Abstract
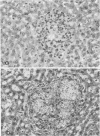
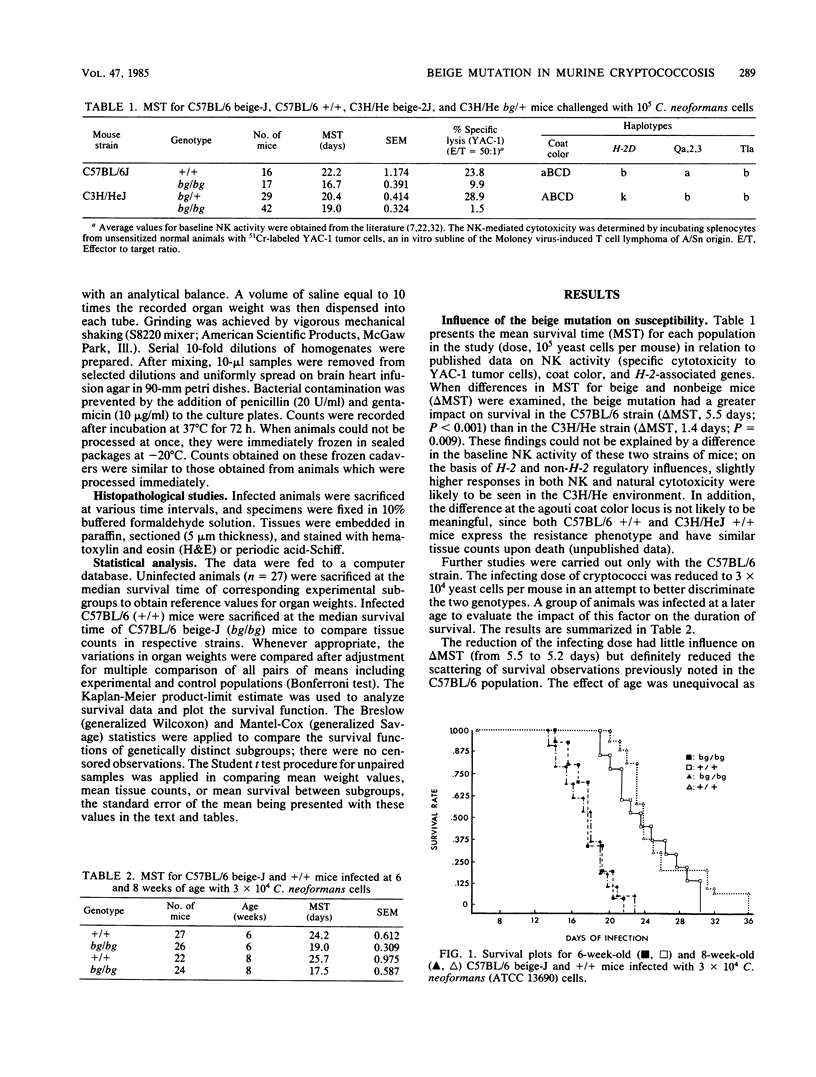
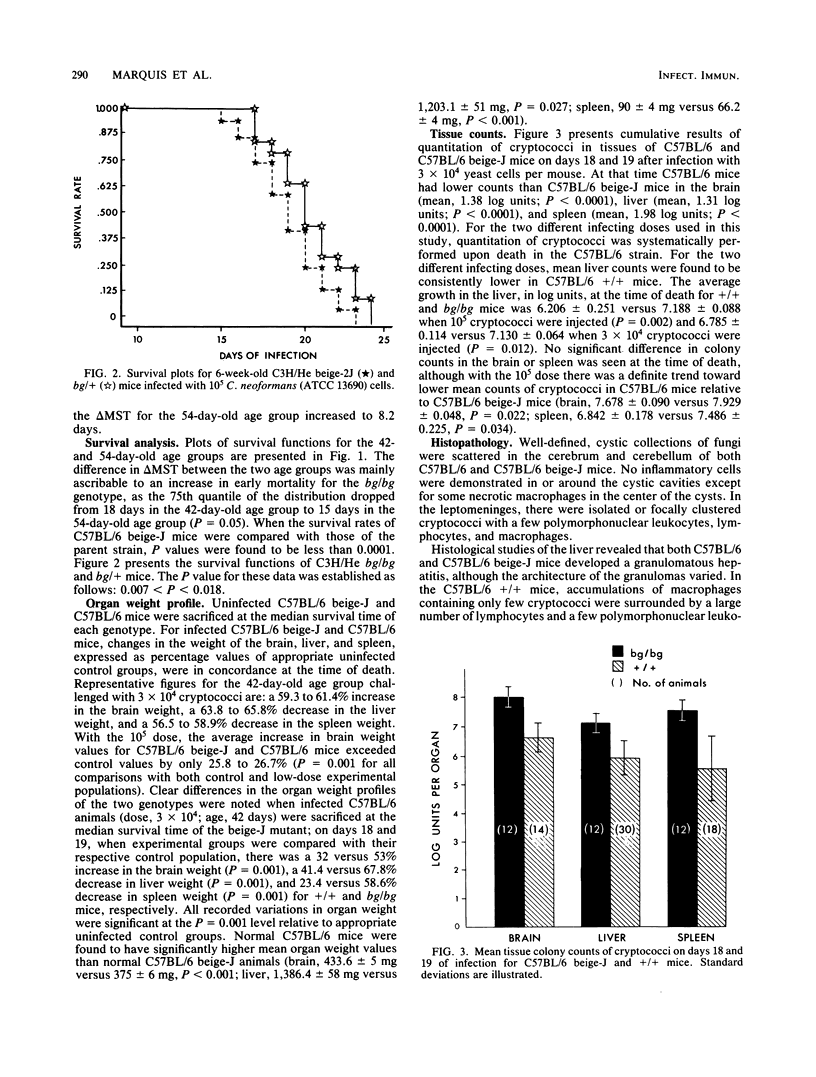
The influence of the bgJ and bg2J mutations on the susceptibility of mice to experimental cryptococcosis was studied in inbred mice of the C57BL/6J and C3H/HeJ strains. Although infected animals with the bg/bg genotype had a significantly shorter lifespan than bg/+ or +/+ animals, C3H/He beige-2J mice were less susceptible than C57BL/6 beige-J mice when compared with nonbeige mice of similar background. On days 18 and 19 after infection, quantitation of cryptococci in the brain, liver, and spleen revealed that the overall burden of organisms in infected C57BL/6 beige-J mice was in excess of one log unit above that found in the brain, liver, and spleen of infected C57BL/6 +/+ mice. At that time, C57BL/6 beige-J mice showed a 53% increase in mean brain weight, a 67.8% decrease in mean liver weight, and a 58.6% decrease in mean spleen weight, when compared with uninfected animals of the same age and genetical lineage. The corresponding figures for C57BL/6 +/+ mice were a 32% increase in mean brain weight, a 41.4% decrease in mean liver weight, and a 23.4% decrease in mean spleen weight. From these data, it is concluded that the beige mutation in mice is associated with increased susceptibility to cryptococcosis, the accrued susceptibility of the beige mutant is related to more rapid changes in the weight profile of the target organs as well as to a higher rate of growth or decreased clearance of Cryptococcus neoformans or both, and other autosomal genes are likely to be involved in the genetic control of susceptibility to murine cryptococcosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. W., Huang K. Y., Albright J. F. Natural killer activity in mice infected with Trypanosoma musculi. Infect Immun. 1983 Jun;40(3):869–875. doi: 10.1128/iai.40.3.869-875.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger P., Marquis G., Dallaire L. Viability assessment by dye exclusion. A fluorescent method for fungal cells. Arch Dermatol. 1979 Oct;115(10):1195–1196. [PubMed] [Google Scholar]
- Bennett J. M., Blume R. S., Wolff S. M. Characterization and significance of abnormal leukocyte granules in the beige mouse: a possible homologue for Chediak-Higashi Aleutian trait. J Lab Clin Med. 1969 Feb;73(2):235–243. [PubMed] [Google Scholar]
