Abstract
The aim of this study was to test the hypothesis that imbalance in patients with a severe deformity of the spine is associated with an increase in the sensory integration disorder. This paper is a case comparison study. Patients were divided into three groups: able-bodied (n = 53), observation (n = 23), and pre-brace (n = 26) groups. Time domain parameters (sway area, position and displacement) and structural posturographic parameters [mean distance (MD) and mean peak (MP)] were calculated from the COP excursion using a force platform. A sensory integration disorder could be an important factor in the progression of the scoliotic curve. Significant differences were found in time domain between observation, pre-brace and able-bodied groups. The results for the structural posturographic parameters showed significant differences between the pre-brace and the able-bodied groups (P = 0.018 MD and P = 0.02 MP) demonstrating a perturbation in sensory integration system by an increase of imbalance. The absence of statistical difference between the observation and the pre-brace groups for the structural posturographic parameters indicates a perturbation of sensory integration system associated with curve progression. Our study has demonstrated that the pre-brace group is less stable than the able-bodied group. The severity of scoliosis in pre-brace scoliotic girls could be related to an increase in the sensory integration disorder.
Keywords: Adolescent idiopathic scoliosis, Standing balance, Sway density plots, Sensory integration
Introduction
Adolescent idiopathic scoliosis (AIS) is often characterized by a lateral deviation of the spine. This three-dimensional deformation is responsible for geometric changes in the trunk [12, 13, 17] and rib cage [28]. Its prevalence is approximately 2–3% in children aged between 10 and 16 years and girls are more at risk for severe progression by a ratio of 3.6–1 [32]. Several aetiologies of AIS are suggested [9, 10, 19]. Among these, Yamada and Yamamoto [34] raised the hypothesis that a deficit in postural balance results in scoliosis.
Herman et al. [16] reported that a sensory rearrangement of the motor system on the representation of the body in space could be associated with idiopathic scoliosis. This could explain the relations reported between standing imbalance in AIS and types of curvature [11], body posture [21] and spinal deformity progression [3, 25]. The inability to recalibrate the position of the center of pressure (COP) in relation to the body’s center of mass (COM) due to a sensory integration disorder could be responsible for balance dysfunction in AIS [18]. This is supported by clinical findings in which differences in neurological responses were reported between AIS and normal subjects [3, 33, 35]. We assumed that a sensory integration disorder could also be an important factor in the progression of the scoliotic curve due to an inability to readjust the position of the COP on a long time scale to counterbalance COM position.
The integrity of the postural control system can be measured in different ways in quiet standing balance. Sensory integration deficits can be estimated by measuring the excursion of the COP during or following visual or proprioceptive deprivation [3, 8, 22, 26, 27, 29, 30]. Body shifting can also be assessed by prolonged testing periods of 30 min without external perturbations [36]. Both of these methods address postural imbalance in the antero-posterior and medio-lateral directions separately and in the time domain. The posturographic analysis designed by Baratto et al. [2] is based on the structural characteristics of the COP excursion (sway density plots) in the horizontal plane. There is no need to perturb the subject since the ability to maintain a stable position or to migrate over a period can be estimated from the COP excursion highlighting problems on quiet standing. This method, which contains similarities with the concept of rambling and trembling of Zatsiorsky and Duarte’s stabilogram decomposition [36, 37], has been used in few studies to investigate the effect of sensory deprivation on standing balance in AIS [26, 27] and in patients with chronic low back pain [23].
The aim of this study is to test the hypothesis that imbalance in patients with a severe deformity of the spine is associated with an increase in the sensory integration disorder.
Methods
In total, 102 girls participated in this study. Among these, 49 girls were diagnosed as AIS according to the definition given by Bunnell [4]. The inclusion criteria were the following: subject aged between 10 and 16 years, a Cobb angle ≥10° and no active treatment of the scoliosis (brace or surgery). All scoliotic subjects were recruited from the orthopaedic clinic of the Sainte-Justine Hospital. According to clinical criteria, scoliotic girls were divided into two groups: the observation and the pre-brace groups. This segregation between scoliotic subjects was done to test if the severity of scoliosis in still non-treated AIS girls is related to an increase in the sensory integration disorder. The orthopaedist’s diagnosis was used as division criterion instead of Cobb angle because this clinical criterion takes into account more factors (such as age, Risser sign, Cobb angle, gibbosity and history of scoliosis in the immediate family) than Cobb angle alone.
