Abstract
Here, we present a detailed characterization of ion binding in the NaK pore using the high resolution structures of NaK in complex with various cations. These structures reveal four ion binding sites with similar chemical environments but vastly different ion preference. The most non selective of all is site 3, which is formed exclusively by backbone carbonyl oxygen atoms and resides deep within the selectivity filter. Additionally, four water molecules in combination with four backbone carbonyl oxygen atoms are seen to participate in K+ and Rb+ ion chelation both at the external entrance and vestibule of the NaK filter, confirming the preference for an octahedral ligand configuration for K+ and Rb+ binding. In contrast, Na+ binding in the NaK filter, particularly at site 4, utilizes a pyramidal ligand configuration requiring the participation of a water molecule in the cavity. Therefore, the ability of the NaK filter to bind both Na+ and K+ ions seemingly arises from the ions' ability to utilize the existing environment in unique ways rather than any structural rearrangements of the filter itself.
Along with channel gating, the ability of ion channels to select for and allow the passage of specific ions through their conduction pores is central to their physiological functioning [1]. Our knowledge of ion selectivity, as this process is known, has come a long way with the determination of several K+ selective ion channel structures over the past decade [2-7]. However, several fundamental issues are yet to be fully understood and remain the focus of ongoing debate within the field. For example, is the snug-fit model alone enough to account for K+ over Na+ selectivity or do ligand chemical environment (eight carbonyl oxygen atoms) and dynamics play the determinant role [8, 9]?
The recently discovered NaK channel from B. cereus has provided a unique structural model for further probing mechanisms of ion selectivity. The channel shares overall structural similarities with KcsA but has been shown to conduct most group 1A cations as well as Ca2+, using a unique mode of Ca2+ chelation with only backbone carbonyl oxygen atoms acting as ligands [10, 11]. This ion non-selectivity is thought to arise from structural differences within the selectivity filter, where only the two most intracellular K+ binding sites of KcsA (sites 3 and 4) are preserved while the upper two are replaced by a vestibular structure. Using the initial NaK structure, an analysis of structural differences among complexes of NaK and its various conducting ions has been difficult due to resolution limits as well as the presence of a high Ca2+ concentration in addition to monovalent cations in crystallization solutions. To decipher the underlying mechanisms by which various monovalent cations are able to permeate NaK, we crystallized a truncated form of the channel, NaKNΔ19, previously used for channel function analysis. Well diffracting crystals were obtained and the structures were determined at resolutions ranging from 1.6 – 2.0 Å, revealing a channel in an open conformation. A detailed analysis of gating mechanics was carried out and is presented in our companion study. Here we use the high resolution structures of NaKNΔ19 in complex with various mono- and divalent cations to visualize the differences in ion binding profiles among various conducting ions at atomic detail. While our structural study reveals ion binding profiles in the NaK filter at atomic detail and reveals several interesting features that underlie NaK ion non-selectivity, fundamental questions remain about how sites with seemingly identical chemical environments can display different ion binding properties.
Results
Na+ binding in NaK selectivity filter
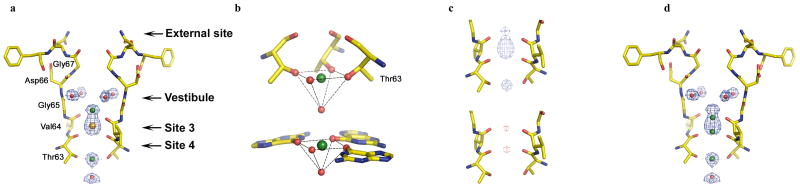
The structure of the NaKNΔ19-Na+ complex was determined at 1.8 Å resolution and its 2Fo-Fc ion omit map reveals three bound ions in the selectivity filter: in the vestibule, and sites 3 and 4 (Fig. 1a). The density in the vestibule and site 4 can be assigned to Na+ ions but that at site 3 cannot, as will be discussed in detail later. The ion in the vestibule is coordinated by four carbonyl oxygen atoms of Val64s with a ligand-ion distance of about 2.9 Å. Four water molecules are clearly seen to reside within the vestibule, each with a distance of about 4 Å to the Na+ ion, suggesting no intimate interaction between the ion and the water molecules. The Na+ ion in site 4 does not reside in the center of the ion binding cage formed by the hydroxyl and backbone carbonyl oxygen atoms from Thr63. Instead it sits in an almost planar conformation with respect to its ligands, namely the four hydroxyl oxygen atoms of Thr63s, with a distance of 2.4 Å. Furthermore, another water molecule in the central cavity, only seen in the Na+ complex, also participates in Na+ coordination with a Na+-O distance of 2.7 Å. This scheme of pyramidal Na+ coordination is reminiscent of what is seen in the Na+ complex of the Guanine tetraplex structure [12], part of which is shown in figure 1b, drawn based on a 0.95 Å crystal structure of the complex. By using the O6 of the guanine base, the Guanine tetraplex generates a set of Na+ binding sites that have a similar chemical environment as in NaK and K+ channels (Supplementary fig 1). Na+ ions have a versatile way of binding and the one near the 3′ end is planar with the tetrad and has a water molecule axial to it (fig. 1b). The distances between Na+ and O6 or water are in the range of 2.32 – 2.4 Å.
