Abstract
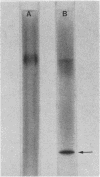
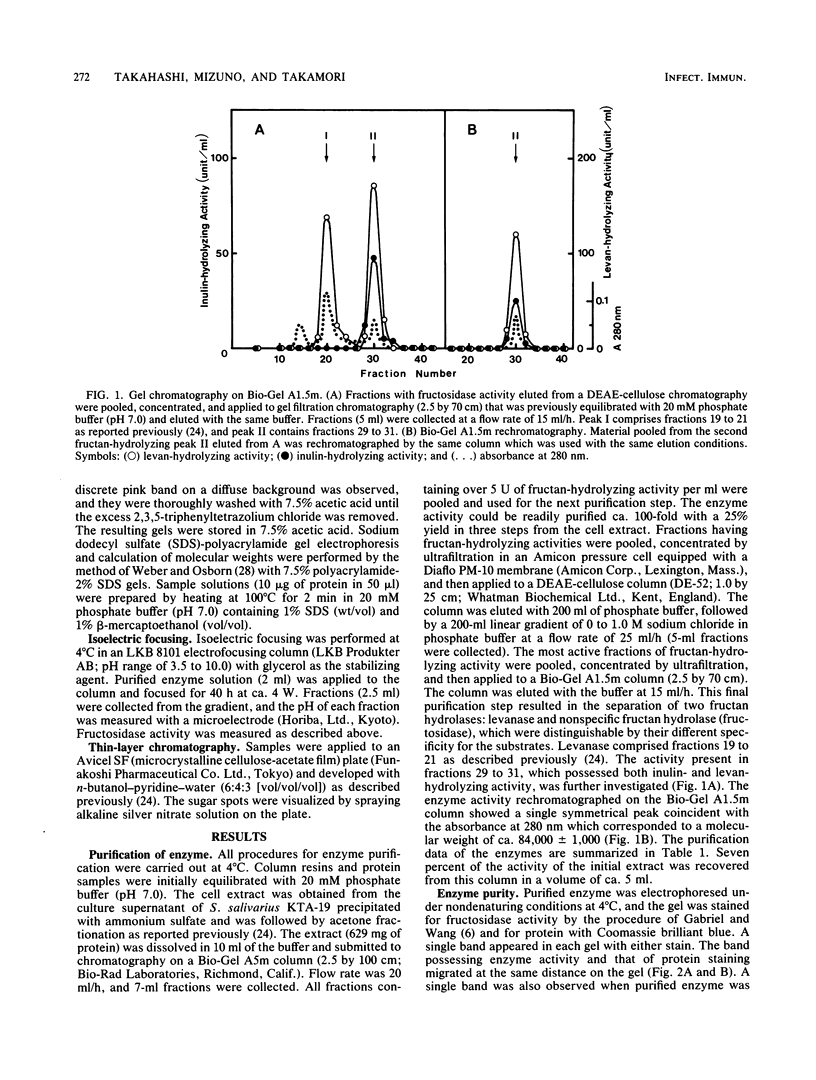
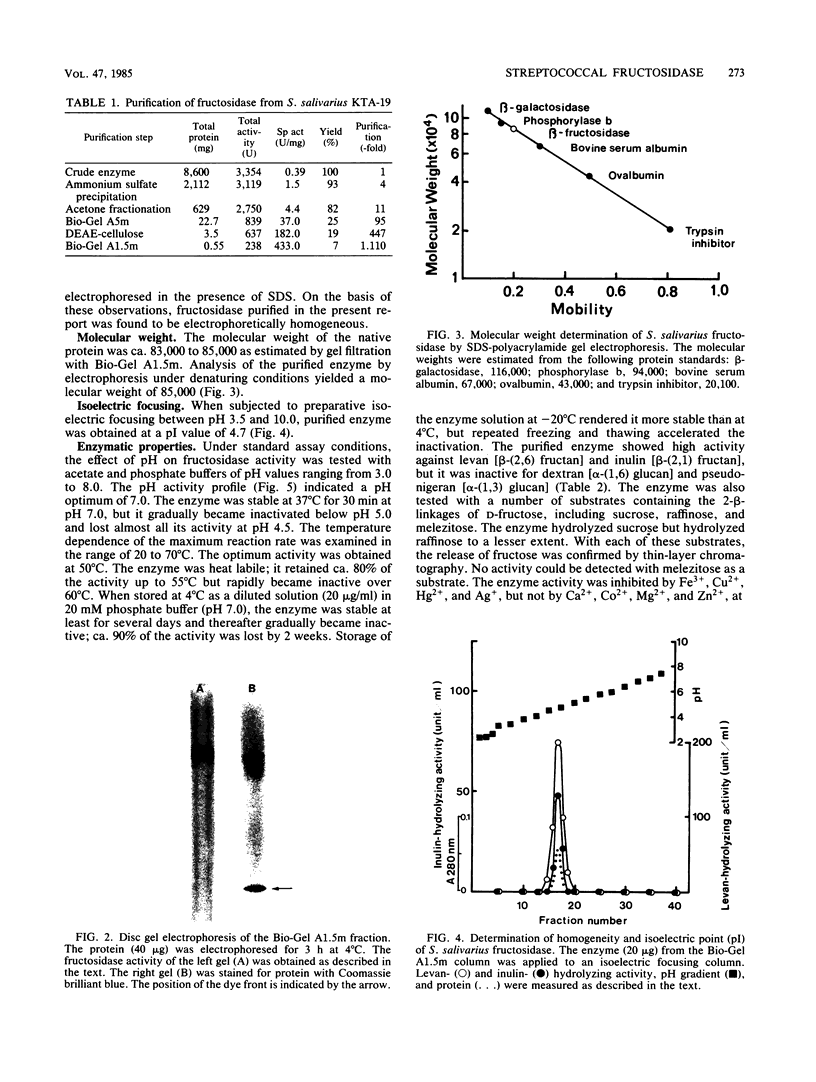
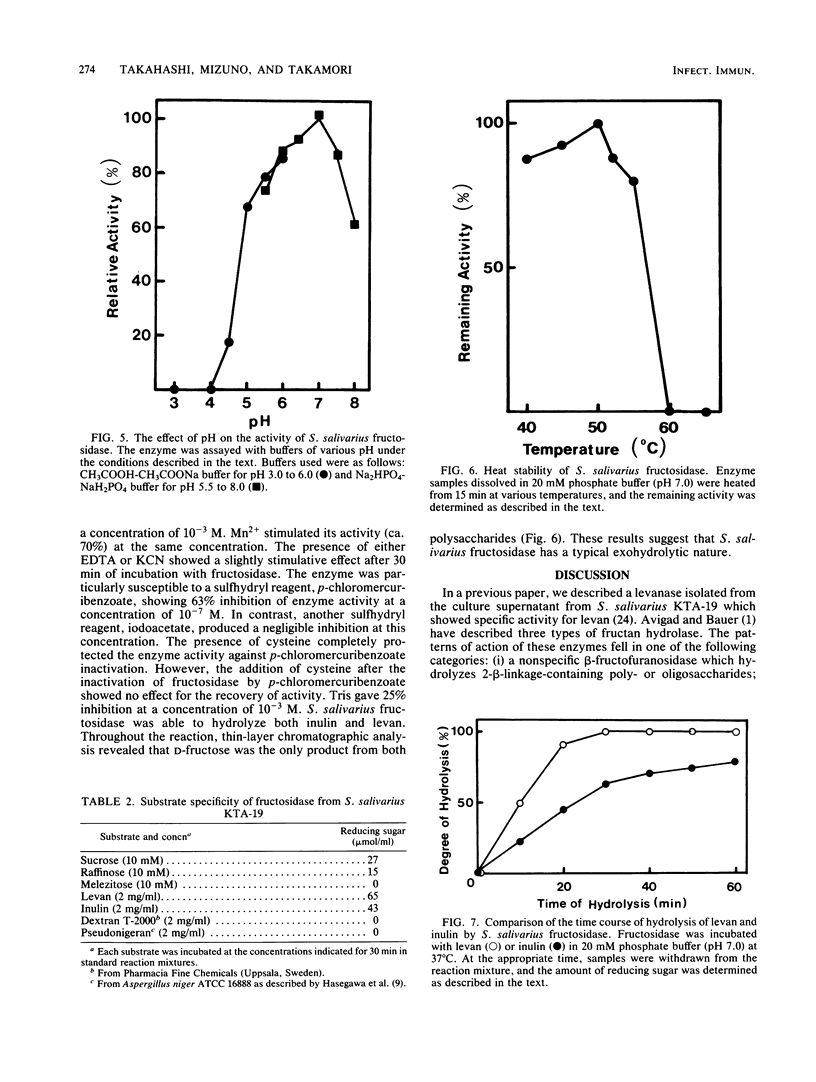
Streptococcus salivarius fructosidase (beta-D-fructan fructohydrolase, EC 3.2.1.80) was purified to homogeneity. The molecular weight of the fructosidase was estimated to be 83,000 to 85,000 by gel filtration and by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The pH optimum of the enzyme was 7.0, and the isoelectric point was pH 4.7. The purified enzyme preparation hydrolyzed levan, inulin, and several 2-beta-linkage-containing oligosaccharides such as sucrose and raffinose, but not melezitose, dextran, and pseudonigeran. The fructosidase was inhibited by Fe3+, Cu2+, Hg2+, and Ag+, but not by Ca2+, Co2+, Mg2+, and Zn2+, at a concentration of 10(-3) M. Mn2+ was particularly effective in stimulating activity at the same concentration. The presence of either EDTA or KCN also increased fructosidase activity by 20 to 30%. The enzyme was susceptible to sulfhydryl reagents since p-chloromercuribenzoate (10(-7) M) produced 63% inhibition of the activity. However, this inhibition was overcome in the presence of cysteine. This enzyme acts as an exofructosidase since thin-layer chromatographic analysis revealed that D-fructose was formed from levan or inulin by the action of the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DaCosta T., Gibbons R. J. Hydrolysis of levan by human plaque streptococci. Arch Oral Biol. 1968 Jun;13(6):609–617. doi: 10.1016/0003-9969(68)90139-8. [DOI] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel O., Wang S. F. Determination of enzymatic activity in polyacrylamide gels. I. Enzymes catalyzing the conversion of nonreducing substrates to reducing products. Anal Biochem. 1969 Mar;27(3):545–554. doi: 10.1016/0003-2697(69)90068-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Gold W., Preston F. B., Lache M. C., Blechman H. Production of levan and dextran in plaque in vivo. J Dent Res. 1974 Mar-Apr;53(2):442–446. doi: 10.1177/00220345740530024401. [DOI] [PubMed] [Google Scholar]