- Clark E. A., Shultz L. D., Pollack S. B. Mutations in mice that influence natural killer (NK) cell activity. Immunogenetics. 1981 Mar 1;12(5-6):601–613. doi: 10.1007/BF01561700. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Elin R. J., Edelin J. B., Wolff S. M. Infection and immunoglobulin concentrations in Chediak-Higashi mice. Infect Immun. 1974 Jul;10(1):88–91. doi: 10.1128/iai.10.1.88-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung P. Y., Murphy J. W. In vitro interactions of immune lymphocytes and Cryptococcus neoformans. Infect Immun. 1982 Jun;36(3):1128–1138. doi: 10.1128/iai.36.3.1128-1138.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GADEBUSCH H. H. Phagocytosis of Cryptococcus neoformans in anemic mice. J Bacteriol. 1959 Aug;78:259–262. doi: 10.1128/jb.78.2.259-262.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Bujak J. S., Patten E., Wolff S. M. Granulocyte function in the Chediak-Higashi syndrome of mice. Blood. 1974 Feb;43(2):201–206. [PubMed] [Google Scholar]
- Gallin J. I., Elin R. J., Hubert R. T., Fauci A. S., Kaliner M. A., Wolff S. M. Efficacy of ascorbic acid in Chediak-Higashi syndrome (CHS): studies in humans and mice. Blood. 1979 Feb;53(2):226–234. [PubMed] [Google Scholar]
- Jones J. F., Hancock G. E. Trypanosomiasis in mice with naturally occurring immunodeficiencies. Infect Immun. 1983 Nov;42(2):848–851. doi: 10.1128/iai.42.2.848-851.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama T. Toxoplasma-induced activities of peritoneal and spleen natural killer cells from beige mice against thymocytes and YAC-1 lymphoma targets. Infect Immun. 1984 Mar;43(3):973–980. doi: 10.1128/iai.43.3.973-980.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. E., Farrell J. P. Leishmaniasis in beige mice. Infect Immun. 1982 Dec;38(3):1208–1216. doi: 10.1128/iai.38.3.1208-1216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M. Fungicidal components of mammalian granulocytes active against Cryptococcus neoformans. J Infect Dis. 1977 Jul;136(1):96–99. doi: 10.1093/infdis/136.1.96. [DOI] [PubMed] [Google Scholar]
- Lutzner M. A., Lowrie C. T., Jordan H. W. Giant granules in leukocytes of the beige mouse. J Hered. 1967 Nov-Dec;58(6):299–300. doi: 10.1093/oxfordjournals.jhered.a107620. [DOI] [PubMed] [Google Scholar]
- Mahoney K. H., Morse S. S., Morahan P. S. Macrophage functions in beige (Chédiak-Higashi syndrome) mice. Cancer Res. 1980 Nov;40(11):3934–3939. [PubMed] [Google Scholar]