1.
Na+ binding in the NaK filter (a) 2Fo-Fc ion omit map contoured at 1.5σ showing density (blue mesh) for Na+ ions, water molecules in the vestibule and central cavity, and the contaminating ion at site 3 (green, red, and orange spheres, respectively). The front and back subunits of the NaK model have been removed for clarity in most figures. (b) Comparison of Na+ binding in NaK at site 4 (upper) and the 3′ side of the guanine tetraplex (lower) showing Na+ binding in plane with 4 of its ligand, with a water molecule sitting axial to it. (c) 2Fo-Fc ion omit map contoured at 2.5σ (blue mesh, upper) showing football shape of electron density at site 3 of NaKNΔ19 in high Na+ (500mM); and the Fo-Fc map with Cs+ at site 3 contoured at 4 σ (red mesh, lower) showing density that likely arises from a contribution of Na+ present on both the upper and lower edges of site 3. (d) Suggested mode of Na+ binding at the upper and lower edges of site 3.
Identification of the bound ion at site 3 was complicated due to its apparent contamination by an unknown heavy atom ion or a mixture of ions. Even though 100 mM NaCl was the only salt present in crystallization conditions, electron density at site 3 is too strong to be accounted for solely by Na+ ions. The bound ion also carries an anomalous signal as shown in the anomalous difference Fourier map (Supplementary fig. 2a). We suspect the source of this contamination is (±)-2-Methyl-2,4-pentanediol (MPD), used as a precipitant at high concentrations in the crystallization of NaKNΔ19 (Methods). MPD treatment with chelating resins carrying moieties for non-selective binding of both monovalent and divalent heavy metal ions can decrease the extent of contamination but, unfortunately, not eliminate it. Contamination from trace metal ions is not surprising judging from the very non selective nature of site 3, as will be discussed in further detail later. For simplicity, in our structural analysis we have assigned this strong density to Cs+ and refined its occupancy to reveal the extent of the contamination. Even though the contamination obscures a direct visualization of possible Na+ binding at site 3, important information can still be extracted from an analysis of electron density distribution. A Flow Na-Fhigh Na difference map between NaKNΔ19 crystals grown in 100 mM NaCl (low Na) and those grown in 500 mM NaCl (high Na) reveals a strong peak at site 3, indicating the replacement of some contaminating heavy atoms by the less electron dense Na+ ions (Supplementary fig. 2b). This is also evident from a decrease in ion occupancy of the heavy atoms (Cs+ for the purpose of refinement) in the High Na+ crystals. We therefore conclude that Na+ does indeed bind at site 3 albeit at low affinity. Recent computational studies have suggested that Na+ ions tend to bind at the upper or lower ends of the ion binding cage at site 3, almost planar with the backbone carbonyls of Val64 or Thr63 [13, 14]. Three observations from NaKNΔ19 structures lead us to believe this is actually the case. First, the football shaped electron density at site 3 in a 2Fo-Fc ion omit map (Fig. 1c upper) indicates the presence of weak scattering elements at both end of the site. Second, a Fo-Fc difference map of the high Na+ crystal reveals unaccounted density on both ends of the site that likely comes from bound Na+ ions (Fig. 1c, lower). This density is weak (4 sigma in figure 1c), as would be expected from a low occupancy and low scattering power of Na+ ions. Finally, a similar planar conformation of a Na+ ion with its ligand is observed at site 4. Thus, 3 distinct Na+ binding positions are likely formed by sites 3 and 4 where the ions can be chelated in plane with their ligands (Fig. 1d), and the observed electron density in the filter is the combined effect of the ion distribution shown in figure 1 a&d.
K+ & Rb+ complex structures of NaKNΔ19
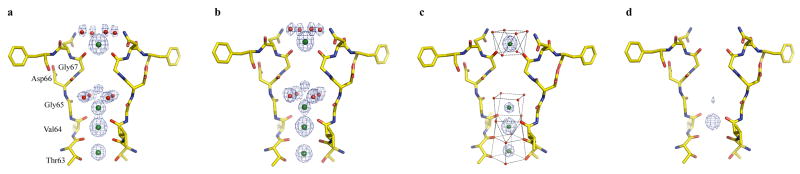
K+- and Rb+-complex structures of NaKNΔ19 were determined at 1.8 Å and 1.7 Å, respectively. While ion binding in the NaK filter is similar between both complexes, the details governing their ion chelation properties are noticeably different from the Na+-complex. Both K+ and Rb+ display a strong preference for maintaining an octahedral arrangement of ligands. Figure 2 shows a 2Fo-Fc ion omit map of the K+(a) and Rb+ (b) complex of NaKNΔ19, revealing four ion binding sites: the external entrance, vestibule, and sites 3 & 4. While no Na+ binding was observed at the external entrance, K+ and Rb+ bind there in a partially hydrated manner with the four carbonyl oxygen atoms from the Gly67 residues and four water molecules acting as ligands. A partially hydrated K+ ion at a similar position has been observed in the KcsA structure[15]. However, the carbonyl oxygen atoms of Gly67, rather than pointing upwards as in KcsA, are oriented more inward, forming a better chelation scheme for K+ and Rb+. Partially hydrated K+ and Rb+ ions are also observed in the vestibule of the filter chelated by the four carbonyl oxygen atoms of Val64s along with 4 water molecules. Thus, the involvement of water molecules allows for the maintenance of an octahedral ligand arrangement (Fig. 2c), much like that seen in KcsA [15]. It is important to note that these four water molecules are also seen within the vestibule of the filter in the Na+ complex but, with ion-ligand distances of ∼4 Å, are unlikely to be involved in ion chelation. K+ and Rb+ binding at sites 3 and 4 is virtually the same as that at equivalent sites in K+ channels both in terms of position and ligand coordination. Heavy atom contamination at site 3 was still observed in the K+ and Rb+ complexes, but to a much lesser extent than in the Na+ complex based on the following observation. A FNa − FK difference map between crystals grown in 100 mM NaCl and KCl reveals strong electron density peak at site 3 (Supplementary fig. 3), despite K being a stronger X-ray scattering element than Na. This alludes to a competitive knockout of a larger fraction of the contaminating ion/ions by K+, akin to a Fheavy-FK map. This suggests that K+ binds at site 3 better than Na+. Taken together with the observation of K+ binding at the external entrance, along with the functional observation that K+ has a larger effect on reducing 86Rb influx into liposomes compared to Na+ [11], it is reasonable to conclude that NaK is more selective for K+ than Na+.
