Abstract
Purpose
Long-term effects of treatment with 9-cis-retinyl acetate (9-cis-R-Ac), an artificial retinoid prodrug, were tested on changes in rod and cone visual functions in mice.
Methods
The acetyl ester of the functional geometric chromophore 9- cis-retinal was delivered by oral gavage to C57BL/6 female mice. In initial experiments, 10-month-old mice were employed for the single treatment with 9-cis-R-Ac or the control vehicle. In long-term experiments, 4-month-old mice were treated with 9-cis-R-Ac monthly for 6 and 10 months. Photoreceptor status was evaluated by various electroretinographic (ERG) techniques, retinoid analyses, and retinal morphology. Opsin, the predicted target of oxidized 9-cis-R-Ac, was purified and its chromophore characterized.
Results
As compared to 4-month-old mice, age-related changes observed in vehicle treated mice at 10 months of age included a progressive decline in ERG responses, such as a decreased rate of dark adaptation, and a lowered rhodopsin/opsin ratio. Administration of 9-cis-R-Ac increased the rhodopsin regeneration ratio, and improved ERG responses, dark adaptation. Compared to vehicle treated controls, 10- and 14-month-old mice treated monthly with 9-cis-R-Ac for 6 or 10 months exhibited improved dark adaptation. For 14-month-old mice treated monthly we observed changes in the expression of retina-specific genes in the eye by mRNA expression profiling, but no significant differences in gene expression were detected in the liver and kidney.
Conclusions
Deteriorating photoreceptor function documented in mice at 10 and 14 versus 4 months of age was improved significantly by long-term, monthly administration of 9-cis-R-Ac. These findings suggest a potential therapeutic approach to prevent age-related retinal dysfunction.
Keywords: rhodopsin, aging, vision, chromophore, phototransduction, retina
INTRODUCTION
To understand why human vision declines with age, much research has focused on the retina, the layer of rod and cone photoreceptors cells that convert light into electrical signals. Studies in mice have shown that age-related decreases in retinal rod cell function cannot be explained by rod cell loss, abnormal retinal plasticity or any signs of retinal disease 1–3. But Jackson and colleagues have reported a dramatic slowing of rod-mediated dark adaptation after light exposure associated with human aging that was related to delayed regeneration of rhodopsin 1.
Inadequate availability and/or processing of vitamin A to the visual chromophore, 11-cis-retinal (reviewed in Refs. 4, 5), can adversely affect vertebrate rhodopsin regeneration and visual transduction (reviewed in Refs. 6–8). As in aging, rhodopsin regeneration after light exposure is more delayed in humans and mice with vitamin A deficiency due either to inadequate dietary intake or intestinal absorption 7. Moreover, treatment with vitamin A and its derivatives may have beneficial effects in aging 9 and retinal diseases such as Sorsby’s fundus dystrophy 9 and retinitis pigmentosa 10.
Retinoid absorption, storage and recycling after bleaching of retinal pigments is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) 11–14 and a null mutation in the human LRAT gene results in early-onset rod-cone dystrophy 15. The latter resembles a form of human Leber’s congenital amaurosis (LCA) in which disabling mutations in the retinal pigment epithelium-specific 65 kDa (RPE65) gene also cause severe rod and cone photoreceptor dysfunction 15. LCA patients carrying mutations in both LRAT and RPE65 genes, like Lrat−/− and Rpe65−/− knockout mice, may lack 11-cis-retinal and rhodopsin, manifest abnormalities in all-trans-retinyl ester levels within RPE cells, show severe impairment of rod and cone photoreceptor functions and exhibit retinal degeneration 11, 12, 16–18.
We previously reported that the biochemical defects causing LCA-like symptoms in Lrat−/− and Rpe65−/− knockout mice could be bypassed by oral gavage with 9-cis-retinal. This treatment resulted in preserved retinal morphology and recovery of normal rod function as assessed by single cell recordings and ERG measurements 12, 17, 18. 9-cis-Retinal forms photoactive isorhodopsin which, when bleached, undergoes conformational changes via the same photoproducts as does rhodopsin that is naturally regenerated from 11-cis-retinal 19. Others have reported that 11-cis-retinal given by intraperitoneal injection also improved vision in Rpe65−/− mice 20.
Recently we found that gastric gavage with a more chemically stable compound than either 9-cis- or 11-cis-retinal, i.e. 9-cis-retinyl acetate (9-cis-R-Ac), produced the same beneficial effects as 9-cis-retinal in Lrat−/− mice 12. Now we report that monthly gavage with 9-cis-R-Ac significantly improved age-related deteriorating photoreceptor function in mice. This compound may have therapeutic potential for humans as well.
METHODS
Animals
Pigmented age-matched C57BL/6 female mice obtained from Charles River Laboratories were maintained on a normal diet in complete darkness or on a 12-hr light/dark cycle. All animal experiments involved procedures approved by the University of Washington and Case Western Reserve University Animal Care Committees and conformed to recommendations of the American Veterinary Medical Association Panel on Euthanasia and the Association of Research for Vision and Ophthalmology.
Single dose and long-term treatment of mice with 9-cis-R-Ac
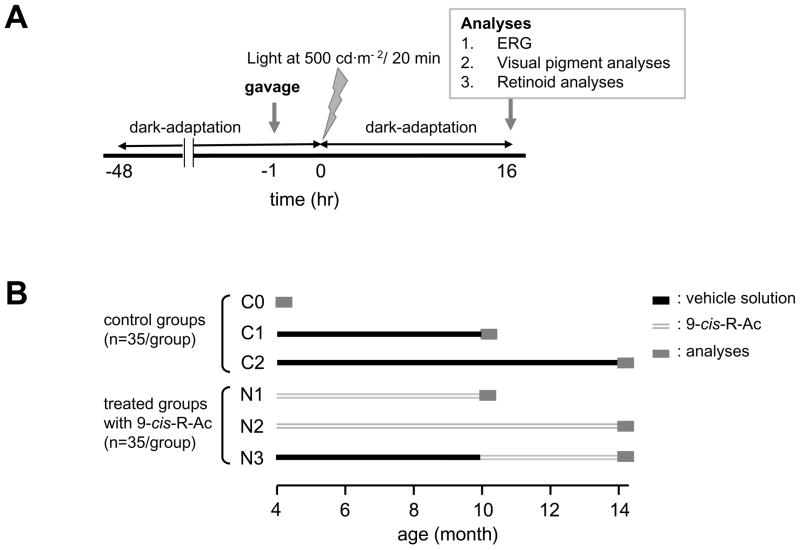
9-cis-R-Ac was prepared as previously described 12, 13. A dose of ~80 mg/kg body weight of 9-cis-R-Ac in 150 μl vegetable oil was administered by gavage to each treated animal. Prior to the single dose experiments (Fig. 1A), 10-month-old mice were dark-adapted for more than 48 hr, gavaged with 9-cis-R-Ac or vehicle control solution 1 hr before bleaching, exposed to light at 500 cd·m−2 for 20 min and dark-adapted for 16 hr before analyses.
Fig. 1. Experimental protocol for single dose treatment of 10-month-old mice with 9-cis-R-Ac and timelines for multiple dose long-term treatment regimes.
(A) Fully dark-adapted (48 hr) 10-month-old mice were gavaged with 9-cis-R-Ac(~80 mg/kg body weight) or control vehicle vegetable oil solution. One hr after gavage, mice were exposed to continuous strong light at 500 cd·m−2 for 20 min (~90% rhodopsin bleach) followed by 16 hr of dark adaptation. Mice then were examined by ERG and eyes were analyzed for rhodopsin and retinoid content. ERGs also were recorded prior to the 9-cis-R-Ac treatment. (B)Mice were gavaged monthly with 9 -cis-R-Ac (~80 mg/kg body weight) or control vehicle solution for 6 or 10 months as described in Methods.
For the long-term treatment studies, mice obtained at 3 months of age were maintained until 4 months of age before experiments were initiated. Then they were gavaged with 9-cis-R-Ac or vehicle solution once a month for different periods. Six groups of mice were studied (Fig. 1B). The first 3 groups (C0, C1 and C2, total n=35 for each group) were treated with vehicle as controls and tested at 4, 10 and 14 months of age. The other 3 groups (N1, N2 and N3, n=35) were gavaged with 9-cis-R-Ac. Group N1 was gavaged for 6 months and tested at 10 months of age. Group N2 was gavaged with 9-cis-R-Ac for 10 months and tested at 14 months of age. Group N3, was gavaged with vehicle until 10 months of age and then received 9-cis-R-Ac monthly until testing at 14 months of age. In another control group not shown in Fig. 1B, 10 mice were gavaged with ~80 mg/kg body weight of all-trans-R-Ac (Sigma-Aldrich, Corp.), a functional vitamin A analog, for 10 months and tested at 14 months of age.
Two weeks after the last gavage with either, 9-cis-R-Ac, all-trans-R-Ac or vehicle, groups of 48 hr dark-adapted mice were tested for single flash ERG and recovery of dark adaptation after light exposure, rhodopsin/opsin and retinoids in the eye and retinal morphology. Mice were exposed to light of intensity 500 cd·m−2 that bleached ~ 75 % of rhodopsin, anesthetized and monitored by ERG for one hr to evaluate recovery of dark adaptation.
Electroretinography (ERG)
ERGs of anesthetized mice were recorded as previously reported 21, 22. Briefly, mice were dark-adapted overnight prior to recording. Then they were anesthetized under a safety light by an intraperitoneal injection of 20 μl/g body weight of 6 mg/ml ketamine and 0.44 mg/ml xylazine diluted with 10 mM sodium phosphate, pH 7.2, containing 100 mM NaCl. Pupils were dilated with 1% tropicamide. A contact lens electrode was placed on the eye and a reference electrode and ground electrode were positioned on the ear and tail. ERGs were recorded with the universal testing and electrophysiologic system UTAS E-3000 (LKC Technologies, Inc.).
