuki M, Takaki S, et al. Functional dissection of the cytoplasmic subregions of the interleukin-5 receptor alpha chain in growth and immunoglobulin G1 switch recombination of B cells. Immunology. 2001;102:289–300. doi: 10.1046/j.1365-2567.2001.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007;179:5886–96. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 68.Nera KP, Lassila O. Pax5--a critical inhibitor of plasma cell fate. Scand J Immunol. 2006;64:190–9. doi: 10.1111/j.1365-3083.2006.01809.x. [DOI] [PubMed] [Google Scholar]
- 69.Engedal N, Gjevik T, Blomhoff R, Blomhoff HK. All-trans retinoic acid stimulates IL-2-mediated proliferation of human T lymphocytes: early induction of cyclin D3. J Immunol. 2006;177:2851–61. doi: 10.4049/jimmunol.177.5.2851. [DOI] [PubMed] [Google Scholar]
- 70.Naderi S, Blomhoff HK. Retinoic acid prevents phosphorylation of pRB in normal human B lymphocytes: regulation of cyclin E, cyclin A, and p21(Cip1) Blood. 1999;94:1348–58. [PubMed] [Google Scholar]
- 71.Guidoboni M, Zancai P, Cariati R, Rizzo S, Dal Col J, Pavan A, et al. Retinoic acid inhibits the proliferative response induced by CD40 activation and interleukin-4 in mantle cell lymphoma. Cancer Res. 2005;65:587–95. [PubMed] [Google Scholar]
- 72.Chen Q, Ross AC. Vitamin A and immune function: retinoic acid modulates population dynamics in antigen receptor and CD38-stimulated splenic B cells. Proc Natl Acad Sci U S A. 2005;102:14142–9. doi: 10.1073/pnas.0505018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 74.Tokuyama H, Tokuyama Y. The regulatory effects of all-trans-retinoic acid on isotype switching: retinoic acid induces IgA switch rearrangement in cooperation with IL-5 and inhibits IgG1 switching. Cell Immunol. 1999;192:41–7. doi: 10.1006/cimm.1998.1438. [DOI] [PubMed] [Google Scholar]
- 75.Tokuyama Y, Tokuyama H. Retinoids as Ig isotype-switch modulators. The role of retinoids in directing isotype switching to IgA and IgG1 (IgE) in association with IL-4 and IL-5. Cell Immunol. 1996;170:230–4. doi: 10.1006/cimm.1996.0156. [DOI] [PubMed] [Google Scholar]
- 76.Lee CG, Kinoshita K, Arudchandran A, Cerritelli SM, Crouch RJ, Honjo T. Quantitative regulation of class switch recombination by switch region transcription. J Exp Med. 2001;194:365–74. doi: 10.1084/jem.194.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Q, Ross AC. Retinoic acid promotes mouse splenic B cell surface IgG expression and maturation stimulated by CD40 and IL-4. Cell Immunol. 2007;249:37–45. doi: 10.1016/j.cellimm.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu MZ, Stavnezer J. Regulation of transcription of immunoglobulin germ-line gamma 1 RNA: analysis of the promoter/enhancer. EMBO J. 1992;11:145–55. doi: 10.1002/j.1460-2075.1992.tb05037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cunningham K, Ackerly H, Alt F, Dunnick W. Potential regulatory elements for germline transcription in or near murine Sgamma1. Int Immunol. 1998;10:527–36. doi: 10.1093/intimm/10.4.527. [DOI] [PubMed] [Google Scholar]
- 80.Lin SC, Stavnezer J. Activation of NF-kappaB/Rel by CD40 engagement induces the mouse germ line immunoglobulin Cgamma1 promoter. Mol Cell Biol. 1996;16:4591–603. doi: 10.1128/mcb.16.9.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delphin S, Stavnezer J. Regulation of antibody class switching to IgE: characterization of an IL-4-responsive region in the immunoglobulin heavy-chain germline epsilon promoter. Ann N Y Acad Sci. 1995;764:123–35. doi: 10.1111/j.1749-6632.1995.tb55815.x. [DOI] [PubMed] [Google Scholar]
- 82.Xu Z, Pone EJ, Al-Qahtani A, Park SR, Zan H, Casali P. Regulation of aicda expression and AID activity: relevance to somatic hypermutation and class switch DNA recombination. Crit Rev Immunol. 2007;27:367–97. doi: 10.1615/critrevimmunol.v27.i4.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cattoretti G, Buttner M, Shaknovich R, Kremmer E, Alobeid B, Niedobitek G. Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells. Blood. 2006;107:3967–75. doi: 10.1182/blood-2005-10-4170. [DOI] [PubMed] [Google Scholar]
- 84.Sayegh CE, Quong MW, Agata Y, Murre C. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat Immunol. 2003;4:586–93. doi: 10.1038/ni923. [DOI] [PubMed] [Google Scholar]
- 85.Gonda H, Sugai M, Nambu Y, Katakai T, Agata Y, Mori KJ, et al. The balance between Pax5 and Id2 activities is the key to AID gene expression. J Exp Med. 2003;198:1427–37. doi: 10.1084/jem.20030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Delogu A, Schebesta A, Sun Q, Aschenbrenner K, Perlot T, Busslinger M. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 2006;24:269–81. doi: 10.1016/j.immuni.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 87.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 88.Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997;16:161–70. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- 89.Nera KP, Kohonen P, Narvi E, Peippo A, Mustonen L, Terho P, et al. Loss of Pax5 promotes plasma cell differentiation. Immunity. 2006;24:283–93. doi: 10.1016/j.immuni.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 90.Calame K. Activation-dependent induction of Blimp-1. Curr Opin Immunol. 2008;20:259–64. doi: 10.1016/j.coi.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 91.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–20. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 92.Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 93.Worm M, Krah JM, Manz RA, Henz BM. Retinoic acid inhibits CD40 + interleukin-4-mediated IgE production in vitro. Blood. 1998;92:1713–20. [PubMed] [Google Scholar]
- 94.Scheffel F, Heine G, Henz BM, Worm M. Retinoic acid inhibits CD40 plus IL-4 mediated IgE production through alterations of sCD23, sCD54 and IL-6 production. Inflamm Res. 2005;54:113–8. doi: 10.1007/s00011-004-1331-8. [DOI] [PubMed] [Google Scholar]
- 95.Worm M, Herz U, Krah JM, Renz H, Henz BM. Effects of retinoids on in vitro and in vivo IgE production. Int Arch Allergy Immunol. 2001;124:233–6. doi: 10.1159/000053721. [DOI] [PubMed] [Google Scholar]


