Abstract
Epithelial cells, once dissociated and placed in two-dimensional (2D) cultures, rapidly lose tissue-specific functions. We showed previously that in addition to prolactin, signaling by laminin-111 was necessary to restore functional differentiation of mammary epithelia. Here, we elucidate two additional aspects of laminin-111 action. We show that in 2D cultures, the prolactin receptor is basolaterally localized and physically segregated from its apically placed ligand. Detachment of the cells exposes the receptor to ligation by prolactin leading to signal transducers and activators of transcription protein 5 (STAT5) activation, but only transiently and not sufficiently for induction of milk protein expression. We show that laminin-111 reorganizes mammary cells into polarized acini, allowing both the exposure of the prolactin receptor and sustained activation of STAT5. The use of constitutively active STAT5 constructs showed that the latter is necessary and sufficient for chromatin reorganization and β-casein transcription. These results underscore the crucial role of continuous laminin signaling and polarized tissue architecture in maintenance of transcription factor activation, chromatin organization, and tissue-specific gene expression.
Introduction
The activity of several transcription factors is essential for mammary gland development and milk protein expression (for review see Rosen et al., 1999). One of these factors, signal transducers and activators of transcription protein 5 (STAT5), is a downstream target of the prolactin receptor (PrlR)–JAK2 signaling pathway (Gouilleux et al., 1994; Watson and Burdon, 1996). Binding of prolactin (Prl) to its receptor induces STAT5 phosphorylation. Phosphorylated STAT5 dimerizes and translocates to the nucleus where it binds specific DNA motifs to induce gene expression (Gouilleux et al., 1994). However, treatment with Prl alone does not activate the JAK2–STAT5 pathway in primary or immortalized mammary epithelial cells in 2D cultures (Streuli et al., 1995a); additional signals from ECM are required for Prl to induce STAT5 phosphorylation and DNA binding (Streuli et al., 1995a; Xu et al., 2007). The data in vivo are consistent with these findings in that β1-integrin signaling contributes to nuclear translocation of STAT5 in the mammary gland (Faraldo et al., 2002). However, despite considerable literature, much of it from our own laboratory, how cells in 2D cultures resist signaling by Prl has not been elucidated.
3D tissue architecture is essential for induction and maintenance of tissue-specific functions of epithelial organs. Both physical and biochemical signals from ECM are necessary for expression of specific cellular functions (Roskelley et al., 1994; Wipff et al., 2007; Alcaraz et al., 2008). Luminal mammary epithelial cells form relatively flat and polarized monolayers when cultured on 2D substrata, but fail to express milk proteins in response to treatment with lactogenic hormones. When cultured in 3D laminin-rich ECM (lrECM) gels, they form polarized acinar structures with a central lumen (Barcellos-Hoff et al., 1989). Under these conditions and in the presence of the lactogenic hormones Prl and hydrocortisone, mammary cells functionally differentiate and express milk proteins, such as β- and γ-caseins.
Laminin-111 (formerly laminin-1) is a major basement membrane (BM) component required for milk protein expression (Streuli et al., 1991; Streuli et al., 1995b; Muschler et al., 1999). Previously, we identified two types of cellular response to laminin-111 in mammary epithelial cells that are necessary for inducing functional differentiation: (1) changes in cytostructure mediated by dystroglycan (DG; Muschler et al., 1999; Weir et al., 2006), which can be mimicked physically by plating cells on the nonadhesive substratum poly(2-hydroxyethylmethacrylate) (polyHEMA; Roskelley et al., 1994), and (2) transmission of biochemical signals mediated by β1-integrin (Streuli et al., 1991; Roskelley et al., 1994; Muschler et al., 1999).
The plasma membrane of epithelial cells is separated by tight junctions into apical and basolateral surfaces with different protein and lipid components (Nelson, 2003). In 2D culture of polarized cells, the basal membrane is adherent to the substrata, and basolaterally localized receptors in tight monolayers of epithelial cells may consequently be prevented from binding to their apically presented ligands (Vermeer et al., 2003). We asked whether mammary epithelial cells fail to differentiate in response to Prl because of the limited accessibility of PrlR. We demonstrate here that this indeed is the case in 2D cultures. But we show also that Prl binding and transient STAT5 activation are not sufficient to activate tissue-specific functions. We show that ECM-dependent sustained activation of STAT5 is necessary for chromatin remodeling of mammary-specific gene loci, allowing tissue-specific gene expression.
Results
Transient STAT5 activation is insufficient to induce mammary-specific functions
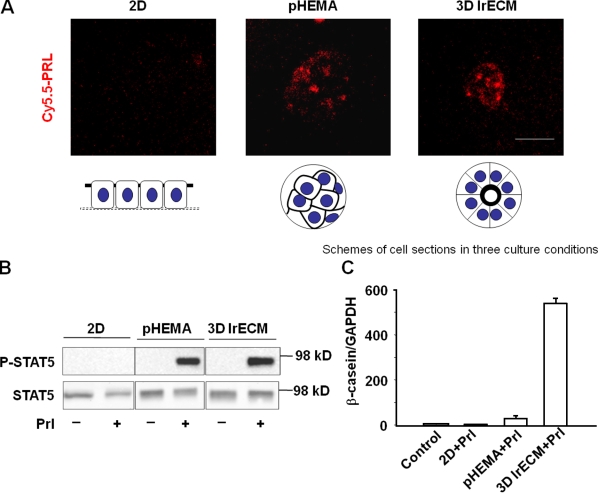
Mammary epithelial cells in 2D cultures fail to undergo functional differentiation even in the presence of the lactogenic hormone Prl. When cells were placed on gels of type I collagen and allowed to float, they reorganized and were shown to contain some caseins (Emerman et al., 1977). We showed subsequently that caseins were induced de novo under these conditions (Lee et al., 1985) and that expression was dependent on the presence of laminin-111 and formation of a BM (Li et al., 1987; Barcellos-Hoff et al., 1989; Streuli et al., 1991; Muschler et al., 1999; Xu et al., 2007). More recently, it was shown that STAT5 is not activated in 2D cultures and that activation in 3D cultures depends on signaling from laminin-111 (Streuli et al., 1995a; Akhtar and Streuli, 2006; Xu et al., 2007). Here, we asked whether PrlR was present and, if so, whether Prl could bind its receptor in cells cultured in monolayers. We showed that the levels of PrlR were similar between cells on plastic and cells treated with lrECM (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200807021/DC1). To show whether or not Prl can bind to its receptor, we conjugated Prl with Cy5.5 and found that indeed the receptor was not available for binding, explaining the failure to induce STAT5 phosphorylation under these conditions (Fig. 1, A and B). However, when cells were cultured either in 3D lrECM or on the nonadhesive and inert substratum polyHEMA (Roskelley et al., 1994), the labeled Prl bound to the receptor and induced rapid phosphorylation of STAT5 (Fig. 1, A and B). Surprisingly, however, only cells in 3D lrECM produced appreciable quantities of β- and γ-casein (Fig. 1 C and not depicted) in response to Prl treatment. These results clearly indicate that the transient STAT5 activation does not require signals from laminin-111, and ligation of Prl by itself, or even the transient activation of STAT5, is not sufficient to induce tissue-specific functions. These findings led to two subsequent questions. (1) Why does Prl fail to bind to its receptor in 2D cultures? (2) What does laminin-111 signaling provide in addition to transient STAT5 phosphorylation to induce milk protein expression?