Single-flash recording
Mice were exposed to a series of white light flash stimuli at a range of intensities (−3.7–2.8 log cd·s·m−2) with each flash duration (from 20 μs to 1 ms) adjusted according to the selected intensity. Two to five recordings were made at sufficient intervals (from 10 s to 10 min) between flash stimuli that allowed mice to recover. Light-adapted ERG responses were recorded after bleaching at 1.4 log cd·m−2 for 15 min. Typically, four to eight animals were used to generate each data point under all conditions. Leading edges of the ERG responses were fitted with a model of rod photoreceptor activation 17.
Dark adaptation after intense constant illumination
Mice were dark-adapted overnight and then bleached by the background light of a Ganzfeld chamber (500 cd·m−2) for 3 min. After the light was turned off, mice were exposed to a single-flash ERG at −0.2 log cd·s·m−2 and recovery of a-wave amplitude was monitored every 5 min for 60 min. The recovery ratio was calculated by normalizing single flash a-wave amplitude responses at various times following bleaching to WT dark-adapted a-wave responses at the identical flash intensity (− 0.2 log cd·s·m−2).
Purification of rhodopsin
Rhodopsin was purified under dim red light as previously described 23. Purified anti-rhodopsin C terminus antibody 1D4 24 was immobilized on CNBr-activated Sepharose 4B and a 4.6 × 12-mm column was packed with 2 mg of 1D4 antibody/ml of Sepharose beads. Mouse whole eyes in 137 mM NaCl, 5.4 mM Na2HPO4, (pH 7.5) were homogenized with a glass-to-glass 2.7 mM KCl and 1.8 mM KH2PO4 homogenizer. After centrifuging the homogenate at 14,000g for 5 min, the pellet was solubilized in buffer containing 1% dodecyl-β-maltoside in 10 mM Bis-Tris propane (pH 7.5) containing 500 mM NaCl. This solution was clarified by centrifugation at 125,000g for 20 min and the supernatant was loaded onto an antibody 1D4-packed immunoaffinity column which then was thoroughly washed at a flow rate of 0.5 ml/min with 10 mM Bis-Tris propane (pH 7.5) containing 500 mM NaCl and 0.1% dodecyl-β-maltoside. Purified mouse rhodopsin was eluted with 100 μM nonapeptide (TETSQVAPA) in 10 mM Bis-Tris propane (pH 7.5) containing 500 mM NaCl and 0.1% dodecyl-β-maltoside at room temperature. The purified rhodopsin concentration was determined at 500 nm and the total amount of opsin and rhodopsin at 280 nm with a Hewlett-Packard 8452A UV-visible spectrophotometer. 25 The purity of eluted rhodopsin was evaluated by SDS-PAGE. Rhodopsin and isorhodopsin coeluted from the immunoaffinity 1D4-column. The vast majority of opsin molecules were regenerated with 11-cis-retinal and less than 10% were regenerated with 9-cis-retinal under these experimental conditions. Therefore, the concentration of isorhodopsin which has a spectrum and absorption maximum similar to rhodopsin was not sufficient to shift the spectrum of this rhodopsin/isorhodopsin mixture dominated by rhodopsin. To confirm whether 9-cis-retinal regenerated opsin as isorhodopsin, we extracted retinoids from purified visual pigment and definitely detected 9-cis-retinal. This result demonstrates that both 11-cis-retinal and 9-cis-retinal recombined with opsin.
Retinoid analyses
All experimental procedures related to extraction, derivatization and separation of retinoids were carried out under dim red light provided by a Kodak No. 1 safelight filter (transmittance >560 nm) as described previously 17, 18, 21, 26. Eluted fractions of purified rhodopsin from 6 mouse eyes were combined (total 3.0 ml) and mixed with an equal volume of 100% methanol. The mixture then was vortexed and incubated on ice for 15 min. Retinoids were extracted twice with an equal volume of 100% hexane (6 ml total). The combined extracts were dried under argon and retinoids were separated by normal phase HPLC (Beckman, Ultrasphere-Si, 5 μm, 4.6 × 250 mm) with 10% ethyl acetate and 90% hexane at a flow rate of 1.4 ml/min and detected at 325 nm by an HP1100 HPLC with a diode array detector and HP Chemstation A.03.03 software. A2E was analyzed as previously described 21.
Analysis of retinoic acid in the liver was carried out as described before 12 with an Agilent 1100 HPLC and two tandem normal phase columns: a Varian Microsorb Silica 3 μm, 4.6 × 100 mm (Varian, Palo Alto, CA) and an Ultrasphere-Si, 5 μm, 4.6 × 250 mm column. An isocratic solvent system of 1000:4.3:0.675 hexane:2-propanol:glacial acetic acid (v/v) was used for elution at a flow rate of 1 ml/min at 20 °C with detection at 355 nm. Calibration was done with standards of all-trans-RA and 9-cis-RA purchased from Sigma-Aldrich.
Immunoblotting
Immunoblotting was done according to standard protocols using Immobilon-P to adsorb proteins (polyvinylidene difluoride; Millipore Corp.). Monoclonal anti-rhodopsin antibody (1D4) was provided by Dr. R. Molday. The anti-LRAT (mAb) 27, anti-transducin (Gt) (mAb) (unpublished), anti-guanylate cyclase 1 (1S4, mAb) 28, anti-guanylate cyclase-activating protein 1 (UW14 pAb) 29, anti-guanylate cyclase-activating protein 2 (UW50 pAb) 30, anti-rhodopsin kinase 31, and anti-retinol dehydrogenase 12 (pAb) 32 were generated in our laboratory. Alkaline phosphatase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG (Promega) were used as secondary antibodies. Protein bands were visualized with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium color development substrate (Promega). Proteins (30 μg per each well) were separated by 12.5% SDS-PAGE.
Retinal Morphology
For light microscopy, mouse eyecups were fixed with 2.5% glutaraldehyde and 1.6% paraformaldehyde in 0.08 M 1,4-piperazinediethanesulfonate buffer (PIPES) (pH 7.4) containing 2% sucrose for ~1 hr at room temperature followed by 23 hr at 4 °C. Eyecups then were washed with 0.13 M sodium phosphate buffer (pH 7.3) and dehydrated through a CH3OH series and embedded in JB4 glycol methacrylate. Sections (6 μm) were stained by immersion in 5% Richardson’s stain for 1.5 – 2 min at room temperature and then destained in 0.13 M sodium phosphate (pH 7.3) until the retinal layers were visible by light microscopy (about 8–15 min). For transmission electron microscopy, mouse eyecups were analyzed as described previously 21, 22. Mice used to evaluate retinal morphology were not exposed to photobleaching prior to analysis.
RNA microarray analysis
RNA was isolated from 10 eyes, 100 mg of liver or 100 mg of kidney from groups of C2 and N2 mice (Fig. 1B) with a RiboPure Kit (Ambion, Austin, TX). Quality of the preparations was verified by RNA agarose gel electrophoresis and the Agilent Bioanalyzer. Aliquots of total RNA isolated from the different tissues and from mice undergoing various treatments were detection-labeled and hybridized on the mouse genomic microarray using a service provided by NimbleGen System Inc. (Madison, WI). The microarray contained all 37,364 genes in the entire mouse transcriptome as represented by the University of California, Santa Cruz database (build HG 17) with a minimum of 11 probes per gene. Gene expression was normalized according to probe signal, and the average signal for each gene was normalized for each sample replicate.
Array data for samples across the whole study were normalized by NimbleGen Systems Inc. (Madison, WI) that employed the robust multichip analysis feature of the data analysis package contained in http://www.bioconductor.org. Project-wide spreadsheets of robust multichip analysis results were exported to Microsoft Excel and expression level ratios were calculated for all the possible pair-wise comparisons comprising one control and one treated sample. These pair-wise ratios were imported to Microsoft Access and mined for credible-fold changes in gene expression. Changes greater than or equal to a 2-fold increase or less than or equal to a 0.5-fold decrease were considered significant. Differentially expressed genes were then exported from Access as Excel files and were assigned functional annotations by Lucidyx Searcher software (http://www.lucidyx.com).
Statistical analyses
Statistical analyses were performed by one-way ANOVA.
RESULTS
Both single dose and longer-term monthly dosage regimens with 9-cis-R-Ac were used to assess the effect of artificial chromophore augmentation on the visual function of mice. 9-cis-R-Ac is a pro-drug that is metabolically converted first to fatty acid esters and consequently to 9-cis-retinal to form isorhodopsin 12. The retinoid dosage regimen was based on our previous studies wherein a single dose of 9-cis-retinoid (about 100 mg/kg) significantly restored ERG responses in mice that were maintained for a month 12, 17, 18. In addition to previous study, no direct assessments of 9-cis-R-Ac pharmacodynamics, pharmacokinetics or bioavailability were made in the current work 12. Twenty 10-month-old mice were employed for the single dose experiments (Fig. 1A), whereas 210 mice analyzed at either 10 or 14 months of age were used for the longer-term monthly 9-cis-R-Ac treatment studies (Fig. 1B). Additionally, four-month-old mice also were gavaged monthly for 10 months with all-trans-R-Ac (n = 10) before selective analyses were performed.