- Hasegawa S., Nordin J. H. Enzymes that hydrolyze fungal cell wall polysaccharides. I. Purification and properties of an endo-alpha-D-(1-3)-glucanase from Trichoderma. J Biol Chem. 1969 Oct 25;244(20):5460–5470. [PubMed] [Google Scholar]
- Higuchi M., Iwami Y., Yamada T., Araya S. Levan synthesis and accumulation by human dental plaque. Arch Oral Biol. 1970 Jun;15(6):563–567. doi: 10.1016/0003-9969(70)90111-1. [DOI] [PubMed] [Google Scholar]
- Kiel R. A., Tanzer J. M., Woodiel F. N. Identification, separation, and preliminary characterization of invertase and beta-galactosidase in Actinomyces viscosus. Infect Immun. 1977 Apr;16(1):81–87. doi: 10.1128/iai.16.1.81-87.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of invertase activity from cariogenic Streptococcus mutans. J Bacteriol. 1973 Sep;115(3):1003–1010. doi: 10.1128/jb.115.3.1003-1010.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manly R. S., Richardson D. T. Metabolism of levan by oral samples. J Dent Res. 1968 Nov-Dec;47(6):1080–1086. doi: 10.1177/00220345680470061301. [DOI] [PubMed] [Google Scholar]
- Maynard M. T., Kuramitsu H. K. Purification and antigenic properties of intracellular invertase from Streptococcus mutans. Infect Immun. 1979 Mar;23(3):873–883. doi: 10.1128/iai.23.3.873-883.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E., Cowman R. A. Invertase activity in Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 1973 Apr;18(4):525–531. doi: 10.1016/0003-9969(73)90073-3. [DOI] [PubMed] [Google Scholar]