Figure 1.
A transient STAT5 activation is not sufficient to induce mammary-specific gene expression. (A) Confocal images of Cy5.5-labeled Prl incubated EpH4 cells. EpH4 cells were cultured in 2D on plastic, on polyHEMA (pHEMA), and in 3D lrECM. Bar, 25 μm. (B) Western blot analysis of STAT5 phosphorylation in EpH4 cells 10 min after Prl treatment. EpH4 cells were cultured in 2D on plastic, on polyHEMA, and in 3D lrECM for 24 h. (C) Quantification of β-casein mRNA levels by real-time RT-PCR. EpH4 cells cultured on plastic, on polyHEMA, and in 3D lrECM were treated with Prl for 24 h before RNA extraction. The bar graph represents the mean ± SEM.
Basolateral localization of PrlR in monolayer cultures prevents interaction with its apically presented ligand
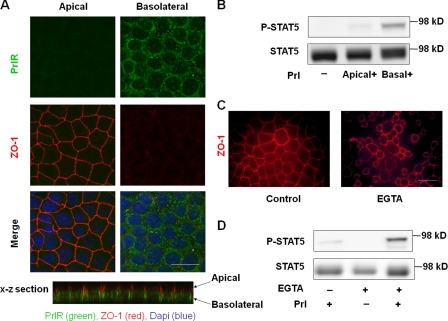
Several receptors are localized basolaterally in epithelial cells and are separated from their apical ligands (Vermeer et al., 2003; Murphy et al., 2004). We reasoned that PrlR was basolaterally localized in 2D cultures and therefore not accessible to its ligand. EpH4 cells were grown to confluence on permeable filters in Transwell plates and stained for both PrlR and the apical marker ZO-1. Confocal analysis showed that PrlR localized mainly to the basolateral membrane (Fig. 2 A). Polarized EpH4 cells were treated with Prl in either the upper (apical) or lower (basal) chambers, and PrlR activation was examined by Western blot analysis with an antibody against phosphorylated STAT5. Whereas adding Prl to the basal chamber activated STAT5 phosphorylation dramatically, adding it to the apical chamber had little or no effect (Fig. 2 B). In cells where tight junctions were disrupted by chelating Ca2+ with EGTA (Fig. 2 C), apical addition of Prl induced STAT5 phosphorylation (Fig. 2 D). These data reveal that it is the accessibility of the receptor to apically added ligand that limits Prl-induced STAT5 activation in monolayer cultures.
Figure 2.
PrlR localizes basolaterally in mammary epithelial cells cultured in 2D. (A) Immunofluorescence analysis of PrlR (green) and the apical marker ZO-1 (red) in monolayers of EpH4 cells demonstrated that PrlR was localized mainly to the basolateral surface. (B) Western blot analysis of STAT5 phosphorylation in response to Prl treatment. Monolayers of EpH4 cells on permeable filters were incubated with Prl added to apical or basal surfaces for 10 min. (C) Immunofluorescence analysis of ZO-1 in monolayers of EpH4 cells after 5 mM EGTA treatment. (D) Western blot analysis of STAT5 phosphorylation. Monolayers of EpH4 cells were incubated with EGTA for 30 and 60 min followed by treatment with Prl for 10 min. Bars, 25 μm.
Sustained reactivation of STAT5 is necessary for the mammary-specific function
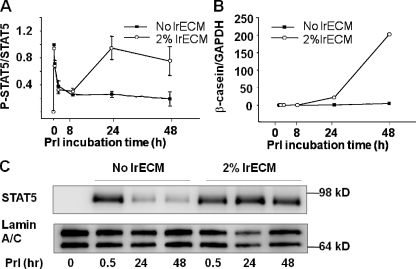
To understand why laminin-111 in addition to Prl binding is required for mammary-specific gene expression, we analyzed the time course of STAT5 activation. When EpH4 cells were treated with Prl in the presence or absence of lrECM on polyHEMA, an initial peak of STAT5 phosphorylation was induced immediately under both conditions, but subsided over the next 2 h irrespective of the presence or absence of lrECM (Fig. 3 A). In the presence of lrECM, however, STAT5 phosphorylation was activated again after 8 h, reached a peak at 24 h and persisted at a relatively high level until at least 48 h (Fig. 3 A). Importantly, the expression of both β- and γ-caseins (Fig. 3 B and not depicted) corresponded with the second wave of STAT5 activation (Fig. 3 C). Consistent with its increased phosphorylation, nuclear levels of STAT5 in lrECM-treated cells were also higher than in the control cultures at 24 and 48 h (Fig. 3 C). We confirmed that in both primary cultures of mammary epithelial cells as well as in SCp2, another mammary epithelial cell line, sustained STAT5 reactivation occurs in a lrECM-dependent manner (Fig. S2, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200807021/DC1).
Figure 3.
β-Casein transcription corresponds with lrECM-induced sustained STAT5 activation. (A) EpH4 cells on polyHEMA were treated with Prl alone or Prl plus 2% lrECM for different intervals. STAT5 phosphorylation was detected by Western blotting. The Western blot results were quantified by AlphaEaseFC software and expressed as relative levels of phosphorylated STAT5 to total STAT5 (n = 3). (B) Quantification of β-casein mRNA levels by real-time RT-PCR. EpH4 cells on polyHEMA were treated with Prl alone or Prl plus 2% lrECM for different time points before RNA extraction. (C) Western blot analysis of nuclear levels of STAT5 in EpH4 cells. Cells on polyHEMA were treated with Prl alone or Prl plus 2% lrECM for 30 min or 24 or 48 h.