Single dose treatment of C57BL/6 female micewith 9-cis-R-Ac
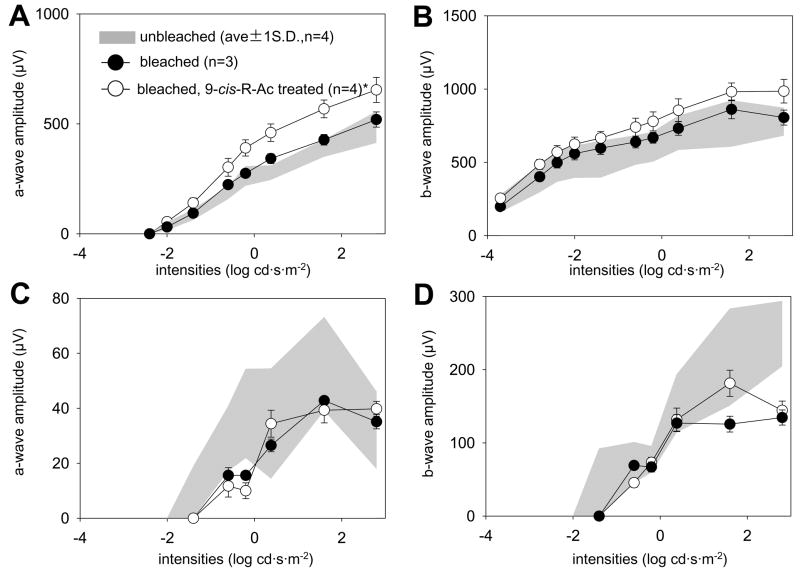
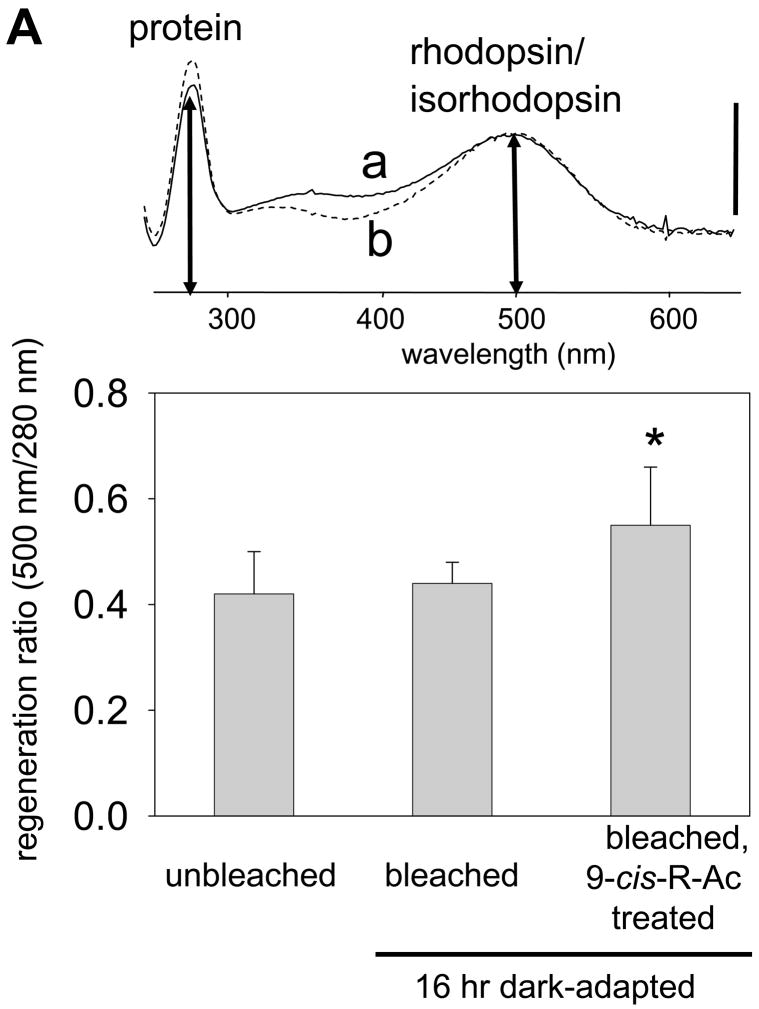
Forty eight hr dark-adapted, 10-month-old mice were gavaged with a single dose (~80 mg/kg body weight) of 9-cis-R-Ac or control vehicle and exposed to strong illumination for 20 min (500 cd·m−2 that bleached ~ 90 % rhodopsin). Then mice were dark-adapted for 16 hr after which various analyses were performed (Fig. 1A). Single-flash ERG conducted on treated and untreated mice showed that functional a- and b-wave amplitudes of 9-cis-R-Ac treated mice in scotopic ERG recordings were slightly increased as compared with the amplitudes in control mice (Fig. 2, panels A and B; a-waves, p<0.01). Photopic ERG recordings were largely unaffected compared with the amplitudes in control mice (Fig. 2, panels C and D). To investigate whether 9-cis-retinal was utilized to form isorhodopsin and assess how much unliganded opsin was present, we purified rhodopsin, isorhodopsin and opsin by immunoaffinity chromatography from the eyes of treated and control groups of mice. Opsin, rhodopsin and isorhodopsin are copurified by this procedure. The molar extinction coefficients of rhodopsin and isorhodopsin are similar but not identical, i.e. 42±2 ×10−3 at 502 nm for rhodopsin and 44±4 ×10−3 at 494 nm for isorhodopsin 33. The absorption spectra shown in Fig. 3A, upper panel, are representative of pooled peak fractions of purified rhodopsin/isorhodopsin/opsin normalized for absorbance at 500 nm. The concentration of isorhodopsin which has a spectrum and absorption maximum close to rhodopsin was not sufficient to shift the spectrum of the copurified rhodopsin/isorhodopsin mixture dominated by rhodopsin. Thus, regeneration ratios calculated from these curves (280 nm/500 nm) yield only an indirect estimate of the total protein represented by rhodopsin/isorhodopsin because determining the exact composition of these purified fractions would require direct biochemical analysis. Absorbance at intermediate wavelengths (about 380 nm) probably emanates from free retinals coeluted with visual pigments. The regeneration ratio of rhodopsin/isorhodopsin to opsin was significantly higher in eyes from treated than from control mice (Fig. 3 A, lower panel). To confirm whether 9-cis-retinal regenerated opsin as isorhodopsin, we extracted retinoids from purified visual pigment and definitely detected 9-cis-retinal. Significant amounts of 9-cis-retinal also were detected in eyes of treated mice exposed to intense light (Fig. 4A, 4B) whereas the amounts of 11-cis-retinal and all-trans-retinyl esters were not significantly affected in control and treated groups of mice (Fig. 4B, 4C). Retinyl esters are present in significant amounts only in the RPE of mouse eyes as reported in many previous studies 34, 35. In treated mice exposed to intense light, the RPE also stored significant amounts of 9-cis-retinyl esters, precursors of the retinal (Fig. 4C). Only trace amounts of 9-cis-retinal and 13-cis-retinal were detected in either control mouse eyes or unbleached eyes from treated mice (Fig. 3B). These small amounts of cis-retinal are likely produce by thermal isomerization during retinoid extraction and HPLC analysis (Fig. 3B). These results clearly demonstrate that 9-cis-R-Ac is trans-esterified to its fatty acid esters and consequently to form functional 9-cis-retinal in wild-type C57BL/6 female mice. After bleaching, 9-cis-retinal binds to opsin even in the presence of a functional retinoid cycle that produces 11-cis-retinal to regenerate rhodopsin. Retinoid levels in eyes from mice treated with all-trans-R-Ac showed no significant changes compared with controls. This result demonstrates that both 11-cis-retinal and 9-cis-retinal recombined with opsin.
Fig. 2. Single flash ERGs of 10-month-old mice treated with a single dose of 9-cis-R-Ac.
Mice were treated with 9-cis-R-Ac or control vehicle by gastric gavage as described in Materials and Methods (Fig. 1A). Animals were dark-adaptated for 16 hr after bleaching with intense light and scotopic (A, B) and photopic (C, D) ERGs were recorded. Bars represent ±1 standard errors of the mean, and gray colored background areas represent responses from pretreated 10-month-old mice. The a-wave amplitudes of 9-cis-R-Ac treated mice increased significantly (A, *; p<0.01) whereas b-wave amplitudes showed slight improvement under scotopic conditions (B). No significant differences were observed under photopic conditions (C, D). Statistical analyses were performed by one-way ANOVA.
Fig. 3. Characterization of purified visual pigments from 10-month-old mice treated with a single dose of 9-cis-R-Ac.
Mouse eye visual pigments; rhodopsin, isorhodopsin and opsin were copurified as described in Methods from 10-month-old animals treated with a single dose of 9-cis-R-Ac as shown in Fig. 1A. The regeneration level of purified rhodopsin/isorhodopsin/opsin was calculated from the ratio of absorbance at 500 nm (opsin with bound chromophore) to that of total opsin at 280 nm. (A). Above. Normalized representative absorbance spectra of purified rhodopsin from a 10-month-old 9-cis-R-Ac treated mouse (a, solid line) and a control mouse (b, dashed line) are shown. The bar indicates 0.02 AU. Below. The regeneration ratio of the 9-cis-R-Ac treated group was slightly higher than the control group (n = 3, p < 0.02), indicating that the treated group had a lower level of unliganded (free) opsin (a). Means ± S.D. are indicated. (B). Retinoids in the purified mouse visual pigment were extracted as described in Methods. 11-cis-Retinal was detected in the visual pigment from WT mice whereas both 11-cis-retinal and 9-cis-retinal (I) were observed in treated mice. Other minor cis-retinoids that could form during sample preparation (i.e. 13-cis-retinal (II)) also were detected in samples from treated and control mice.