2.
K+ and Rb+ binding in the NaK selectivity filter. The 2Fo-Fc ion omit maps (1.5σ) show electron density of ion binding in the K+ complex (a) and Rb+ complex (b) of NaKNΔ19. K+ and Rb+ ions are colored green with water molecules represented as red spheres. c) The maintenance of an octahedral ligand arrangement in the K+ complex, which also holds true for the Rb+ complex. Oxygen atoms from the front and back subunits chelating the ions are shown as red spheres. d) FCs soak − FK difference map contoured at 10 σ showing Cs+ binding at site 3.
Analysis of Cs+ binding in NaK filter
Our analysis of monovalent cation binding in NaK was also extended to the larger Cs+ ions. Even though we failed to produce crystals of NaKNΔ19 grown in 100 mM CsCl, the structure of a K+ complex of NaKNΔ19 soaked in a stabilization solution containing 67 mM KCl and 33 mM CsCl (Methods) was determined at 1.8 Å resolution. The FCs soak − FK difference map between this soaked crystal and the native K+ complex crystal revealed a strong peak at site 3 (Fig. 2d). No K+ replacement by Cs+ was observed at the external entrance or site 4, indicating that Cs+ appears to have a strong preference for site 3 with no detectable binding at the extracellular entrance and site 4 in the presence of K+.
Divalent cation binding in the NaK filter
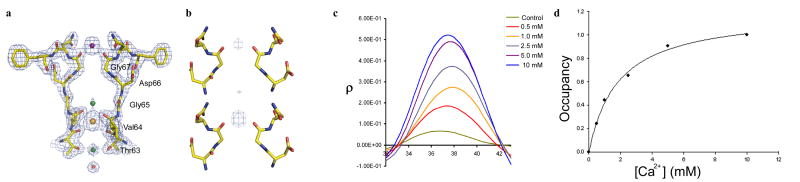
Although we were unable to crystallize NaKNΔ19 in CaCl2 alone, we co-crystallized NaKNΔ19 in the presence of CaCl2 in addition to 100 mM NaCl. In the presence of 10 mM Ca2+, clear density observed at the external entrance in a 2Fo-Fc ion omit map (Fig. 3a) which, considering no obvious Na+ binding at this site, can be attributed to Ca2+ binding. To probe the binding affinity of Ca2+ at this site, we performed a crystallographic titration assay by obtaining NaKNΔ19 crystals at different Ca2+ concentrations (0.5, 1.0, 2.5, 5.0 and 10 mM). Figure 3b shows two examples of the resulting difference maps between crystals with and without Ca2+ (F0.5mM Ca2+ − F0mM Ca2+ and F5mM Ca2+ − F0mM Ca2+), revealing stronger electron density from bound Ca2+ at the external site at higher [Ca2+]. Since the only variable in crystallization conditions from which all crystals were obtained was the Ca2+ concentration and all diffraction data were scaled against that of the same native crystal, the difference in intensity of the electron density at the external entrance reflects the extent of Ca2+ binding, or occupancy, at the external site. Figure 3c shows a one-dimensional electron density profile corresponding to Ca2+ binding at the external entrance plotted along the central axis of the filter (Methods). An increase in Ca2+ occupancy is clearly seen with increasing Ca2+ concentrations. Crystals grown in 5 and 10 mM Ca2+ display a similar intensity of electron density, pointing to a saturation of Ca2+ binding at 10mM. This can be further verified by refining Ca2+ occupancy of the NaKNΔ19 co-crystallized with 10 mM Ca2+, which gives rise to a value of 0.99. Assuming a spherical distribution of electron density from the bound Ca2+, the integration of the area under each peak of the 1-D electron density profile and plotting these values against Ca2+ concentration (Fig. 3d) yields a Kd of 1.8 mM for the external site at pH 7.5 and in the presence of 100 mM Na+, conditions under which the associated crystals were obtained. Ca2+ has also been shown to bind site 3 previously [10]. However its affinity cannot be determined by a similar titration assay because of competition from Na+ and trace element binding at this site. Nevertheless Ca2+ binding at site 3 can be further confirmed by analyzing the effect of increasing Ca2+ concentration on the electron density profile at site 3. It is clear that increasing Ca2+ concentrations cause a corresponding decrease in electron density at this site, indicated by stronger peaks in the F0 Ca2+-F+ Ca2+ difference maps (supplementary fig. 4). This points to an increase in the replacement of contaminating heavy atom by the lighter Ca2+ ions upon increasing Ca2+ concentrations.