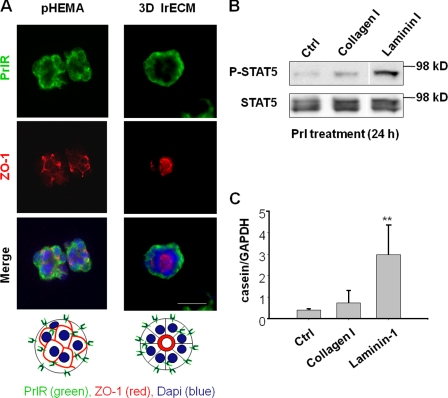
PrlR staining showed that the localization of the receptor did not change after lrECM treatment (Fig. 4 A), but the majority of colonies established apical (Fig. 4 A) and basal polarity (not depicted) in the presence of lrECM after 24 h. We had shown previously that expression of β-casein correlated with formation of the endogenous BM (Streuli and Bissell, 1990). Not surprisingly, we find that it is the laminin component of lrECM that is responsible for sustained activation of STAT5 (Fig. 4 B). Laminin-111, but not collagen I, activated casein transcription (Fig. 4 C). However, neither laminin-111 nor collagen I affected the transient phosphorylation of STAT5 in response to Prl treatment, confirming that the transient activation is independent of an ECM signal (unpublished data).
Figure 4.
Laminin-111 regulates sustained STAT5 activation and casein transcription. (A) Immunofluorescence analysis of PrlR and ZO-1 in EpH4 cells on polyHEMA that were treated with Prl alone (pHEMA) or Prl plus 2% lrECM (3D lrECM) for 24 h. Bar, 25 μm. (B) Western blot analysis of phosphorylated STAT5 in EpH4 cells. The cells on polyHEMA were treated with Prl plus collagen or laminin-111 at 120 μg/ml for 24 h. (C) Quantification of β-casein mRNA levels by real-time RT-PCR. EpH4 cells on polyHEMA were treated with Prl plus collagen or laminin-111 at 120 μg/ml for 24 h. The bar graph represents the mean ± SEM. **, P < 0.01; n = 4.
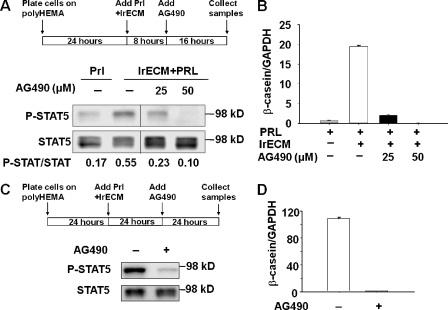
The JAK2 tyrosine kinase phosphorylates STAT5 in response to Prl (Gouilleux et al., 1994), and blocking JAK-2 activity with AG490, a specific inhibitor of JAK2, suppresses Prl-regulated STAT5 activation in mammary epithelial cells (Selvaraj et al., 2000). Addition of AG490 to mammary cells in the presence of Prl and lrECM blocked sustained STAT5 activation (Fig. 5 A) and significantly inhibited β- (Fig. 5 B) and γ-casein (not depicted) transcription. Even after sustained phosphorylation of STAT5 was achieved, the inhibitor could reverse activation (Fig. 5 C) and casein expression (Fig. 5 D). The data suggest that continuous activation of STAT5 is required to maintain the transcription of milk protein genes. It is noteworthy that in vivo STAT5 phosphorylation starts in mid- to late pregnancy, remains high during lactation, and drops shortly after involution (Liu et al., 1996), suggesting the need for sustained activation also in vivo during milk production.
Figure 5.
Sustained activation of STAT5 is necessary for the induction of β-casein transcription. (A and B) Blocking sustained STAT5 activation with AG490 inhibited β-casein transcription. After EpH4 cells on polyHEMA were incubated with Prl and 2% lrECM for 8 h, AG490 or DMSO vehicle alone were added to the media at different concentrations. (A) Western blot analysis of STAT5 reactivation in control and AG490-treated cells. (B) Quantification of β-casein mRNA levels in control and AG490-treated cells by real-time RT-PCR. (C and D) Interrupting the sustained STAT5 reactivation with AG490 significantly inhibited β-casein transcription. EpH4 cells on polyHEMA were incubated with Prl and 2% lrECM for 24 h to induce the sustained STAT5 activation; the cells were then treated with 25 μM AG490 or DMSO vehicle alone for another 24 h. (C) Western blot analysis of the sustained STAT5 activation in control and AG490-treated cells. (D) Real-time RT-PCR measuring β-casein mRNA levels after the sustained STAT5 activation was interrupted. The bar graph represents the mean ± SEM.
Sustained activation of STAT5 induces chromatin remodeling
Transcription of mammary-specific genes requires not only activation of transcription factors but also chromatin remodeling (Xu et al., 2007). We asked whether sustained STAT5 phosphorylation is necessary for histone acetylation in the promoters of β- and γ-caseins. Using chromatin immunoprecipitation (ChIP), we show here that levels of acetylated H3 and H4 in the β- (Fig. 6 A) and γ-casein (not depicted) promoters were not significantly different from control cultures after 30 min of Prl treatment. In contrast, acetylated histone accumulated in the casein promoters after 24 h of treatment with Prl and lrECM (Fig. 6 A), paralleling the peak of reactivation of STAT5 and expression of casein genes. These data indicate one important reason why transient activation of STAT5 is insufficient to induce milk protein expression: there has to be chromatin remodeling of casein genes by laminin-111 through sustained STAT5 activation. When sustained STAT5 activation was blocked by AG490 (Fig. 5 A), histone acetylation in the β- (Fig. 6 B) and γ-casein (not depicted) promoters was inhibited. The mouse casein genes, including α-, β-, γ-, δ-, and κ-casein, all contain STAT5 binding sites and cluster at a single gene locus on chromosome 5 (Rijnkels et al., 1997). Therefore, the sustained activation of STAT5 may be essential for chromatin remodeling in the entire gene locus.
Figure 6.