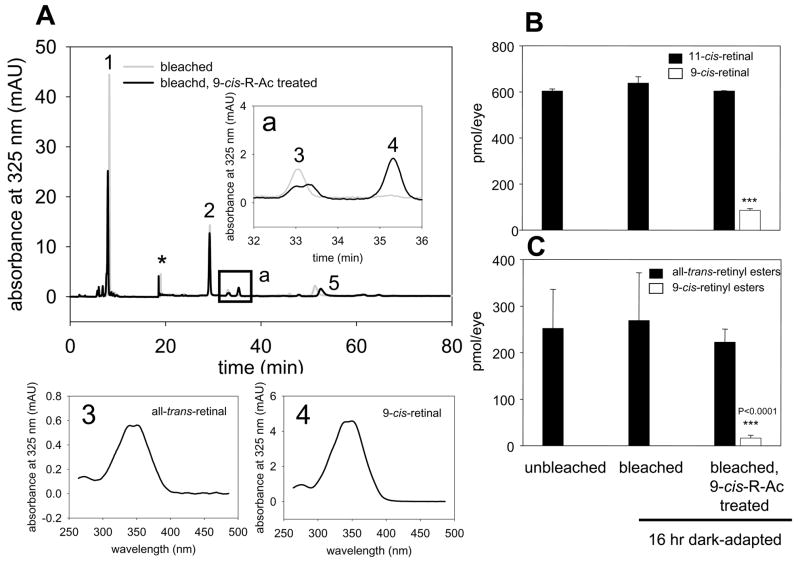
Fig. 4. Retinoid levels in eyes from 10-month-old mice gavaged with a single dose of 9-cis-R-Ac and exposed to intense light followed by full dark-adaptation.
(A) HPLC separation of retinoids from 9-cis-R-Ac treated and vehicle treated control mice. Fatty acid all-trans-retinyl esters eluted first (peak 1) followed by syn-11-cis-retinal oxime (2), syn-all-trans-retinal oxime (3), syn-9-cis-retinal oxime (4) and all-trans-retinol (5). Minor peaks on the chromatograms represent syn-oximes and the asterisk (*) indicates a spike related to a solvent change. Inset(a), an expanded scale of the chromatogram shows peaks 3 and 4 corresponding to levels of syn-all-trans-retinal and syn-9-cis-retinal oximes. Online spectra of these oximes are shown below (3 and 4). Retinoid levels in the eyes from treated and control mice (Fig. 1A) were analyzed by HPLC. Extraction procedures and derivatization with hydroxylamine to improve the recovery of retinals are described in Methods. (B and C) Quantification of retinals and esters. Amounts of 11-cis-retinal and all-trans-retinyl esters were similar in eyes of 9-cis-R-Ac treated and control mice but 9-cis-retinal and 9-cis-retinyl esters were detected only in the eyes of treated mice (n = 3, p < 0.0001). Levels of other non-polar retinoids were similar in 9-cis-R-Ac treated and untreated animals. A significant amount of 9-cis-retinal (peak 4) was detected in the samples from treated mice. Means ± S.D. are indicated.
Long-term treatment of C57BL/6 female mice with 9-cis-R-Ac
For long-term treatment studies (Fig. 1B), C57BL6 female mice were gavaged monthly with 9-cis-R-Ac, all-trans-R-Ac or control vehicle for 6 or 10 months with one exception. Group N3 4-month-old mice received vehicle control for 6 months and then 9-cis-R-Ac for only 4 months before analysis. We chose 14-month-old mature mice as the oldest mice for this study because they are still sturdy and able to tolerate repetitive anesthesia and multiple gavages.
Single flash ERG analyses of long-term treated mice with 9-cis-R-Ac
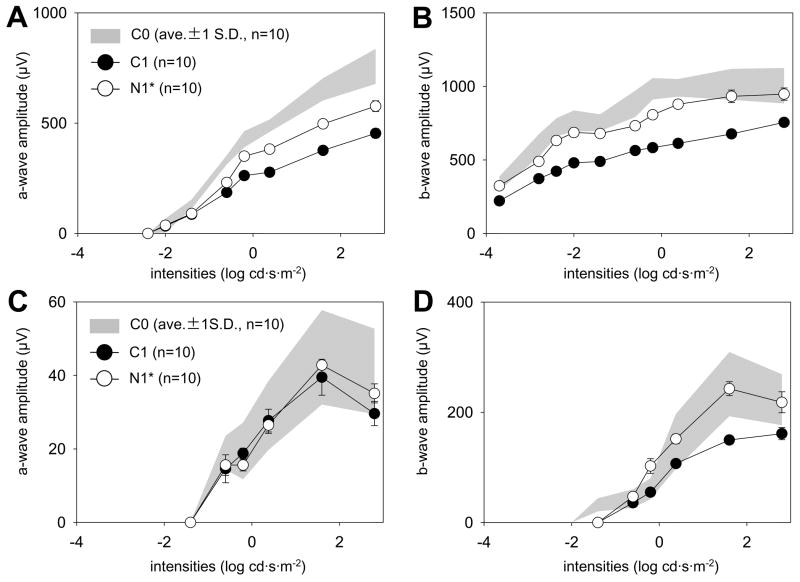
Mice were examined by non-invasive ERG methods to evaluate rod- and cone-mediated light responses after long-term 9-cis-R-Ac treatment. ERG responses of 4-month-old C0 mice were used as the baseline for comparing ERG data obtained from C1, C2, N1, N2 and N3 groups of mice. To indicate statistical differences between C0 and other plots, the C0 data are shown as a gray colored background area representing the C0 mean +/− 1 SD. The bars shown in C1 and N1 represent ±1 standard error of the mean (n = 10). Two to five recordings were obtained at intervals starting at 10 s and increasing to 10 min (see Methods). The first set of analyses was done in mice at 4 and 10 months of age. Under scotopic conditions, the amplitudes of a-waves were decreased in 10-month-old as compared to 4-month-old mice, especially at high flash intensities (C1 versus C0 group, Fig. 5A), and changes in b-waves also were evident (Fig. 5B). Under photopic conditions, no differences were observed in either a- or b-waves (Fig. 5C, 5D). When 4-month-old groups of mice gavaged monthly with either 9-cis-R-Ac or vehicle for 6 months were compared (N1 versus C1), improvement was observed for the N1 group with respect to a- and b-waves at high flash strengths (p < 0.01, one-way ANOVA) under both photopic and scotopic conditions except for a-waves noted under photopic conditions (Fig. 5A, B, C, D).
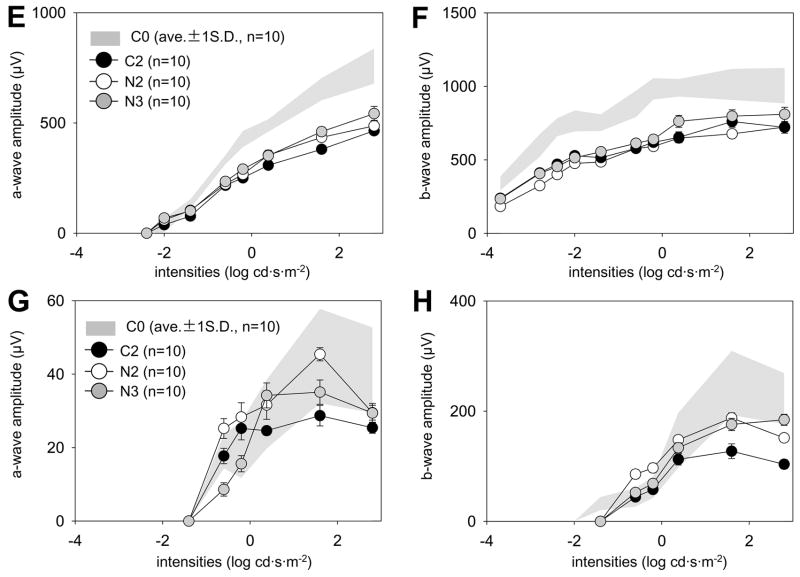
Fig. 5. ERG responses of 10- and 14-month-old mice treated monthly with 9-cis-R-Ac or control vehicle for various periods.
Scotopic and photopic ERGs were recorded from dark-adapted mice as described in Methods. The a- and b-wave amplitudes are plotted as a function of light intensity and error bars are shown (n = 10) and gray colored background areas represent responses from C0 mice. Compared to control 10-month-old mice (C1), responses of 10-month-old mice treated monthly for 6 months with 9-cis-R-Ac (N1) were increased significantly under scotopic (A, B) and photopic (D) conditions (p<0.01) except for a-wave amplitudes under photopic conditions (C). No significant differences between 14-month-old mice treated monthly with 9-cis-R-Ac for either 10 or 4 months (groups N2 and N3) relative to vehicle treated controls (C3) were observed under either scotopic (E, F) or photopic (G, H) conditions.
The second set of analyses was done in 14-month-old mice. No significant differences were found in a- and b-wave amplitudes between control (C2) and treated (N2 and N3) groups of mice under either scotopic or photopic conditions (Fig. 5E, F and G). Slight improvements in b waves were observed under photopic conditions (Fig. 5H). Maximal a-wave amplitudes estimated from a-wave maximal responses in dark-adapted mice also did not differ significantly between treated and control mice (C2: 720,57 ± 162.42 μV, N2: 723.02 ± 92.53 μV, N3: 809.50 ± 180.07 μV, P>0.2).