3.
Ca2+ binding in the NaK selectivity filter. (a) 2Fo-Fc ion omit map (1.5σ) of the NaKNΔ19-Na+ complex co-crystallized with 10mM CaCl2, showing electron density for a Ca2+ ion (purple sphere) at the external entrance. Na+ and the contaminant at site 3 are modeled as green and orange spheres, respectively. Electron density for the protein backbone is also shown as a blue mesh. (b) F0.5mM Ca − F0mM Ca (upper) and F5mM Ca2+ − F0mM Ca2+ (lower) difference map contoured at 10 σ showing increasing intensity of Ca2+ binding upon increasing Ca2+ concentration. Dotted lines indicate the position of central axis along which the 1-D electron density was calculated. (c) 1-D electron density profile of Ca2+ binding at the external entrance showing increased Ca2+ binding at increasing concentrations. The control is from a difference map between two native crystals grown in the absence of Ca2+. (d) Occupancy of Ca2+ binding at the external site was calculated by integration of the peaks in 1-D electron density profile and normalizing them against the 10 mM Ca2+ peak. The plot of occupancy against Ca2+ concentration give rise to a Kd value of about 1.8 mM upon fitting the curve to a single component Langmuir equation.
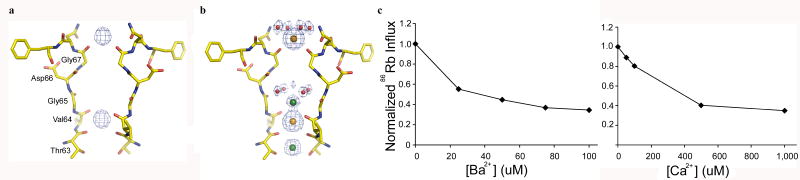
Attempts at crystallizing NaKNΔ19 in the presence of Ba2+ also failed to yield high quality crystals. Consequently, we soaked a crystal of the K+-complex of NaKNΔ19 in a stabilization solution containing 67 mM KCl and 33 mM BaCl2 (Methods) to analyze Ba2+ binding and determined its structure at 2.0 Å resolution. The FBa2+ soak − FK difference map between the Ba2+ soaked crystal and the native K+ complex crystal confirms two Ba2+ binding positions, the external entrance and site 3, as was observed before[11] (Fig. 4a). Even though Ba2+ is present at half the concentration of K+, refinement of the ion at the external site as Ba2+ in the soaked crystal gives rise to an occupancy of 0.97. This indicates an almost complete replacement of K+ at the external site, suggesting a higher affinity for Ba2+ than K+. Ba2+ coordination at the external site is similar to that of K+ and Rb+, with 4 water molecules and 4 carbonyl oxygen atoms of Gly67s serving as the 8 ligands (Fig. 4b). This is different from Ca2+ binding, where no hydration shell was observed in external Ca2+ chelation (fig. 3a). This does not necessary mean that water molecules do not participate in external Ca2+ chelation. It could simply be that because Ca2+ prefers 6 or 7 ligands as in other Ca2+ binding motifs like the EF-hand [16-18], the symmetry mismatch between the channel and the configuration of Ca2+ chelation leads to a 4-fold reduction in occupancy of the water molecules. It is also interesting to note that even though sites 3 & 4 in KcsA have a virtually identical chemical environment as the corresponding sites in NaK, Ba2+ only binds at the external entrance and site 3 in NaK instead of sites 2 and 4 as in KcsA [19, 20].
4.
Ba2+ binding in NaK filter. a) FBa soak − FK difference map (blue mesh) contoured at 10 σ showing the two Ba2+ binding sites. (b) 2Fo-Fc ion omit maps of Ba2+-soaked crystal contoured at 1.5 σ. The electron density at external entrance and site 3 was modeled as Ba2+ (orange spheres) and water molecules are modeled as red spheres. (c) Ba2+ (left) and Ca2+ (right) blocking of 86Rb influx in liposomes loaded with NaCl.
Although we were unable to carry out an analysis of Ba2+ affinity at both sites, we expect it to be fairly high for at least one of them based on results from 86Rb flux assays. Figure 4c shows an analysis of Ca2+ and Ba2+ blocking of 86Rb influx in liposomes loaded with NaCl. It is clear that Ba2+ displays much stronger concentration dependent blocking of 86Rb influx compared to Ca2+ (Fig. 4c).