Sustained activation of STAT5 is required for histone acetylation in the β-casein promoter. (A and B) ChIP assays measuring the acetylated histone levels in the β-casein promoter. (A) EpH4 cells on polyHEMA were treated with Prl or Prl plus 2% lrECM for different times before the analysis. (B) EpH4 cells were treated with AG490 to block the sustained STAT5 activation as shown in Fig. 4 A before the ChIP analysis. The PCR results were quantified by AlphaEaseFC software, and the values of ChIP DNA were normalized to input DNA. Fold enrichments were determined by dividing the normalized values from treated cells by that of untreated cells.
A constitutively active STAT5 is sufficient to induce mammary-specific function
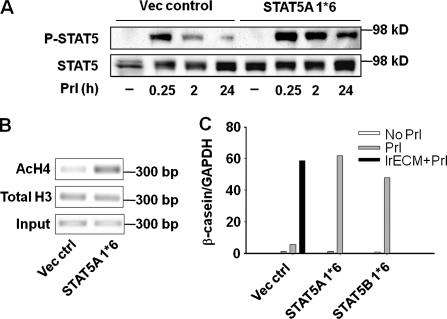
Given the necessity of sustained STAT5 activation, we wondered whether this activation would be sufficient to induce mammary-specific function in the absence of laminin-111 signals. Two amino acid substitutions, H299R and S711F, produce a constitutively active STAT5 (STAT5A/B 1*6) by inducing prolonged tyrosine phosphorylation and nuclear translocation upon cytokine stimulation (Onishi et al., 1998). We introduced either isoform A or B of the constitutively activated STAT5 into mammary cells because they both are present in the mammary gland and are activated during lactation (Liu et al., 1996). Transfected cells on polyHEMA were then treated with Prl. The STAT5 phosphorylation persisted significantly longer in either STAT5A or B 1*6–expressing cells than in control cultures after 24 h of Prl treatment (Fig. 7 A and not depicted). We found that introduction of constitutively active STAT5 induced histone acetylation at the β-casein promoter (Fig. 7 B) and led to high levels of casein expression on polyHEMA in the absence of laminin-111 (Fig. 7 C).
Figure 7.
Constitutively active STAT5 induces chromatin remodeling and β-casein expression in the absence of laminin-111 signals. (A–C) Introducing constitutively activated STAT5A/B (STAT5A/B 1*6) is sufficient to induce chromatin remodeling and β-casein transcription in the absence of lrECM on polyHEMA. EpH4 cells infected with retrovirus (vector control) or expressing STAT5A/B 1*6 were treated with Prl and 2% lrECM on polyHEMA for 24 h. (A) Phosphorylation of STAT5 was detected by Western blot assays. (B) ChIP analysis of levels of acetylated histone H4 at β-casein promoter. (C) The mRNA levels of β-casein were measured by real-time RT-PCR.
DG ligation is required for sustained activation of STAT5
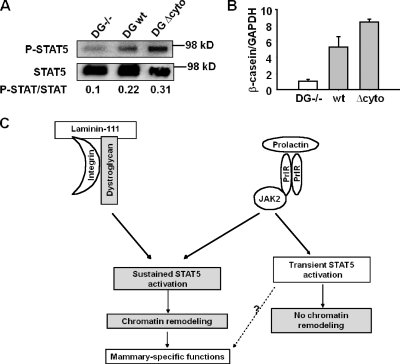
We had shown previously that DG, a laminin-111 receptor that plays an important role in BM assembly (Henry and Campbell, 1998), is expressed by mammary epithelial cells and its deletion impairs mammary acinar morphogenesis and inhibits milk protein expression (Weir et al., 2006). We measured STAT5 phosphorylation in DG-positive and -negative (DG−/−) mammary epithelial cell lines established from DG knockout mice (Weir et al., 2006). In DG−/− cells, Prl failed to induce the sustained activation of STAT5 even in the presence of lrECM (Fig. 8 A). Reexpression of wild-type or cytoplasmic domain–deleted DG in DG−/− cells rescued the sustained STAT5 activation (Fig. 8 A) and induced casein transcription (Fig. 8 B). These results indicate that interaction between laminin-111 and DG is required for sustained activation of STAT5.
Figure 8.
DG ligation regulates sustained activation of STAT5. (A and B) Expression of wild-type (wt) or cytoplasmic domain–deleted DG (DG Δ cyto) in DG−/− cells restored the sustained STAT5 activation (A) and β-casein transcription (B). (C) A scheme showing that the integrated signals from laminin-111 and Prl regulate mammary-specific function. Gray portion shows the differences in molecular mechanisms between our current results and previous studies (Tourkine et al., 1995).
Discussion
In vivo, Prl-induced STAT5 phosphorylation increases rapidly in mid- to late pregnancy, persists throughout lactation, and declines during involution (Liu et al., 1996). Nonetheless, past studies of mammary epithelial cells in culture have mainly focused on the transient activation of STAT5. We show here that the transient activation is not sufficient for induction of milk protein expression and that sustained STAT5 activation is required for activation and maintenance of mammary-specific functions. But more importantly, our results provide a mechanism and a physiological rationale for why STAT5 activation appears to be sustained throughout lactation.
Our finding that there is no or limited accessibility of the basolaterally localized PrlR to its ligand in the apical space solves the puzzle of why Prl fails to induce STAT5 activation in mammary epithelial cells cultured on 2D surfaces (Streuli et al., 1995a; Xu et al., 2007). This phenomenon can likely be generalized to other epithelia and other tissue-specific genes. Segregation of receptor and ligand regulates several epithelial and lymphocyte functions, and asymmetrical localization of proteins in and out of the plasma membrane is important for tissue homeostasis (Schwartz et al., 1985; Vermeer et al., 2003; Porter et al., 2008). In polarized airway epithelium, erbB2 and erbB4 are basolaterally localized and are physically separated from their apical ligand, heregulin (Vermeer et al., 2003). This physical segregation prevents receptor activation except when epithelial integrity is disrupted, as is the case in injury and cancer; in the former case, the ligation would ensure rapid restoration of the epithelia and tissue polarity (Vermeer et al., 2003), but, in the latter, the continuous disruption of architectural integrity prevents down-modulation of the receptor activity with dire consequences (Muthuswamy et al., 2001; Liu et al., 2004). Converse examples also occur: ligands that are inhibitory must be kept segregated from their receptors in order for the target tissue-specific gene to be expressed, as we showed several years ago for whey acidic protein, another milk protein gene, and its inhibitor TGF-α. Unlike β-casein, whey acidic protein is not expressed even in cells treated with lrECM in 3D cultures until all the cells form complete acini with tight junctions, allowing separation of TGF-α from its receptor (Chen and Bissell, 1989; Lin et al., 1995).