Changes in dark adaptation of long-term treated mice with 9-cis-R-Ac
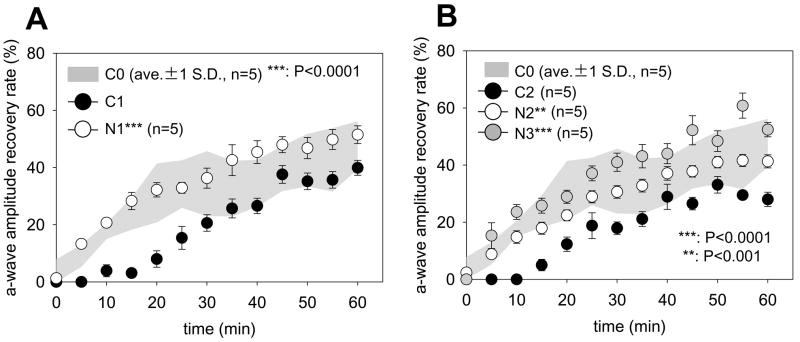
Recovery of the ERG response (dark adaptation) following bleach was measured by monitoring the amplitude of a-waves after retinal exposure to intense constant illumination for 3 min (500 cd·m−2, bleaching ~ 75 % rhodopsin). Recovery of this response was significantly faster in the 9-cis-R-Ac treated groups compared with the control groups of mice at both 10 months (N1 vs. C1, Fig. 6A) and 14 months of age (N2 and N3 vs. C2, Fig. 6B)(p < 0.001, one-way ANOVA). To indicate statistical differences between C0 and other plots, the C0 data are shown as a gray colored background area representing the C0 mean +/− 1 SD. Retinoid content in the eyes of each group was quantitatively evaluated at four time points during dark adaptation (0, 10, 30, and 60 min) after the 3 min bleach. The amount of regenerated 11-cis-retinal in the treated group of 10-month-old mice was significantly higher than in the control group at 30 and 60 min after bleach as measured by retinoid analysis. In 14-month-old mice, the amount of regenerated 11-cis-retinal was slightly higher in the N3 group whereas no significant difference was found between the C2 and N2 groups (data not shown). No differences were observed in the kinetics of all-trans-retinal and all-trans-retinyl-esters in the eye between treated (both N2 and N3) and untreated (C2) mice at 14 months of age and neither 9-cis-retinal nor 9-cis-retinyl esters were detected in eyes from untreated groups (data not shown).
Fig. 6. Recovery of dark adaptation after intense light bleaching by 10- and 14-month-old mice treated monthly with 9-cis-R-Ac or control vehicle for various periods.
(A) Recovery of 10-month-old mice from intense retinal bleaching. Different groups of 48 hr dark-adapted mice were bleached with intense constant illumination (500 cd·m−2) for 3 min and a-wave amplitude recovery was monitored by recording single-flash ERGs (−0.2 log cd·s·m−2) over a 60 minute dark adaptation period. The rate of recovery was significantly higher in 10-month-old mice treated monthly with 9-cis-R-Ac (N1) than in similarly aged mice gavaged with control vehicle (C1). Moreover, treatment with 9-cis-R-Ac even restored the rate of recovery to that seen in 4-month-old mice. Gray colored background areas represent recovery responses from C0 mice. (B) Recovery of 14-month-old mice from intense retinal bleaching. A significantly higher recovery rate occurred in 14-month-old 9-cis-R-Ac treated mice (N2 and N3) than in similarly aged vehicle gavaged (C2) animals (**, n=5; p<0.001; ***, p<0.0001, respectively). Again, 9-cis-R-Ac treated mice showed the same response as did young 4-month-old mice. Error bars are indicated.
Effects of long-term administration of all-trans-R-Ac on visual function
In additional control experiments, 4-month-old mice were gavaged with the inactive isomer, all-trans-R-Ac (n = 10) for 10 months and evaluated at 14 months age. Examination revealed no significant differences as compared with vehicle controls (C2) in single flash ERG a- and b-wave analyses or in dark adaptation recovery (n = 3). These results indicate that ERG effects are specific for the 9-cis isomer.
Analyses of rhodopsin, A2E and retinoid acids in control and long-term treated mice with 9-cis-R-Ac
In long-term experiments, regeneration ratios (rhodopsin/opsin) and total amounts of purified rhodopsin showed no significant differences between control and treated groups of mice at both 10 and 14 months of age. Amounts of purified protein recovered from the treated groups at 14 months of age (N2 and N3) were significantly lower than those from control mice (C2), whereas the corresponding amounts were only slightly less in 10-month-old treated as compared to control mice (N1 versus C1) (data not shown).
A2E and iso-A2E accumulation were measured in the eye (Fig. 7A) to evaluate the safety of long-term administration of 9-cis-R-Ac. This ester potentially could be converted to retinal at low levels in the treated mice, and in turn, retinal could spontaneously condense to A2E compounds 36, 37. A2E and iso-A2E were detected at similar levels in treated and control mice at both 10- and 14 months of age (Fig. 7B) whereas A2E accumulation was below detectable levels in 4-month-old pre-treatment controls (CO, Fig. 7B). Although long-term treatment with retinyl esters might produce elevated levels of mitogenic retinoic acids in the liver; however, we found that hepatic retinoic acid levels were below detectable levels in all groups of mice (data not shown).
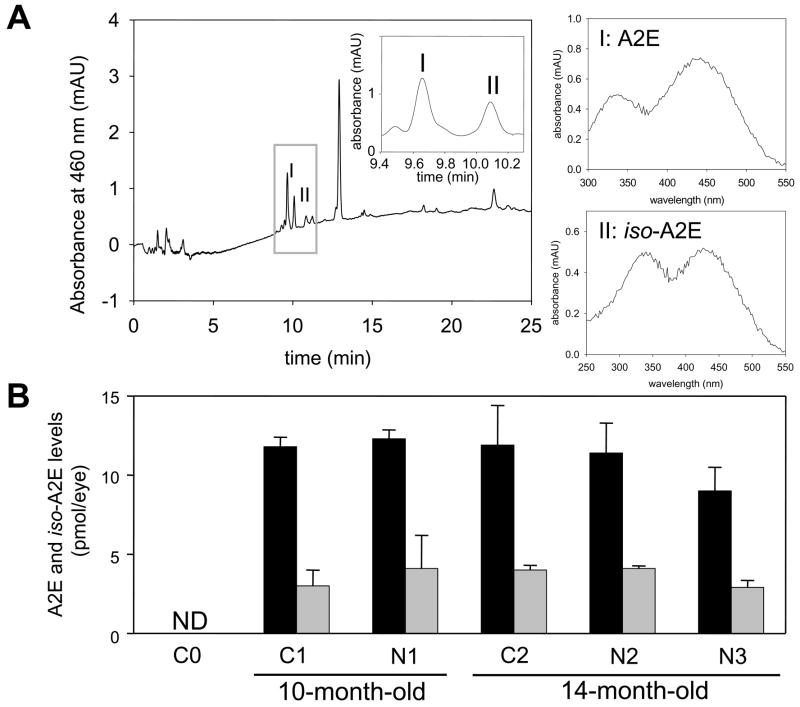
Fig. 7. A2E accumulation in the eyes of control and long-term 9-cis-R-Ac treated mice.
(A) Chromatographic separation and spectra of A2E and iso-A2E. A representative HPLC chromatogram of eluted A2E and iso-A2E is shown from a group of 10-month-old N1 mice treated monthly with 9-cis-R-Ac for 6 months (left panel in A). Inset. Magnified elution profiles of A2E and iso-A2E are highlighted. Spectra of these peaks (I and II) represent A2E and iso-A2E, respectively (top right). (B) Amounts of A2E (black bars) and iso-A2E (gray bars) from different experimental groups are shown. Amounts of A2E did not differ significantly among all groups of mice with the exception of N3, where they were slightly decreased (p<0.05). Iso-A2E levels were similar among all groups. Neither compound was detected at significant levels in young 4-month-old untreated mice (C0). Means ± S.D. are indicated (n = 5), ND, not determined.
Health status and retinal morphology of long-term treated micewith 9-cis-R-Ac
Animals were evaluated weekly for activity and changes in coat and skin appearance. No changes in these parameters were observed during the experimental period aside from those due to natural aging. Body weights obtained before treatment and at 10 months and 14 months of age showed no significant differences between vehicle control (C1–2) and treated groups of mice (N1–3) (data not shown).
Light microscopy revealed no major abnormalities in the retinas of vehicle, 9-cis-R-Ac or all-trans-R-Ac (n = 2) treated mice at 10 and 14 months of age. Moreover, retinas from the two 9-cis-R-Ac treated groups were indistinguishable. Lengths of rod outer segments (ROS) were similar in control and treated groups at 10- and 14 months of age (Fig. 8B; n = 5) but were significantly decreased as compared to ROS lengths of 4-month-old mice (C0) whose retinal histology is shown in Fig. 8A. The thickness of each major layer in the retina also was similar in control and treated groups. TEM analysis of the outer retina and RPE layer revealed no gross differences between control and treated mice nor did higher resolution of the interface between the RPE and ROS reveal any abnormalities (data not shown).
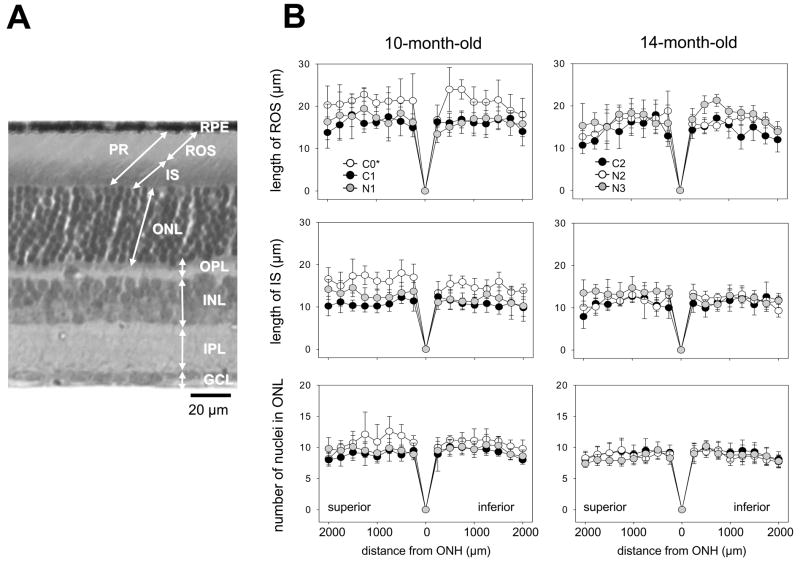
Fig. 8. Retinal morphology of 9-cis-R-Ac gavaged mice.