Discussion
The four well defined ion binding sites in the NaK filter can be divided into three subtypes based on the nature of their ligands: carbonyl-water for the external site and vestibule, carbonyl-carbonyl for site 3, and carbonyl-hydroxyl for site 4. Despite their similar chemical environments, these sites display a different ion binding preference as summarized in Table 1.
Table 1. Ion Binding Properties of individual Sites In the NaK Selectivity Filter.
| Site | Ligand Type | Ion-ligand distance (Å) | Selectivity | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Na+ | K+ | Rb+ | Ba2+ | Ca2+ | |||
|
| |||||||
| External | H2O | 3.06 | 2.77 | 2.94 | K+, Rb+, Ca2+, Ba2+ | ||
| C=O (Gly67) | 2.52 | 2.50 | 2.73 | 2.43 | |||
|
| |||||||
| Vestibule | H2O | 4.02 | 2.96 | 2.85 | Na+, K+, Rb+ | ||
| C=O (Val64) | 2.94 | 3.00 | 3.11 | ||||
|
| |||||||
| Site 3 | C=O (Val64) | 2.80 | 2.97 | Na+, K+, Rb+, Cs+, Ca2+, Ba2+, others* | |||
| C=O (Thr63) | 2.83 | 2.94 | |||||
|
| |||||||
| Site 4 | C=O (Thr63) | 2.97 | 2.98 | Na+, K+, Rb+ | |||
| -OH (Thr63) | 2.38 | 2.79 | 2.89 | ||||
refers to unknown contaminating ions.
The external site, formed by 4 carbonyl oxygen atoms from Gly 67 along with extracellular water molecules can bind both mono (K+ and Rb+) and divalent (Ca2+ and Ba2+) cations. Interestingly, no obvious Na+ binding was observed at this site. This site is interesting in its apparently greater selectivity for divalent cations, which arises from the through space interaction between Asp66 and the Gly67-Asn68 peptide bond as reported previously [10].
With the involvement of four water molecules, the position of ion binding in the vestibule of the NaK selectivity filter is equivalent to that of site 2 in KcsA. However, the mobility of the water molecules imparts greater flexibility in ion binding, allowing the site to accommodate various monovalent cations (Na+, K+, Rb+). Thus, even though the highly K+ selective sites 1 & 2 of K+ channels are absent, the channel is still equipped for optimal K+ conduction through the use of water molecules that help create a ligand geometry similar to that in K+ channel selectivity filters. These four water molecules have longer ion-water distances in the Na+-complex than the K+-complex and do not form an inner hydration shell around Na+ ions. This might be a consequence of Na+ having a much smaller radius than K+ or Rb+, and its preference for smaller hydration shells with 5∼6 water molecules [21, 22], which could spatially prevent an octahedral arrangement of ligands for Na+ coordination.
Sites 3 and 4 in NaK are virtually identical to those in K+ channels in terms of structure and ligand type as shown in the superimposition of their selectivity filter regions (Fig 5a). However, they exhibit vastly different ion binding properties. Site 3 in NaK is of particular interest as it appears to be the most non-selective, accommodating both mono- and divalent cations with wide ranging radii. The non-selective nature of this site also makes it inevitably susceptible to contamination from trace elements, leading to the presence of unknown species of ions at this site. Three major differences in ion binding at sites 3 and 4 between NaK and K+ channels are obvious. First, Ba2+, a well known K+ channel blocker [23-26], binds at site 4 in K+ channels but at site 3 in NaK. Second, Cs+ can bind at both sites in K+ channels but prefers to bind at site 3 in NaK in the presence of K+. Finally, while Na+ binds at both sites in NaK, no Na+ binding was observed in K+ channels. In light of no obvious structural differences in sites 3 and 4 between NaK and K+ channels, these differences in Ba2+ and Cs+ binding are intriguing and the underlying features that give rise to them require further investigation.
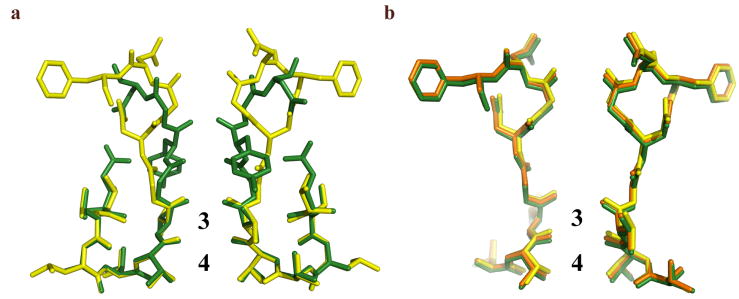
5.
Comparison of the selectivity filter structures of NaK and KcsA and between various ion complexes of NaK. a) Superimposition of the NaK selectivity filter (yellow) in complex with K+ with that of KcsA (green, PDB code 1K4C). b) Superimposition of the selectivity filters of the NaKNΔ19 channel in complex with Na+ (yellow), K+ (green), and Rb+ (orange).