We show here that whereas physical changes in 3D cultures, such as cell rounding and clustering, lead to exposure of PrlR allowing binding to its ligand and induction of a transient STAT5 activation, milk protein genes are expressed only when laminin-111 is present to allow the sustained activation of STAT5 (Fig. 8 C). Introduction of constitutively activated STAT5A/B induces gene expression in the absence of laminin-111 (Fig. 6 C), suggesting that sustained STAT5 activation is the key to induce and maintain mammary-specific function. In normal mammary gland, laminin-111 would be present in the BM around the acini at all times from puberty to lactation. What fluctuates is Prl, which is highly expressed and secreted into the interstitium and blood supply during late-pregnancy and lactation (Ben-Jonathan et al., 1996; Goffin et al., 2002) and would have access to the basolaterally localized PrlR.
It is well established that BM integrity is necessary for tissue homeostasis and function. At involution, BM is degraded by a dramatic increase of MMPs, with concomitant loss of tissue inhibitors of MMPs and subsequent reduction in β-casein expression (Talhouk et al., 1991, 1992). Inhibition of MMPs in the involuting gland delays BM degradation and maintains milk protein production (Talhouk et al., 1992). Conversely, when BM is degraded by targeted expression of MMP3, milk proteins are no longer produced even during lactation (Sympson et al., 1994). Our results here suggest that BM integrity is crucial for the duration of transcription factor activation, and this novel regulatory phenomenon is critical to maintaining tissue homeostasis during lactation.
Why would tissue-specific functions require sustained activation of transcription factors? ChIP analysis and photobleaching techniques have demonstrated that transcription factors on target promoters are exchanged rapidly, inducing dynamic and cyclical histone acetylation and RNA polymerase II recruitment (McNally et al., 2000; Metivier et al., 2003). STAT5 cooperates with other transcription factors to induce chromatin remodeling and activate Prl-induced transcription (Kabotyanski et al., 2006; Xu et al., 2007). Here our ChIP data show that histone acetylation in the β-casein promoter depends on the sustained activation of STAT5. Therefore, it is most likely that sustained activation of transcription factors is the key to maintaining the dynamic interplay of ECM and chromatin organization necessary for gene expression.
Whereas transient STAT5 activation is not sufficient to permit casein expression, our preliminary data show that blocking transient activation partially inhibited transcription of β-casein gene (Fig. S3, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200807021/DC1), indicating that the transient activation may contribute in some fashion to expression of mammary-specific genes. Indirect evidence also suggests that the transient activation of STAT5 may play a role in mammary gland development because Prl is released transiently in the form of two daily surges early in pregnancy and during estrus (Freeman et al., 1974). It has been reported that transient Prl secretion induces STAT5A activation during estrus, which parallels a small amount of milk production (Liu et al., 1996). In addition, knocking out JAK2 in mammary gland impairs development of alveolar precursors in virgin mice (Wagner et al., 2004), implicating that STAT5 activation during estrus may be involved in mammary gland morphogenesis. However, none of these studies addressed how long the STAT5 activation persists at early pregnancy and estrus cycles. Therefore, the function of transient STAT5 activation, if indeed it may occur during mammary gland development in vivo, still needs further clarification.
The cooperative action between integrin- and growth factor–dependent signals regulates a variety of biological processes, such as cell proliferation and migration (Schwartz and Baron, 1999; Yamada and Even-Ram, 2002). We have shown previously that the cross-modulation of β1-integrin and EGF receptor signaling regulates acinar morphogenesis through the MAPK pathway (Weaver et al., 1997; Wang et al., 1998). The cross talk between integrins and lactogenic hormone receptors is required for the activation of tissue-specific function in the mammary gland (Streuli et al., 1995b). Deletion of β1 integrins impairs STAT5 phosphorylation and nuclear translocation in epithelial cells during lactation (Naylor et al., 2005), suggesting that β1-integrin, which is important for laminin-111 signaling (Streuli et al., 1991; Muschler et al., 1999), cooperates with PrlR to induce the biochemical signals necessary for sustained STAT5 activation. We find that phosphoinositide 3-kinase localization and activity is also regulated by laminin-111 signaling (unpublished data). Blocking phosphoinositide 3-kinase inhibits Rac1 activation, sustained STAT5 phosphorylation, and milk protein expression (unpublished data). Rac1 has been demonstrated as a downstream target of β1-integrin to induce rapid (15 min) STAT5 phosphorylation in cells cultured on lrECM (Akhtar and Streuli, 2006). However, laminin signaling is not required for transient STAT5 activation, suggesting that transient activation either does not depend on β1-integrin or that its involvement in this process is also transient.
An important further finding here is that one of the laminin-111 receptors, DG, is a mediator in sustained STAT5 activation and induction of milk protein expression. Deletion of DG in mammary epithelial cells inhibits laminin-regulated polarization (Weir et al., 2006), STAT5 activation (Fig. 8 A), and casein expression (Weir et al., 2006). Deleting its cytoplasmic domain has no effect on polarization or casein expression (Weir et al., 2006). Consistent with the casein expression data, we found that the cytoplasmic domain of DG was not required for the sustained STAT5 activation (Fig. 8, A and B). Therefore, the binding of laminin-111 to DG does not appear to induce the canonical “outside-in signaling” directly; rather, DG assists in assembling laminin-111 into the BM around the acini (Weir et al., 2006), allowing further canonical signaling by laminin to integrin, which regulates sustained STAT5 activation.
These results provide an important link between ECM-dependent signaling, transcription factor activation, and chromatin organization. Given the potential plasticity of cells from different organs as demonstrated again recently (Takahashi and Yamanaka, 2006; Boulanger et al., 2007), the mechanisms presented here may shed additional light on why the integrity of ECM microenvironment is necessary to integrate signaling by hormones and cytokines to maintain the differentiated state.