The observation group consisted of 23 girls (12.5 ± 2.4 years) in which 20 subjects had a right thoracic curve and the remaining had a right (n = 3) lumbar curve. Subjects in the observation group had a mean height and weight of 151.5 ± 10.7 cm and 43.5 ± 10.9 kg, respectively and a mean Cobb angle of 18.9 ± 7.1°. Because of their relatively small scoliotic curve, these subjects were clinically followed at regular intervals of three months but bracing was not recommended.
Twenty-six girls (12.2 ± 1.4 years) to whom a Boston brace was prescribed formed the pre-brace group. These subjects were not under active brace treatment before their postural evaluation. According to the Scoliosis Research Society, the Boston brace was indicated for the adolescent with moderate scoliosis (curvature > 30°) and/or having a significant risk for progression of the scoliosis. The pre-brace group had an average Cobb angle of 27.2 ± 12.4° and their average height and weight were 152.3 ± 10.3 cm and 42.1 ± 8.3 kg, respectively. Twenty-five subjects had a right thoracic curve and the remaining had a right (n = 1) lumbar curve. A significant difference (P = 0.001) was found between the Cobb angle of the observation and the pre-brace groups. It should be noted that this difference is not imperative since the division criterion of this study is based on clinical criteria instead of the Cobb angle only.
Fifty-three young healthy adolescent girls without spinal deviation volunteered to form the able-bodied group were selected from nearby schools. Age and sex were the main inclusion criteria while the diagnosis of scoliosis or other neuromuscular disorders as well as a recent health problem that could affect standing balance were the exclusion criteria. Their average age was 13.8 ± 1.9 years, their weight 49.9 ± 9.8 kg and their height 159.2 ± 9.3 cm. No significant differences (P > 0.05) were found between the age, weight and height for the three groups. The adolescents and families were informed of the purpose of the experiments and gave their informed consent.
Standing balance was tested using an AMTI force platform (AMTI, Newton, MA, USA). Each subject was asked to stand as quiet as possible on the force plate in a standardized position with the heels spaced by approximately 20 cm and the midline of the feet were pointing externally by 15° as described by McIlroy and Maki [20]. They were asked to keep their eyesight focused on a target placed at eye level, 1.2 m ahead with their arms by their sides. Each subject completed three trials of 64 s at a sampling rate of 64 Hz [1, 6]. The participants had 2 min of rest between each trial to limit the effect of fatigue. The COP excursion was calculated from the ground reaction forces and moments obtained from force platform. The COP corresponds to the point of application of the resultant force acting on the platform and its excursions are indicative of subject’s stability [7].
In all, seven balance parameters were calculated from the excursion of the COP. Five of them were time dependant balance parameters (global posturographic parameters). The mean value of the COP indicates the position of the COM. A positive value in the antero-posterior direction (COPAP) corresponds to a forward displacement of the COP measured from the back of the heels, while a positive medio-lateral (COPML) displacement is a shift of the COP towards the left limb from its central body position. Body oscillations are represented by the surface covered by the COP and is called sway area. A large area reflects greater neuromuscular demand to regulate body oscillations. The last two time dependant parameters are the sum of the COP displacements in the antero-posterior (LAP) and medio-lateral (LML).