The unique ability of the NaK selectivity filter to utilize water molecules in sync with carbonyl oxygen atoms has important consequences for ion chelation. The ability to form octahedral ion chelation schemes seemingly makes the NaK selectivity filter more optimized for K+ binding than Na+. This hypothesis is supported by data from competition assays where externally added K+ has a more pronounced effect on reducing 86Rb accumulation in liposomes compared to Na+ [11]. Theoretical studies of K+ selectivity based on the KcsA structure have also suggested that an octahedral arrangement of oxygen ligands in the channel pore is more favorable for K+ than Na+([27-29]. However, this K+ over Na+ selectivity in NaK clearly does not occur to the same extent as in K+ channels and NaK is able to conduct Na+ well. Overall, we believe two factors may be key contributors to the ion selectivity differences observed between NaK and K+ channels. The first is the apparent need for K+ ions to stabilize the selectivity filter of K+ channels in the ‘conducting’ state. Replacement of K+ with Na+ leads to a ‘collapsed’ filter which yields the channel non conductive [30]. Such a dependence on K+ ions for maintaining the structural integrity of the selectivity filter is not observed in the NaK channel, which maintains an almost identical structure in complex with Na+, K+, or Rb+ as shown in figure 5b. It is worth noting that the presence of contaminating ions at site 3 makes it difficult to make any definitive assertions regarding subtle structural differences in the NaK selectivity filter. However, it is still clear that major rearrangements do not occur upon binding of different ions, as a mixture of proteins with two conformations at the filter region is not expected to yield well diffracting crystals. The structural stability of the NaK selectivity filter can be partly attributed to strong H-bonding interactions between Asp66 and the backbone amide of Asn68. Another cause could be related to how well Na+ ions bind within the selectivity filter. In a recent study, mutation of a conserved Glycine (Gly77) residue to D-Ala in KcsA yielded a channel that maintained a conductive selectivity filter even in the presence of Na+ [31]. The mutant channel can conduct Na+ but is still very selective for K+, suggesting that the 4 equivalent K+ binding sites in a K+ channel selectivity filter are not energetically favorable for Na+ binding. The replacement of K+ channel sites 1&2 with a water-filled vestibule in NaK may overcome this energy barrier and make Na+ binding less unfavorable. Na+ ions display versatility in relative positioning and number of ligands, as shown in the NaKNΔ19-Na+ complex. Ions are not restricted to the middle of the ion binding sites, but bind at the upper and lower edges instead, in plane with 4 carbonyl or hydroxyl oxygen atoms. A well ordered water molecule in the cavity just below the selectivity filter participates in a pyramidal Na+ chelation at site 4. This ligand geometry effectively reduces the ion-ligand distance and the coordination number of Na+ to 2.4 Å and 5, respectively, consistent with the statistics of Na+ coordination commonly observed in water, small molecules, or proteins [21, 32]. This planar configuration could very well be the case for a Na+ ion at site 3 as the ion could be stabilized by a water molecule in the vestibule. Therefore, by having partial exposure to solvent, Na+ can bind both at site 3 and 4 in a favorable planar configuration as also has been suggested in the recent computational studies of ion selectivity and conduction in NaK [13, 14].
Methods
Protein expression, purification, and crystallization
Detailed experimental procedures are described in our companion paper. Na+, K+, and Rb+ complexes of NaKNΔ19 were purified with the corresponding salts (100 mM NaCl, KCl or RbCl) and crystallized over a well solution containing 55-70% (v/v)(±)-2-Methyl-2,4-pentanediol (MPD) and 100mM Hepes pH 7.5. All crystals used for the Ca2+ titration assay were obtained by using proteins purified in 100 mM NaCl and with the addition of 0.5-10mM CaCl2 to the crystallization well solution. For soaking experiments, crystals of the NaKNΔ19-K+ complex were soaked in stabilization solutions containing 67 mM KCl and 33 mM CsCl or BaCl2 for one day.
A majority of the crystals suffered from either partial or perfect twinning [33]. Several data sets were therefore collected for each of the structures reported here and in our companion study to obtain at least one untwinned data set of high quality. The only exception was the data set collected for NaKND19-Rb+ complex, which has a twinning fraction of 20% and was detwinned in CCP4 [34] before being used for refinement. All statistics of data collection and refinements are listed in Table 2.
Table 2. Data collection and refinement statistics for NaKNΔ19 in complex with various cations.