- McGarry M. P., Brandt E. J., Swank R. T. Eosinophil and neutrophil granulocyte exudation in the Chediak-Higashi (beige) mouse. Am J Pathol. 1976 Dec;85(3):685–692. [PMC free article] [PubMed] [Google Scholar]
- McKinnon K. P., Hale A. H., Ruebush M. J. Elicitation of natural killer cells in beige mice by infection with vesicular stomatitis virus. Infect Immun. 1981 Apr;32(1):204–210. doi: 10.1128/iai.32.1.204-210.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S., Rosenstreich D. L. Defect in macrophage effector function confers Salmonella typhimurium susceptibility on C3H/HeJ mice. Cell Immunol. 1982 Mar 1;67(2):325–333. doi: 10.1016/0008-8749(82)90224-6. [DOI] [PubMed] [Google Scholar]
- Rhodes J. C., Wicker L. S., Urba W. J. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect Immun. 1980 Aug;29(2):494–499. doi: 10.1128/iai.29.2.494-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Wigzell H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J Immunol. 1979 Nov;123(5):2174–2181. [PubMed] [Google Scholar]
- Roder J. C. The beige mutation in the mouse. I. A stem cell predetermined impairment in natural killer cell function. J Immunol. 1979 Nov;123(5):2168–2173. [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Defective T-cell response in beige mutant mice. Nature. 1982 Jan 21;295(5846):240–241. doi: 10.1038/295240a0. [DOI] [PubMed] [Google Scholar]
- Skamene E., Stevenson M. M., Lemieux S. Murine malaria: dissociation of natural killer (NK) cell activity and resistance to Plasmodium chabaudi. Parasite Immunol. 1983 Nov;5(6):557–565. doi: 10.1111/j.1365-3024.1983.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Stutman O., Cuttito M. J. Normal levels of natural cytotoxic cells against solid tumours in NK-deficient beige mice. Nature. 1981 Mar 19;290(5803):254–257. doi: 10.1038/290254a0. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Granelli-Piperno A., Griscelli C., Reich E. Specific protease deficiency in polymorphonuclear leukocytes of Chédiak-Higashi syndrome and beige mice. J Exp Med. 1978 Apr 1;147(4):1285–1290. doi: 10.1084/jem.147.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R., Taylor B. A. The response of recombinant inbred strains of mice to bacterial lipopolysaccharides. J Immunol. 1977 Jun;118(6):2088–2093. [PubMed] [Google Scholar]
- Welsh R. M., Jr, Kiessling R. W. Natural killer cell response to lymphocytic choriomeningitis virus in beige mice. Scand J Immunol. 1980;11(4):363–367. doi: 10.1111/j.1365-3083.1980.tb00001.x. [DOI] [PubMed] [Google Scholar]