(A) A representative cross- section of retina from a 4-month-old untreated mouse (C0) is shown. RPE, retinal pigment epithelium; PR, photoreceptors, ROS, rod outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; and GCL, ganglion cell layer. (B) Thicknesses of the ROS and IS and numbers of ONL nuclei in retinas of control and 9-cis-R-Ac treated mice of various ages are shown. Data points are plotted as a function of the distance from the optic nerve head (ONH). Lengths of the ROS and IS of young 4-month-old control mice (C0) were significantly greater than those of all other groups of 10- and 14-month-old mice (*, n=5; p<0.01). No other significant differences were detected. Means ± S.D. are indicated.
Changes in RNA expression profiles with 9-cis-R-Ac administration
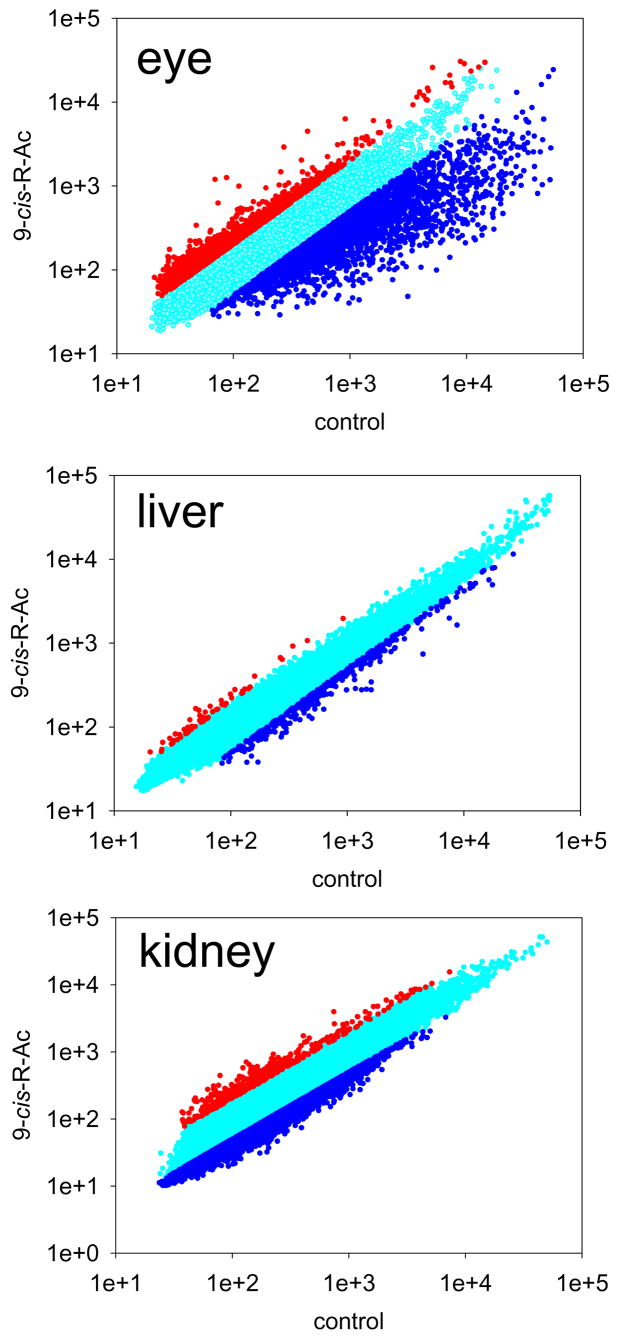
RNA microarray analyses were used to document possible changes in gene expression profiles after long-term treatment with 9-cis-R-Ac. Expression levels of mRNA in the eye, liver and kidney were determined and compared between treated (N2) and control (C2) groupsusing a 37,364 gene array provided by NimbleGen System Inc. Changes greater than or equal to a 2-fold increase or less than or equal to a 0.5-fold decrease were considered significant. Representative normalized values of mRNA from three different tissues, eye, liver and kidney were plotted (Fig. 9). Selected changes commonly observed between C2 and N2 are shown in supplemental Table 1, 2. In the eye, 9-cis-R-Ac treatment elevated expression of 290 genes by a factor of 2 or more and attenuated expression of more than 1057 genes by a factor of 0.5 or less (Fig. 9; Table S1). But in the liver of treated mice, expression of only 7 genes was increased by a factor of 2 or more and that of only 20 genes was suppressed by a factor of 0.5 or less (Fig. 9; Table S2). In the kidney of treated mice, 90 genes increased their expression by a factor of 2 or more and 3 genes suppressed it by a factor of 0.5 or less (Fig. 9; Table S2). Several phototransduction-specific, retinoid processing and functionally relevant mRNAs in the eye whose expression was affected are listed in Table S2. Protein levels of transducin (Gt), rhodopsin kinase, guanylate cyclase-activating proteins 1 and 2, guanylate cyclase 1, retinol dehydrogenase 12 and LRAT were not affected in all groups of mice as assessed by immunoblotting (data not shown).
Fig. 9. RNA array analyses of control and long-term 9-cis-R-Ac treated mice.
Expression levels of 37,364 genes from the eye, liver and kidney of two groups of 10-month-old mice, C2 and N2, were examined with cDNA arrays (Nimblegen). Two independent RNA samples were prepared for microarray hybridization. Representative normalized values of mRNA expression were plotted (control vs. the 9-cis-R-Ac treated group) as scatter plots with Sigma Plot v9.0. Genes expressed more than 2.0 fold and less than 0.5 fold are indicated in red and blue respectively. Further information is available from supplemental Tables S1 and S2. Immunoblots of eye extracts from different groups of mice were probed with various antibodies as described in Methods.
DISCUSSION
Based on experiments in mice, this is the first report that age-related deterioration of dark adaptation is ameliorated by artificial cis-retinoid treatment. This finding probably relates to analogous age-related decline in human vision manifested by dramatic slowing of rod-mediated dark adaptation that has been directly attributed to delayed rhodopsin regeneration 1. Two different types of studies were done here. After single dose studies revealed that 9-cis-retinoids could enter the eye, we undertook the set of experiments showing that long-term administration of 9-cis-R-Ac significantly decreased deterioration of retinal function in aging mice.
Single dose experiments
In agreement with earlier studies, the ERG response in mice measurably declined between 4 and 10 months of age and small but detectible amounts of free opsin were present in the older animals. These experiments were designed to test whether 9-cis-retinoids can enter the eyes of 10-month-old mice and affect visual function. Clearly 9-cis-retinal had entered the eye by the time these treated mice were tested 16 hr after intense light exposure. But the vast majority of opsin molecules still were regenerated with 11-cis-retinal leaving less than 10% that were regenerated with 9-cis-retinal under these experimental conditions. Importantly, when retinoids were extracted from purified proteins to identify bound chromophore, significant amounts of 9-cis-retinal were detected in samples from bleached mice treated with 9-cis-R-Ac. This suggests that 9-cis-retinal was used to regenerate opsin by forming isorhodopsin (Fig. 3B). To evaluate changes of retinoid content in the treated mice, we extracted retinoids from whole eyes. As predicted, significant amounts of 9-cis-retinal also were detected in eyes of treated mice exposed to intense light (Fig. 4A and B) whereas the amounts of 11-cis-retinal and all-trans-retinyl esters were virtually unchanged in control and treated animals (Fig. 4B and C).
Under fully dark-adapted conditions, opsin present in ROS from either control mouse eyes or unbleached eyes from treated mice mostly recombines with 11-cis-retinal. Therefore, the demand for exogenous regeneration of visual pigment is low in control and treated unbleached eyes. Levels of retinyl esters can differ quite markedly between individual mice because these intermediates vary more than any other retinoid in the eye. Importantly, in WT mice, all-trans-retinyl esters comprise one of the major retinoids in the eye whereas 9-cis-retinyl esters are undetectable 34, 35. However, during light exposure or dark-adaptation after bleaching, regeneration of opsin was supplemented by 9-cis-retinal present in the RPE of treated mice. Moreover, there was no statistically significant difference in all-trans-retinyl esters between control mice and mice treated with a single dose of 9-cis-R-Ac. In bleached and treated mice, 9-cis-retinoids are seized into the eye and significant amounts of 9-cis-retinyl esters were stored in the RPE. These precursor compounds are converted to the visual chromophore 9-cis-retinal, the active compound that regenerates isorhodopsin and improves retinal function without isomerization in the RPE. In fact, a-wave amplitudes were significantly improved in treated mice (Fig. 2A). These results encouraged us to investigate the effects of long-term treatment of aging mice with 9-cis-R-Ac.
Long-term treatment of mice with 9-cis-R-Ac
Based on the positive results of this single dose study (Fig. 2, 3, 4), we then evaluated the effects of long-term administration of 9-cis-R-Ac. Prior to our ERG evaluation, these mice were completely dark-adapted for 48 hours. Therefore, rhodopsin was maximally regenerated by endogenous 11-cis-retinal. Then our ERG procedure bleached less than 0.5% of total pigment under scotopic conditions so improvement of retinal function might not be detectible by a single flash ERG. ERG responses to a single light flash were significantly improved in 10-month-old mice that were treated for the previous 6 months with 9-cis-R-Ac as compared with the oil-treated controls (Fig. 5A, B, C, D). But these therapeutic effects of 9-cis-R-Ac were not observed in 14-month-old mice treated for previous 10 months (Fig. 5 E, F, G, H). However, these older mice did display a significant treatment effect involving dark adaptation (Fig. 6B, N2 and N3 groups). Recovery from strong illumination exposure was delayed in untreated mice, consistent with a delayed regeneration of rhodopsin due to slower production of chromophore 6. Visual pigments were characterized by the same procedures used for the single-dose study two weeks after the last gavage to allow complete renewal of ROS. In the 10- and 14-month-old mice there were no significant differences in the amounts of purified rhodopsin/isorhodopsin whereas the purified pigments significantly decreased in treated mice at both ages. From these observations, we suggest that the mechanism(s) for dark-adaptation improvement observed in the longer-term studies differ from those involved in the single dose study.