| NaKNΔ19-complex Conentrations (mM) | Na+
100 |
Na+
500 |
K+
100 |
Rb+
100 |
Na+/Ca2+
100/10 |
K+/Ba2+
67/33 |
|---|---|---|---|---|---|---|
| Data collection | ||||||
|
| ||||||
| Space group | I4 | I4 | I4 | I4 | I4 | I4 |
| Cell dimensions | ||||||
| a=b(Å) | 68.050 | 68.056 | 68.194 | 68.134 | 67.990 | 68.226 |
| c (Å) | 89.251 | 89.326 | 89.294 | 89.359 | 89.168 | 89.548 |
| Resolution (Å) | 50 - 1.8 | 50 - 1.8 | 50 - 1.8 | 50 - 1.7 | 50 - 2.0 | 50 - 2.0 |
| Rsym (%) | 5.1 (65.5) | 6.4 (82.1) | 4.9 (56) | 6.2 (59.9) | 5.7 (34.3) | 8.4 (60.4) |
| I/σI | 36 (2) | 30 (1.5) | 41 (2) | 31 (2) | 38 (2.5) | 26 (2.0) |
| Completeness (%) | 99.3(99.3) | 99.6(99.7) | 99.6(99.6) | 96.6(71.3) | 97.2(81.6) | 99.9(99.4) |
| Redundancy | 6.8 (5.9) | 6.8 (5.4) | 7.0 (5.1) | 6.8 (4.4) | 6.6 (4.7) | 6.9 (5.1) |
| Refinement | ||||||
| Resolution (Å) | 1.8. | 1.8 | 1.8 | 1.7 | 2.0 | 2.0 |
| No. Reflections | 18808 | 18965 | 18948 | 21705 | 13398 | 13987 |
| Rwork / Rfree | 21.3/24.8 | 21.5/23.6 | 21.2/23.4 | 22.0/22.9 | 20.0/23.5 | 20.1/24.8 |
| No. atoms | ||||||
| Protein | 1462 | 1462 | 1462 | 1462 | 1462 | 1462 |
| MPD/ Ion | 24/6 | 0/5 | 32/10 | 32/11 | 24/8 | 16/10 |
| Water | 144 | 111 | 96 | 94 | 52 | 57 |
| B-factors | ||||||
| Protein | 37.60 | 36.06 | 34.692 | 30.583 | 49.385 | 39.206 |
| Ligand/ion | 49.03 | 30.592 | 50.317 | 51.79 | 67.043 | 51.841 |
| Water | 60.855 | 56.592 | 53.276 | 47.63 | 70.243 | 54.492 |
| R.m.s.d | ||||||
| Bond lengths (Å) | 0.0060 | 0.0084 | 0.0053 | 0.0059 | 0.0062 | 0.0060 |
| Bond angles (°) | 1.089 | 1.260 | 1.070 | 1.116 | 1.099 | 1.068 |
Values in parentheses are for highest-resolution shell.
One dimensional electron density profiles of external Ca2+ binding were obtained as described [35]. All data from Na+ complexes co-crystallized with 0.5 – 10 mM Ca2+ were scaled to 2.1 Å using the native NaKND19-Na+ crystal (without Ca2+) as a reference. 1-D profiles were obtained by sampling the F0.5-10mM Ca-F0mM Ca difference maps along the z/central axis at the external site using MAPMAN [36]. Ion occupancy was obtained by integrating the area under each peak on the 1-D profile and normalizing it against that of the 10 mM Ca2+ peak.
86Rb Competition assays
The divalent cation (Ca2+ and Ba2+) blockage of the NaK channel was studied by analyzing the competing effect of Ca2+ and Ba2+ in 86Rb flux assay. In this competition assay, the tested ions (Ca2+ and Ba2+) were added directly into the flux buffer at appropriate concentrations to give the desired final concentrations upon addition of liposomes. Ion flux was then allowed to proceed for 10 minutes before radioactivity levels were counted as described.
Coordinates
The atomic coordinates and structure factors of NaKNΔ19 have been deposited in the Protein Data Bank with accession numbers of 3E8H (K+ complex), 3E8F (K+/Ba2+ complex), 3E8B (Rb+ complex), 3E89(100mM Na+ complex,), 3E83 (500mM Na+ complex), and 3E8G (100mM Na+/10mM Ca2+ complex)
Supplementary Material
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.
Acknowledgments
Use of the Argonne National Laboratory Structural Biology Center beamlines at the Advanced Photon Source was supported by the US Department of Energy, Office of Energy Research. We thank the beamline staff for assistance in data collection. This work was supported by Howard Hughes Medical Institute and by grants from the NIH/NIGMS (RO1 GM079179), David and Lucile Packard Foundation and McKnight Endowment for Neuroscience. A.A. was supported by National Institutes of Health Training Grant T32 GM008297.
Footnotes
Author contributions. A.A performed the research. A.A & Y.J designed the research, analyzed data, and wrote the manuscript.
Competing interests statement: The authors declared no competing interests.