- Miller C. H. Degradation of sucrose by whole cells and plaque of Actinomyces naeslundii. Infect Immun. 1974 Dec;10(6):1280–1291. doi: 10.1128/iai.10.6.1280-1291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. H., Somers P. J. Degradation of levan by Actinomyces viscosus. Infect Immun. 1978 Oct;22(1):266–274. doi: 10.1128/iai.22.1.266-274.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. B., Creamer H. R. Contribution of plaque polysaccharides to growth of cariogenic microorganisms. Arch Oral Biol. 1971 Aug;16(8):855–862. doi: 10.1016/0003-9969(71)90175-0. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Sund M. L., Linder L. Cell-surface bound beta-fructofuranosidase (invertase) of the oral bacterium Streptococcus mitis. Arch Oral Biol. 1980;25(8-9):573–578. doi: 10.1016/0003-9969(80)90070-9. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Mizuno F., Takamori K. Isolation and properties of levanase from Streptococcus salivarius KTA-19. Infect Immun. 1983 Oct;42(1):231–236. doi: 10.1128/iai.42.1.231-236.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Brown A. T., McInerney M. F., Woodiel F. N. Comparative study of invertases of Streptococcus mutans. Infect Immun. 1977 Apr;16(1):318–327. doi: 10.1128/iai.16.1.318-327.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J., Hare M. D., Morrey-Jones J. G. Activity of fructanase in batch cultures of oral streptococci. Carbohydr Res. 1983 Feb 16;113(1):101–112. doi: 10.1016/0008-6215(83)88222-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wood J. M. The amount, distribution and metabolism of soluble polysaccharides in human dental plaque. Arch Oral Biol. 1967 Jul;12(7):849–858. doi: 10.1016/0003-9969(67)90107-0. [DOI] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Levan degradation by streptococci isolated from human dental plaque. Arch Oral Biol. 1968 Jul;13(7):827–830. doi: 10.1016/0003-9969(68)90102-7. [DOI] [PubMed] [Google Scholar]