That long-term gavage of 14-month-old mice with all-trans-R-Ac had no effect on measured ERG parameters is not surprising because only the cis-form of the chromophore can recombine with opsin (reviewed in Ref. 4, 5). Thus, all-trans-retinoid would only add substrate for the isomerization reaction but not supplement the active chromophore if isomerization was attenuated. In this study, mice were fed regular mouse chow containing high levels of retinol. Thus, we did not expect that supplementation with all-trans-retinoid would have any effect on retinoid status. The observed effects in our experiments instead were purely due to 9-cis-retinoid. Interestingly, Owsley and colleagues reported mild improvement of dark-adaptation in humans after all-trans-retinol administration 38. Such findings could originate from improvements in vitamin A homeostasis rather than from exogenous chromophore supplementation as noted in our work.
Long-term treatment (6–10 months) with 9-cis-R-Ac was well tolerated by C57BL/6 female mice. Age-related morphological changes in the retina observed in both treated and control mice were not affected by treatment. Importantly, potentially toxic byproducts of retinoid treatment, A2E and retinoic acid, did not accumulate in these mice even though genetically modified mice 21, 39 are capable of accumulating abnormal amounts of A2E. It has been suggested that the mechanism of A2E accumulation is due to a significant buildup of all-trans-retinal after light exposure, resulting in generation of N-retinylidene-phosphatidylethanolamine, the precursor of A2E 39. As shown in this study, 9-cis-R-Accan be hydrolyzed, oxidized and incorporat ed into free opsin within the retina resulting in an increased regeneration ratio of rod pigments. However, the amount of all-trans-retinal generated by physiological light is constant regardless of the amounts of rod pigments and the regeneration ratio. Accordingly, it is not surprising that A2E levels were unaffected by 9-cis-R-Ac administration. Indeed, the beneficial effect of cis-retinoid supplementation seems more advantageous from this perspective than from any anti-oxidant effects generally attributed to retinoids 40.
Long–term treatment with retinoids could lead to changes in gene expression, because the end-products of retinol oxidation are toxic retinoic acids that might accumulate to excess. But we did not detect any such retinoic acid accumulation, consistent with the fact that their expression levels are tightly regulated by both synthesis and degradation 41, 42. Moreover, the expression profile of only a small number of mRNAs was modified in the liver and kidney whereas mRNA expression was changed in more than 1,300 genes in the eyes of WT mice treated with 9-cis-R-Ac. Several phototransduction mRNAs were affected, but the relation to the protein level is unclear. These changes could results in higher degradation of mRNAs or higher stability of proteins during aging. As a result, improvement of retinal function was observed in 14-month-old mice treated with 9-cis-R-Ac. Our previous work demonstrated that 13-cis-retinoic acid, a potent transcriptional regulator, disregulated the expression of many more RNAs when administered to mice 43. Interestingly, the expression levels of inflammatory and complement component mRNAs were reduced. The mechanisms of these effects are still unclear, but they are likely a positive result of long-term treatment with 9-cis-R-Ac. We speculate that such regulation of phototransduction gene expression by long-term treatment with 9-cis-R-Ac. This regulation might induce improvement of retinal function and homeostasis in retina and RPE. Consistent with this idea is the observation that lowering expression levels of Gnat1+/−, rhodopsin+/− and Rhd12+/− in heterozygotic mice fails to significantly affect ERG responses (Maeda T, Palczewski unpublished data; and Ref. 44). These results may be a consequence of reducing stress and improving dark-adaptation by treatment with 9-cis-retinoid. Indeed, mRNA expression levels of inflammatory and complement component proteins were remarkably decreased in 14-month-old mice as compared with control animals. These results suggest that 9-cis-retinoid treatment reduced age-dependent inflammatory reactions observed in mouse RPE that are similar to those recently proposed as one of the potential causes of human AMD 45. Taking into account that certain visual functions improved, some of these alternations in expression must relate to molecular changes in the eye.
That 9-cis-R-Ac preserved visual functions in mice is consistent with previous work on vitamin A deprivation and also with “the equivalent light hypothesis” proposed to explain the age-related decline in dark adaptation noted in vertebrates 46, 47. Rhodopsin regeneration should be more compromised in animals and humans deprived of vitamin A, an essential substrate for the production of the visual chromophore, 11-cis-retinal, via the visual (retinoid) cycle (reviewed in Ref. 6). “The equivalent light hypothesis” proposes that unliganded opsins (rhodopsins lacking a chromophore) trigger phototransduction events that ultimately lead to the demise of photoreceptor cells. Thus, death of photoreceptor and RPE cells in the aging eye could result from the accumulation of opsins devoid of chromophore 48. Hence, supplementation of the native chromophore needed for visual pigment regeneration could be highly beneficial 12, 49.
Possible prophylactic uses of 9-cis-retinoids
Humans begin to lose their ability to dark adapt beginning in the 3rd-4th decade 50. This decline in visual function is manifest by reduction in the ability to perform activities such as driving at night and reading in darkened environments 51. Such symptoms become more debilitating with age and can result in reduced independence and activity. Thus, a safe therapy that improves dark adaptation and photoreceptor sensitivity would be welcome. In our experiments, mice were treated with 9-cis-retinoids orally for 10 months without significant side effects whereas acute and chronic side effects of high vitamin A (all-trans-retinol) have been reported in human studies 52 that advocate further safety studies for dose and delivery systems. Results of our experiments suggest that oral 9-cis-retinoid administration may provide such a benefit, both as a long-term prophylactic agent and possibly as a therapeutic compound as well.
The beneficial effects and relative safety of 9-cis-retinoids may extend to age-related macular degeneration, the leading cause of legal blindness in the US and Europe 53. Although many factors may contribute to this disease, little attention has been devoted to age related biochemical defects in the visual cycle. Indeed, dark-adaptation in age-related maculopathy patients manifests as a delayed recovery of dark adaptation as well as an elevated absolute visual threshold54–57. Here, we have shown that there are biochemical changes in the visual cycle that occur with age, namely an increase in free opsin and the opsin/rhodopsin ratio. As noted above, when such changes are excessive they can lead to retinal degeneration such as seen in LCA 48. We also know that free opsin results in spontaneous initiation of the visual cascade in rod photoreceptors 46, 58–62. In addition to decreasing the signal-to-noise ratio in the visual system, this may also promote a metabolic overload in the RPE. In fact, dark-adaptation in retina is a highly ATP-dependent process which has been estimated to consume up to four times more oxygen under scotopic conditions63. That in turn may result in the formation of more waste products, free radicals and, eventually, drusen.63 So, decreasing free opsin with 9-cis-retinoid therapy may lead to a reduction of those precursors believed to initiate AMD. This proposition and the profound impact it may have on prevention of AMD warrants further study.
Although the transparency of lens and cornea also decline with age 64, the increase in retinal sensitivity observed here could be, at least partially, a more important factor in improving visual function associated with aging. A strong argument can be made that lens/cornea problems can often be corrected by surgery but surgery would do little to relieve the age-related decline in retinal function described here.
Supplementary Material
Acknowledgments
We thank Dr. A. R. Moise for help with the gene array experiments, Dr. Robert Molday (University of British Columbia) for providing the anti-rhodopsin antibody, Dr. Leslie T. Webster, Jr., and the members of the laboratory for the comments on the manuscript. This research was supported by NIH grants EY09339 and P30 EY11373, and a grant from Foundation Fighting Blindness. The Gene Expression and Genotyping Facility of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals of Cleveland (P30 CA43703) also contributed. University of Washington and QLT Inc. may commercialize some of the technology described in this work. KP, TM, AM, and DAS are consultants for QLT Inc.
Abbreviations
- 9-cis-R-Ac
9-cis-retinyl acetate
- A2E
pyridinium bisretinoid
- ERG
electroretinogram
- LRAT
lecithin:retinol acyltransferase
- ROS
rod outer segments
- RPE
retinal pigmented epithelium
- RPE65
RPE-specific 65 kDa protein
Footnotes
Commercial Relationships: University of Washington and QLT Inc. may commercialize some of the technology described in this work. KP, TM, and DAS are consultants for QLT Inc.