References
- 1.Hille B. Ion Channels of Excitable Membranes. 3rd. Sinauer Associates, Inc.; Sunderland, MA: 2001. [Google Scholar]
- 2.Long SB, et al. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450(7168):376–82. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 3.Kuo A, et al. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300(5627):1922–6. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, et al. The open pore conformation of potassium channels. Nature. 2002;417(6888):523–6. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, et al. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423(6935):33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 6.Long SB, Campbell EB, Mackinnon R. Crystal Structure of a Mammalian Voltage-Dependent Shaker Family K+ Channel. Science. 2005 doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 7.Nishida M, et al. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. Embo J. 2007;26(17):4005–15. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noskov SY, Berneche S, Roux B. Control of ion selectivity in potassium channels by electrostatic and dynamic properties of carbonyl ligands. Nature. 2004;431(7010):830–4. doi: 10.1038/nature02943. [DOI] [PubMed] [Google Scholar]
- 9.Gouaux E, Mackinnon R. Principles of selective ion transport in channels and pumps. Science. 2005;310(5753):1461–5. doi: 10.1126/science.1113666. [DOI] [PubMed] [Google Scholar]
- 10.Alam A, Shi N, Jiang Y. Structural insight into Ca2+ specificity in tetrameric cation channels. Proc Natl Acad Sci U S A. 2007;104(39):15334–9. doi: 10.1073/pnas.0707324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi N, et al. Atomic structure of a Na+- and K+-conducting channel. Nature. 2006;440(7083):570–4. doi: 10.1038/nature04508. [DOI] [PubMed] [Google Scholar]
- 12.Phillips K, et al. The crystal structure of a parallel-stranded guanine tetraplex at 0.95 A resolution. J Mol Biol. 1997;273(1):171–82. doi: 10.1006/jmbi.1997.1292. [DOI] [PubMed] [Google Scholar]
- 13.Vora T, Bisset D, Chung SH. Conduction of Na+ and K+ through the NaK channel: molecular and Brownian dynamics studies. Biophys J. 2008;95(4):1600–11. doi: 10.1529/biophysj.107.126722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noskov SY, Roux B. Importance of hydration and dynamics on the selectivity of the KcsA and NaK channels. J Gen Physiol. 2007;129(2):135–43. doi: 10.1085/jgp.200609633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, et al. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414(6859):43–8. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 16.Katz AK, et al. Calcium Ion Coordination: A Comparison with That of Beryllium, Magnesium, and Zinc. J Am Chem Soc. 1996;118(24):5752–5763. [Google Scholar]
- 17.Pidcock E, Moore GR. Structural characteristics of protein binding sites for calcium and lanthanide ions. J Biol Inorg Chem. 2001;6(5-6):479–89. doi: 10.1007/s007750100214. [DOI] [PubMed] [Google Scholar]
- 18.Lewit-Bentley A, Rety S. EF-hand calcium-binding proteins. Curr Opin Struct Biol. 2000;10(6):637–43. doi: 10.1016/s0959-440x(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, MacKinnon R. The barium site in a potassium channel by x-ray crystallography. J Gen Physiol. 2000;115(3):269–72. doi: 10.1085/jgp.115.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockless SW, Zhou M, MacKinnon R. Structural and thermodynamic properties of selective ion binding in a K+ channel. PLoS Biol. 2007;5(5):e121. doi: 10.1371/journal.pbio.0050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varma S, Rempe SB. Coordination numbers of alkali metal ions in aqueous solutions. Biophys Chem. 2006;124(3):192–9. doi: 10.1016/j.bpc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Anan Tongaarm KRL, Rode Bernd M. Born-Oppenheimer ab Initio QM/MM Dynamics Simulations of Na+ and K+ in Water: From Structure Making to Structure Breaking Effects. J Phys Chem A. 1998;102:10340–10347. [Google Scholar]
- 23.Neyton J, Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel. J Gen Physiol. 1988;92(5):549–67. doi: 10.1085/jgp.92.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neyton J, Miller C. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+ -activated K+ channel. J Gen Physiol. 1988;92(5):569–86. doi: 10.1085/jgp.92.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong CM, Swenson RP, Jr, Taylor SR. Block of squid axon K channels by internally and externally applied barium ions. J Gen Physiol. 1982;80(5):663–82. doi: 10.1085/jgp.80.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong CM, Taylor SR. Interaction of barium ions with potassium channels in squid giant axons. Biophys J. 1980;30(3):473–88. doi: 10.1016/S0006-3495(80)85108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varma S, Sabo D, Rempe SB. K+/Na+ selectivity in K channels and valinomycin: over-coordination versus cavity-size constraints. J Mol Biol. 2008;376(1):13–22. doi: 10.1016/j.jmb.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varma S, Rempe SB. Tuning ion coordination architectures to enable selective partitioning. Biophys J. 2007;93(4):1093–9. doi: 10.1529/biophysj.107.107482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bostick DL, Brooks CL., 3rd Selectivity in K+ channels is due to topological control of the permeant ion's coordinated state. Proc Natl Acad Sci U S A. 2007;104(22):9260–5. doi: 10.1073/pnas.0700554104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, MacKinnon R. The occupancy of ions in the K+ selectivity filter: charge balance and coupling of ion binding to a protein conformational change underlie high conduction rates. J Mol Biol. 2003;333(5):965–75. doi: 10.1016/j.jmb.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Valiyaveetil FI, et al. Ion selectivity in a semisynthetic K+ channel locked in the conductive conformation. Science. 2006;314(5801):1004–7. doi: 10.1126/science.1133415. [DOI] [PubMed] [Google Scholar]
- 32.Harding MM. Metal-ligand geometry relevant to proteins and in proteins: sodium and potassium. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 5):872–4. doi: 10.1107/s0907444902003712. [DOI] [PubMed] [Google Scholar]
- 33.Yeates TO. Detecting and overcoming crystal twinning. Methods Enzymol. 1997;276:344–58. [PubMed] [Google Scholar]
- 34.Collaborative Computational Project, N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 35.Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414(6859):37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 36.Kleywegt GJ, Jones TA. xdlMAPMAN and xdlDATAMAN - programs for reformatting, analysis and manipulation of biomacromolecular electron-density maps and reflection data sets. Acta Crystallogr D Biol Crystallogr. 1996;52(Pt 4):826–8. doi: 10.1107/S0907444995014983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.