Materials and methods
Antibodies and reagents
The following antibodies and culture reagents were obtained as indicated: STAT5 and lamin A/C (Santa Cruz Biotechnology, Inc.); histone H3 (Abcam); phosphorylated STAT5, AcH4, AcH3, and Rac1 pulldown kit (Millipore); α6-integrin (Millipore); ZO-1 (a gift from M. Itoh, Dokkyo University, Saitama, Japan); laminin-111 (Trevigen); Matrigel (BD); AG490 (Sigma-Aldrich). Polyclonal rabbit anti-Prl receptor serum (M2.5) was made with affinity-purified Prl receptor from mouse livers as antigen (Das et al., 1993).
Cell culture
EpH4 cells were originally isolated from the mammary tissue of a mid-pregnant Balb/c mouse (Reichmann et al., 1989) and were a gift from E. Reichmann (Institut Suisse de Recherches, Epalinges, Switzerland). Cells were maintained in DME/F12 (University of California San Francisco Cell Culture Facility) supplemented with 2% fetal bovine serum (Invitrogen), 50 μg/ml gentamycin, and 5 μg/ml insulin (Sigma-Aldrich), were plated at a density of 10,000 cells/cm2, and were allowed to attach for 16–24 h. The cells were then cultured in DME/F12 medium supplemented with 5 μg/ml insulin and 1 μg/ml hydrocortisone (Sigma-Aldrich; GIH medium) in the presence or absence of sheep Prl (Sigma-Aldrich). We measured β-casein levels at different concentrations of Prl and found Prl induced the maxim of expression at 3 μg/ml (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200807021/DC1). DG−/− clones were obtained by limiting dilution of partial DG−/− mammary epithelial cell populations and screened by immunostaining to ensure the absence of DG expression. Constructs containing STAT5A 1*6, STAT5B 1*6 (a gift of T. Kitamura, University of Tokyo, Tokyo, Japan), wild-type DG, and cytoplasmic-deleted DG were transfected in Phoenix packaging cells (Weir et al., 2006), and retroviral stocks were produced according to standard protocols. Mammary epithelial cells were infected at 40–50% confluence. For transwell experiments, EpH4 cells were plated on 12-mm Transwell polycarbonate membrane plates (Corning). To allow monolayer formation, cells were grown for an additional 24 h after confluence. To disrupt tight junctions and adherent junctions, the cells were treated with 5 mM EGTA in DME/F12 medium. Medium containing EGTA was removed and Prl was added to either the apical or basal chamber of the wells.
3D lrECM and suspension culture assay
Tissue culture plates were coated with lrECM at room temperature for 30 min. EpH4 cells plated on the coated plates attached to lrECM within 60 min. Medium was replaced with fresh GIH supplemented with 2% lrECM (vol/vol) and incubated for 24 h. Cells were treated with 3 μg/ml Prl for different times before harvest. To make nonadhesive substrata, polyHEMA was dissolved in 95% ethanol at 6 mg/ml and plates were coated at 0.25 mg/cm2 as previously described (Roskelley et al., 1994). EpH4 cells were plated on polyHEMA-coated plates at 30,000 cells/cm2. 24–48 h after plating, cells were collected by centrifugation and resuspended in DME/F12 medium containing insulin, hydrocortisone, and relevant ECM components. AG490 was dissolved in DMSO, and vehicle treatment was used as a negative control.
Immunostaining and confocal analysis
Cells grown on filters and tissue culture plastic were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. Cells in lrECM gel were smeared on slides, dried briefly, and fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. Cell clusters on polyHEMA-coated plates were processed for immunofluorescence analysis as follows: cells were pelleted by centrifugation at 500 g for 2 min and resuspended in PBS. The clusters were plated on slides, dried briefly, and then fixed with 4% paraformaldehyde. Stained samples were imaged using a Spot RT camera attached to an upright epifluorescence microscope (Carl Zeiss, Inc.) or an intensified charge-coupled device camera (XR/Mega-10; Stanford Photonics) attached to a spinning disk confocal system (Solamere Technology Group) comprised of an inverted microscope (Axiovert 200M; Carl Zeiss, Inc.). Pictures were taken using a 63× oil immersion objective with QED InVivo imaging software at room temperature. The digital images were pseudocolored, overlaid, and merged using ImageJ 1.38 or Photoshop 7.0 (Adobe).
Cell isolation from 3D cultures and Western blot analysis
Cells grown on plastic and filters were lysed in situ in RIPA buffer (1% Nonidet P-40, 0.5% deoxycholate, 0.2% SDS, 150 mM sodium chloride, and 50 mM Tris-HCl, pH 7.4, containing phosphatase and protease inhibitor cocktails [EMD]). Cells in 3D lrECM were isolated as colonies using ice-cold PBS plus 5 mM EDTA and thereafter lysed in RIPA buffer as described previously (Wang et al., 1998). Equal amounts of protein lysates were subjected to SDS gel electrophoresis, immunoblotted, and detected with an ECL system (Thermo Fisher Scientific).
RT-PCR and real time PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen). cDNA was synthesized using Superscript first strand synthesis kit (Invitrogen) from 0.5–1.0-μg RNA samples. Quantitative real-time PCR analysis was performed with the Lightcycler System using the Lightcycler FastStart DNA Master SYBR Green I kit (Roche). The following primers were used to amplify β-casein and GAPDH cDNA sequences: forward primer of the β-casein gene, 5′-GCTCAGGCTCAAACCATCTC-3′, reverse primer, 5′-TGTGGAAGGAAGGGTGCTAC-3′; forward primer of the GAPDH gene, 5′-CCCCTGGCCAAGGTCATCCATGAC-3′, reverse primer, 5′-CATACCAGGAAATGAGCTTGACAAAG-3′. The following Lightcycler PCR amplification protocol was used: 95°C for 10 min of initial denaturation and 45 amplification cycles (95°C for 5 s, 60°C for 10 s, and 72°C for 5 s). Amplification was followed by melting curve analysis to verify the presence of a single PCR product (Xu et al., 2007).
ChIP
The ChIP assay was performed based on the Millipore ChIP protocol (Nelson et al., 2004) with a few modifications. After formaldehyde cross-linking, nuclei were isolated with a nuclei isolation kit (Sigma-Aldrich) and resuspended in ChIP lysis buffer (1% SDS, 10 mM EDTA, and 50 mM Tris-HCl, pH 8.0) containing protease inhibitor cocktail. Protein DNA complexes were immunoprecipitated as per the Millipore protocol. Isolated DNA was then analyzed by semiquantitative PCR using the following primers: forward primer of the β-casein promoter, 5′-GTCCTCTCACTTGGCTGGAG-3′, reverse primer, 5′-GTGGAGGACAAGAGAGGAGGT-3′.