References
- 1.Jackson GR, Owsley C, McGwin G., Jr Aging and dark adaptation. Vision research. 1999;39:3975–3982. doi: 10.1016/s0042-6989(99)00092-9. [DOI] [PubMed] [Google Scholar]
- 2.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investigative ophthalmology & visual science. 1992;33:1–17. [PubMed] [Google Scholar]
- 3.Jackson GR, Owsley C, Cordle EP, Finley CD. Aging and scotopic sensitivity. Vision research. 1998;38:3655–3662. doi: 10.1016/s0042-6989(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 4.Palczewski K. G protein-coupled receptor rhodopsin. Annual review of biochemistry. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filipek S, Stenkamp RE, Teller DC, Palczewski K. G protein-coupled receptor rhodopsin: A Prospectus. Annu Rev Physiol. 2003;65:851–879. doi: 10.1146/annurev.physiol.65.092101.142611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 7.Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases Caused by Defects in the Visual Cycle: Retinoids as Potential Therapeutic Agents. Annu Rev Pharmacol Toxicol. 2006 doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson SG, Cideciyan AV, Regunath G, et al. Night blindness in Sorsby's fundus dystrophy reversed by vitamin A. Nature genetics. 1995;11:27–32. doi: 10.1038/ng0995-27. [DOI] [PubMed] [Google Scholar]
- 10.Berson EL, Rosner B, Sandberg MA, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Archives of ophthalmology. 1993;111:761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 11.Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. The Journal of cell biology. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batten ML, Imanishi Y, Tu DC, et al. Pharmacological and rAAV Gene Therapy Rescue of Visual Functions in a Blind Mouse Model of Leber Congenital Amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batten ML, Imanishi Y, Maeda T, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Byrne SM, Wongsiriroj N, Libien J, et al. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson DA, Li Y, McHenry CL, et al. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nature genetics. 2001;28:123–124. doi: 10.1038/88828. [DOI] [PubMed] [Google Scholar]
- 16.Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nature genetics. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 17.Van Hooser JP, Aleman TS, He YG, et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Hooser JP, Liang Y, Maeda T, et al. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J Biol Chem. 2002;277:19173–19182. doi: 10.1074/jbc.M112384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshizawa T, Wald G. Photochemistry of lodopsin. Nature. 1967;214:566–571. doi: 10.1038/214566a0. [DOI] [PubMed] [Google Scholar]
- 20.Ablonczy Z, Crouch RK, Goletz PW, et al. 11-cis-retinal reduces constitutive opsin phosphorylation and improves quantum catch in retinoid-deficient mouse rod photoreceptors. J Biol Chem. 2002;277:40491–40498. doi: 10.1074/jbc.M205507200. [DOI] [PubMed] [Google Scholar]
- 21.Maeda A, Maeda T, Imanishi Y, et al. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005;280:18822–18832. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda T, Lem J, Palczewski K, Haeseleer F. A Critical Role of CaBP4 in the Cone Synapse. Investigative ophthalmology & visual science. 2005;46:4320–4327. doi: 10.1167/iovs.05-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Jang GF, Jastrzebska B, et al. A naturally occurring mutation of the opsin gene (T4R) in dogs affects glycosylation and stability of the G protein-coupled receptor. J Biol Chem. 2004;279:53828–53839. doi: 10.1074/jbc.M408472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacKenzie D, Arendt A, Hargrave P, McDowell JH, Molday RS. Localization of binding sites for carboxyl terminal specific anti-rhodopsin monoclonal antibodies using synthetic peptides. Biochemistry. 1984;23:6544–6549. doi: 10.1021/bi00321a041. [DOI] [PubMed] [Google Scholar]
- 25.Palczewski K, Carruth ME, Adamus G, McDowell JH, Hargrave PA. Molecular, enzymatic and functional properties of rhodopsin kinase from rat pineal gland. Vision research. 1990;30:1129–1137. doi: 10.1016/0042-6989(90)90170-p. [DOI] [PubMed] [Google Scholar]
- 26.Van Hooser JP, Garwin GG, Saari JC. Analysis of visual cycle in normal and transgenic mice. Methods in enzymology. 2000;316:565–575. doi: 10.1016/s0076-6879(00)16750-3. [DOI] [PubMed] [Google Scholar]
- 27.Moise AR, Golczak M, Imanishi Y, Palczewski K. Topology and membrane association of lecithin: retinol acyltransferase (LRAT) J Biol Chem. 2006 doi: 10.1074/jbc.M608315200. [DOI] [PubMed] [Google Scholar]
- 28.Haire SE, Pang J, Boye SL, et al. Light-driven cone arrestin translocation in cones of postnatal guanylate cyclase-1 knockout mouse retina treated with AAV-GC1. Investigative ophthalmology & visual science. 2006;47:3745–3753. doi: 10.1167/iovs.06-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorczyca WA, Polans AS, Surgucheva IG, Subbaraya I, Baehr W, Palczewski K. Guanylyl cyclase activating protein. A calcium-sensitive regulator of phototransduction. J Biol Chem. 1995;270:22029–22036. doi: 10.1074/jbc.270.37.22029. [DOI] [PubMed] [Google Scholar]
- 30.Otto-Bruc A, Fariss RN, Haeseleer F, et al. Localization of guanylate cyclase-activating protein 2 in mammalian retinas. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4727–4732. doi: 10.1073/pnas.94.9.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, Huang J, Khani SC, Palczewski K. Molecular forms of human rhodopsin kinase (GRK1) J Biol Chem. 1998;273:5124–5131. doi: 10.1074/jbc.273.9.5124. [DOI] [PubMed] [Google Scholar]
- 32.Maeda A, Maeda T, Imanishi Y, et al. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crouch R, Purvin V, Nakanishi K, Ebrey T. Isorhodopsin II: artificial photosensitive pigment formed from 9,13-dicis retinal. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:1538–1542. doi: 10.1073/pnas.72.4.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeda A, Maeda T, Imanishi Y, Golczak M, Moise AR, Palczewski K. Aberrant metabolites in mouse models of congenital blinding diseases: formation and storage of retinyl esters. Biochemistry. 2006;45:4210–4219. doi: 10.1021/bi052382x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 36.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Isolation and onestep preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owsley C, McGwin G, Jackson GR, et al. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Investigative ophthalmology & visual science. 2006;47:1310–1318. doi: 10.1167/iovs.05-1292. [DOI] [PubMed] [Google Scholar]
- 39.Radu RA, Mata NL, Nusinowitz S, Liu X, Sieving PA, Travis GH. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt's macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4742–4747. doi: 10.1073/pnas.0737855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maxwell S, Greig L. Anti-oxidants-- a protective role in cardiovascular disease? Expert Opin Pharmacother. 2001;2:1737–1750. doi: 10.1517/14656566.2.11.1737. [DOI] [PubMed] [Google Scholar]
- 41.Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. [PubMed] [Google Scholar]
- 42.Ross AC. Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic Acid oxidation. J Nutr. 2003;133:291S–296S. doi: 10.1093/jn/133.1.291S. [DOI] [PubMed] [Google Scholar]
- 43.Maeda A, Maeda T, Golczak M, et al. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Molecular pharmacology. 2006;70:1220–1229. doi: 10.1124/mol.106.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang Y, Fotiadis D, Maeda T, et al. Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J Biol Chem. 2004;279:48189–48196. doi: 10.1074/jbc.M408362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, Liu B, Lukas TJ, Neufeld AH. The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration. PLoS One. 2008;3:e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lisman J, Fain G. Support for the equivalent light hypothesis for RP. Nature medicine. 1995;1:1254–1255. doi: 10.1038/nm1295-1254. [DOI] [PubMed] [Google Scholar]
- 47.Fain GL, Lisman JE. Photoreceptor degeneration in vitamin A deprivation and retinitis pigmentosa: the equivalent light hypothesis. Experimental eye research. 1993;57:335–340. doi: 10.1006/exer.1993.1132. [DOI] [PubMed] [Google Scholar]
- 48.Fan J, Woodruff ML, Cilluffo MC, Crouch RK, Fain GL. Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice. J Physiol. 2005;568:83–95. doi: 10.1113/jphysiol.2005.091942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeda A, Maeda T, Palczewski K. Improvement in rod and cone function in mouse model of Fundus albipunctatus after pharmacologic treatment with 9-cis-retinal. Investigative ophthalmology & visual science. 2006;47:4540–4546. doi: 10.1167/iovs.06-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson GR, McGwin G, Jr, Phillips JM, Klein R, Owsley C. Impact of aging and age-related maculopathy on inactivation of the a-wave of the rod-mediated electroretinogram. Vision research. 2006;46:1422–1431. doi: 10.1016/j.visres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Schilling OK, Wahl HW. Modeling late-life adaptation in affective well-being under a severe chronic health condition: the case of age-related macular degeneration. Psychol Aging. 2006;21:703–714. doi: 10.1037/0882-7974.21.4.703. [DOI] [PubMed] [Google Scholar]
- 52.Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. The American journal of clinical nutrition. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- 53.Zack DJ, Dean M, Molday RS, et al. What can we learn about age-related macular degeneration from other retinal diseases? Molecular vision. 1999;5:30. [PubMed] [Google Scholar]
- 54.Brown B, Adams AJ, Coletta NJ, Haegerstrom-Portnoy G. Dark adaptation in age-related maculopathy. Ophthalmic Physiol Opt. 1986;6:81–84. [PubMed] [Google Scholar]
- 55.Brown B, Tobin C, Roche N, Wolanowski A. Cone adaptation in age-related maculopathy. Am J Optom Physiol Opt. 1986;63:450–454. doi: 10.1097/00006324-198606000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Owsley C, Jackson GR, White M, Feist R, Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001;108:1196–1202. doi: 10.1016/s0161-6420(01)00580-2. [DOI] [PubMed] [Google Scholar]
- 57.Dimitrov PN, Guymer RH, Zele AJ, Anderson AJ, Vingrys AJ. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:55–65. doi: 10.1167/iovs.06-1048. [DOI] [PubMed] [Google Scholar]
- 58.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 59.Surya A, Foster KW, Knox BE. Transducin activation by the bovine opsin apoprotein. J Biol Chem. 1995;270:5024–5031. doi: 10.1074/jbc.270.10.5024. [DOI] [PubMed] [Google Scholar]
- 60.Hofmann KP, Pulvermuller A, Buczylko J, Van Hooser P, Palczewski K. The role of arrestin and retinoids in the regeneration pathway of rhodopsin. J Biol Chem. 1992;267:15701–15706. [PubMed] [Google Scholar]
- 61.Palczewski K, Jager S, Buczylko J, et al. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- 62.Jager S, Palczewski K, Hofmann KP. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996;35:2901–2908. doi: 10.1021/bi9524068. [DOI] [PubMed] [Google Scholar]
- 63.de Gooyer TE, Stevenson KA, Humphries P, et al. Rod photoreceptor loss in Rho−/− mice reduces retinal hypoxia and hypoxia-regulated gene expression. Invest Ophthalmol Vis Sci. 2006;47:5553–5560. doi: 10.1167/iovs.06-0646. [DOI] [PubMed] [Google Scholar]
- 64.Glasser A, Campbell MC. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision research. 1999;39:1991–2015. doi: 10.1016/s0042-6989(98)00283-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.