Statistics
All of the data analysis was performed using Sigma Plot. The bar graphs represent the means ± SEM.
Online supplemental material
Fig. S1 shows that lrECM treatment has little effect on PrlR expression. Fig. S2 shows that sustained STAT5 phosphorylation is activated in both primary cultures of mammary epithelial cells as well as in SCp2 in response to lrECM and Prl treatment. Fig. S3 shows that blocking the transient STAT5 activation inhibits β-casein transcription. Fig. S4 shows that Prl induces β-casein expression in a dose-dependent manner in 3D lrECM. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200807021/DC1.
Supplementary Material
Acknowledgments
We thank Dr. Toshio Kitamura for the generous gift of STAT5A/B 1*6 constructs.
This work was supported by the Office of Biological and Environmental Research (OBER) of the Department of Energy (DOE; DOE-AC03-76SF00098), the National Institutes of Health (R01CA057621) to Z. Werb and M.J. Bissell, the National Cancer Institute (5R01CA64786) to M.J. Bissell, and the Breast Cancer Research Program (BCRP) of the Department of Defense (DOD; Innovator Award) to M.J. Bissell. M.J. Bissell is a Distinguished Scientist of the OBER of the DOE. Support was also provided by DOD BCRP postdoctoral fellowships DAMD17-02-1-0441 and W81XWH-04-1-0582 to R. Xu and C.M. Nelson. C.M. Nelson holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
Abbreviations used in this paper: BM, basement membrane; ChIP, chromatin immunoprecipitation; DG, dystroglycan; lrECM, laminin-rich ECM; polyHEMA, poly(2-hydroxyethyl methacrylate); Prl, prolactin; PrlR, prolactin receptor; STAT5, signal transducers and activators of transcription protein 5.
References
- Akhtar, N., and C.H. Streuli. 2006. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J. Cell Biol. 173:781–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz, J., R. Xu, H. Mori, C.M. Nelson, R. Mroue, V.A. Spencer, D. Brownfield, D.C. Radisky, C. Bustamante, and M.J. Bissell. 2008. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 27:2829–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff, M.H., J. Aggeler, T.G. Ram, and M.J. Bissell. 1989. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 105:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan, N., J.L. Mershon, D.L. Allen, and R.W. Steinmetz. 1996. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr. Rev. 17:639–669. [DOI] [PubMed] [Google Scholar]
- Boulanger, C.A., D.L. Mack, B.W. Booth, and G.H. Smith. 2007. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc. Natl. Acad. Sci. USA. 104:3871–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L.H., and M.J. Bissell. 1989. A novel regulatory mechanism for whey acidic protein gene expression. Cell Regul. 1:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, R.B., R. Biswas, and B.K. Vonderhaar. 1993. Characteristics of a membrane-associated antilactogen binding site for tamoxifen. Mol. Cell. Endocrinol. 98:1–8. [DOI] [PubMed] [Google Scholar]
- Emerman, J.T., J. Enami, D.R. Pitelka, and S. Nandi. 1977. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc. Natl. Acad. Sci. USA. 74:4466–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraldo, M.M., M.A. Deugnier, S. Tlouzeau, J.P. Thiery, and M.A. Glukhova. 2002. Perturbation of beta1-integrin function in involuting mammary gland results in premature dedifferentiation of secretory epithelial cells. Mol. Biol. Cell. 13:3521–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, M.E., M.S. Smith, S.J. Nazian, and J.D. Neill. 1974. Ovarian and hypothalamic control of the daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology. 94:875–882. [DOI] [PubMed] [Google Scholar]
- Goffin, V., N. Binart, P. Touraine, and P.A. Kelly. 2002. Prolactin: the new biology of an old hormone. Annu. Rev. Physiol. 64:47–67. [DOI] [PubMed] [Google Scholar]
- Gouilleux, F., H. Wakao, M. Mundt, and B. Groner. 1994. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 13:4361–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M.D., and K.P. Campbell. 1998. A role for dystroglycan in basement membrane assembly. Cell. 95:859–870. [DOI] [PubMed] [Google Scholar]
- Kabotyanski, E.B., M. Huetter, W. Xian, M. Rijnkels, and J.M. Rosen. 2006. Integration of prolactin and glucocorticoid signaling at the beta-casein promoter and enhancer by ordered recruitment of specific transcription factors and chromatin modifiers. Mol. Endocrinol. 20:2355–2368. [DOI] [PubMed] [Google Scholar]
- Lee, E.Y., W.H. Lee, C.S. Kaetzel, G. Parry, and M.J. Bissell. 1985. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc. Natl. Acad. Sci. USA. 82:1419–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M.L., J. Aggeler, D.A. Farson, C. Hatier, J. Hassell, and M.J. Bissell. 1987. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc. Natl. Acad. Sci. USA. 84:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.Q., P.J. Dempsey, R.J. Coffey, and M.J. Bissell. 1995. Extracellular matrix regulates whey acidic protein gene expression by suppression of TGF-α in mouse mammary epithelial cells: studies in culture and in transgenic mice. J. Cell Biol. 129:1115–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., D.C. Radisky, F. Wang, and M.J. Bissell. 2004. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J. Cell Biol. 164:603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., G.W. Robinson, and L. Hennighausen. 1996. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 10:1496–1506. [DOI] [PubMed] [Google Scholar]
- McNally, J.G., W.G. Muller, D. Walker, R. Wolford, and G.L. Hager. 2000. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science. 287:1262–1265. [DOI] [PubMed] [Google Scholar]
- Metivier, R., G. Penot, M.R. Hubner, G. Reid, H. Brand, M. Kos, and F. Gannon. 2003. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 115:751–763. [DOI] [PubMed] [Google Scholar]
- Murphy, S.J., J.J. Dore, M. Edens, R.J. Coffey, J.A. Barnard, H. Mitchell, M. Wilkes, and E.B. Leof. 2004. Differential trafficking of transforming growth factor-beta receptors and ligand in polarized epithelial cells. Mol. Biol. Cell. 15:2853–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschler, J., A. Lochter, C.D. Roskelley, P. Yurchenco, and M.J. Bissell. 1999. Division of labor among the alpha6beta4 integrin, beta1 integrins, and an E3 laminin receptor to signal morphogenesis and beta-casein expression in mammary epithelial cells. Mol. Biol. Cell. 10:2817–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy, S.K., D. Li, S. Lelievre, M.J. Bissell, and J.S. Brugge. 2001. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor, M.J., N. Li, J. Cheung, E.T. Lowe, E. Lambert, R. Marlow, P. Wang, F. Schatzmann, T. Wintermantel, G. Schuetz, et al. 2005. Ablation of β1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J. Cell Biol. 171:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, E.A., S.R. Walker, J.V. Alvarez, and D.A. Frank. 2004. Isolation of unique STAT5 targets by chromatin immunoprecipitation-based gene identification. J. Biol. Chem. 279:54724–54730. [DOI] [PubMed] [Google Scholar]
- Nelson, W.J. 2003. Adaptation of core mechanisms to generate cell polarity. Nature. 422:766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi, M., T. Nosaka, K. Misawa, A.L. Mui, D. Gorman, M. McMahon, A. Miyajima, and T. Kitamura. 1998. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol. Cell. Biol. 18:3871–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, J.C., M. Falzon, and A. Hall. 2008. Polarized localization of epithelial CXCL11 in chronic obstructive pulmonary disease and mechanisms of T cell egression. J. Immunol. 180:1866–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann, E., R. Ball, B. Groner, and R.R. Friis. 1989. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J. Cell Biol. 108:1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnkels, M., D.A. Wheeler, H.A. de Boer, and F.R. Pieper. 1997. Structure and expression of the mouse casein gene locus. Mamm. Genome. 8:9–15. [DOI] [PubMed] [Google Scholar]
- Rosen, J.M., S.L. Wyszomierski, and D. Hadsell. 1999. Regulation of milk protein gene expression. Annu. Rev. Nutr. 19:407–436. [DOI] [PubMed] [Google Scholar]
- Roskelley, C.D., P.Y. Desprez, and M.J. Bissell. 1994. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl. Acad. Sci. USA. 91:12378–12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, G.J., J. Barasch, and Q. Al-Awqati. 1985. Plasticity of functional epithelial polarity. Nature. 318:368–371. [DOI] [PubMed] [Google Scholar]
- Schwartz, M.A., and V. Baron. 1999. Interactions between mitogenic stimuli, or, a thousand and one connections. Curr. Opin. Cell Biol. 11:197–202. [DOI] [PubMed] [Google Scholar]
- Selvaraj, N.G., E. Omi, G. Gibori, and M.C. Rao. 2000. Janus kinase 2 (JAK2) regulates prolactin-mediated chloride transport in mouse mammary epithelial cells through tyrosine phosphorylation of Na+-K+-2Cl- cotransporter. Mol. Endocrinol. 14:2054–2065. [DOI] [PubMed] [Google Scholar]
- Streuli, C.H., and M.J. Bissell. 1990. Expression of extracellular matrix components is regulated by substratum. J. Cell Biol. 110:1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli, C.H., N. Bailey, and M.J. Bissell. 1991. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J. Cell Biol. 115:1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli, C.H., G.M. Edwards, M. Delcommenne, C.B. Whitelaw, T.G. Burdon, C. Schindler, and C.J. Watson. 1995. a. Stat5 as a target for regulation by extracellular matrix. J. Biol. Chem. 270:21639–21644. [DOI] [PubMed] [Google Scholar]
- Streuli, C.H., C. Schmidhauser, N. Bailey, P. Yurchenco, A.P. Skubitz, C. Roskelley, and M.J. Bissell. 1995. b. Laminin mediates tissue-specific gene expression in mammary epithelia. J. Cell Biol. 129:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson, C.J., R.S. Talhouk, C.M. Alexander, J.R. Chin, S.M. Clift, M.J. Bissell, and Z. Werb. 1994. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 125:681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., and S. Yamanaka. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676. [DOI] [PubMed] [Google Scholar]
- Talhouk, R.S., J.R. Chin, E.N. Unemori, Z. Werb, and M.J. Bissell. 1991. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 112:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk, R.S., M.J. Bissell, and Z. Werb. 1992. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J. Cell Biol. 118:1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourkine, N., C. Schindler, M. Larose, and L.M. Houdebine. 1995. Activation of STAT factors by prolactin, interferon-gamma, growth hormones, and a tyrosine phosphatase inhibitor in rabbit primary mammary epithelial cells. J. Biol. Chem. 270:20952–20961. [DOI] [PubMed] [Google Scholar]
- Vermeer, P.D., L.A. Einwalter, T.O. Moninger, T. Rokhlina, J.A. Kern, J. Zabner, and M.J. Welsh. 2003. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 422:322–326. [DOI] [PubMed] [Google Scholar]
- Wagner, K.U., A. Krempler, A.A. Triplett, Y. Qi, N.M. George, J. Zhu, and H. Rui. 2004. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol. Cell. Biol. 24:5510–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., V.M. Weaver, O.W. Petersen, C.A. Larabell, S. Dedhar, P. Briand, R. Lupu, and M.J. Bissell. 1998. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA. 95:14821–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, C.J., and T.G. Burdon. 1996. Prolactin signal transduction mechanisms in the mammary gland: the role of the Jak/Stat pathway. Rev. Reprod. 1:1–5. [DOI] [PubMed] [Google Scholar]
- Weaver, V.M., O.W. Petersen, F. Wang, C.A. Larabell, P. Briand, C. Damsky, and M.J. Bissell. 1997. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137:231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, M.L., M.L. Oppizzi, M.D. Henry, A. Onishi, K.P. Campbell, M.J. Bissell, and J.L. Muschler. 2006. Dystroglycan loss disrupts polarity and beta-casein induction in mammary epithelial cells by perturbing laminin anchoring. J. Cell Sci. 119:4047–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff, P.J., D.B. Rifkin, J.J. Meister, and B. Hinz. 2007. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 179:1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, R., V.A. Spencer, and M.J. Bissell. 2007. Extracellular matrix-regulated gene expression requires cooperation of SWI/SNF and transcription factors. J. Biol. Chem. 282:14992–14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, K.M., and S. Even-Ram. 2002. Integrin regulation of growth factor receptors. Nat. Cell Biol. 4:E75–E76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.