Two additional parameters were calculated from the shape of the COP trajectory. The sway density plot is computed by counting the number of consecutive samples during which the postural oscillations remain inside a 2.5 mm radius. The sway density curve is digitally filtered with a fourth-order Butterworth filter (2.5 Hz low pass cut-off frequency with dual-pass to remove phase shift) in order to perform a better peak extraction of the two structural parameters. The peaks of the sway density curve correspond to time instants in which the COP is relatively stable and valleys correspond to time instants in which the COP rapidly shifts from one stable value to another. The mean value of all peaks (MP: mean peak) and the mean value of all distances (MD: mean distance) between peaks have been extracted from the sway density curve. More specifically, MP represents the time spent by the COP inside the 2.5 mm radius circle centered at the time of peak and estimates the degree of stabilization. It is measured in seconds though its name reflects a number of events rather than time. On the other hand, MD is related to the amplitude of COP excursion from one center of stabilization to another. These parameters have been suggested to reflect the capacity of the central nervous system (CNS) to integrate the sensory information and anticipate physiological internal delays in order to keep the vertical alignment of the whole body [2]. An increase in the MD and a decrease in the MP are indicative of inadequate postural balance commands since the subject sways larger and passed less time in each center of stabilization in the posturogram [2].
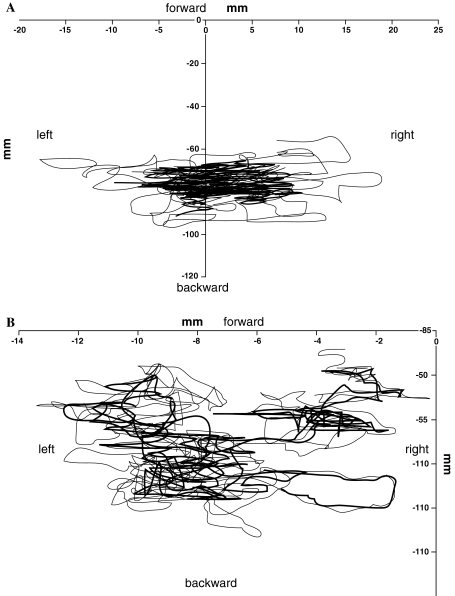
Figure 1 represents the excursion of the COP for an able-bodied girl and a pre-brace subject measured for a trial. In Fig. 1a, the COP is contained inside a single and large area which is characteristic of able-bodied individuals, whereas pre-brace subjects, who have a tendency to shift position even in quiet standing, will display two or more areas representing intermediary stable COP positions as illustrated in Fig. 1b. One of the parameter related to sway density plots, mean distance, characterizes COP shifts.
Fig. 1.
Excursion of the COP a for an able-bodied subject and b a scoliotic girl
In order to corroborate that imbalance in scoliotic patients during quiet standing is associated with a sensory integration disorder and to examine if these deficits are related to the severity of spine deformity, analysis of covariance (ANCOVA) was performed between the three groups for the time-dependant and structural posturographic parameters with the age, weight, height and Cobb angle as co-variables. When the ANCOVAs were significant (P < 0.05), the means were compared using Tukey correction for the unequal n.
Results
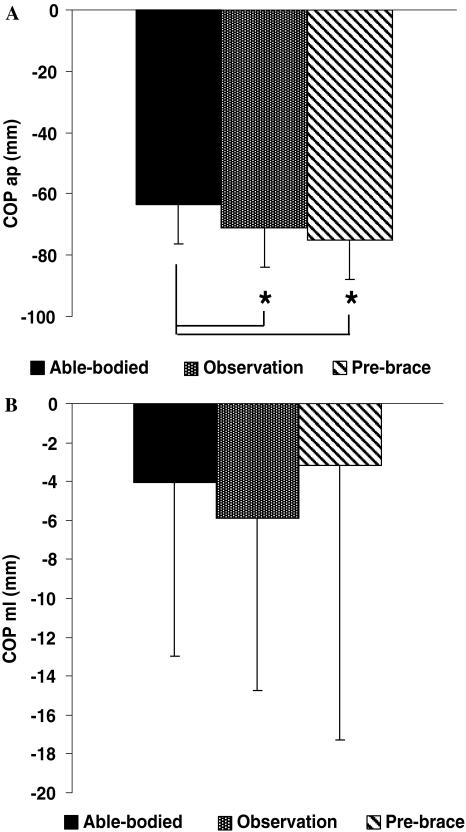
Figure 2 illustrates the mean position of the COP for the able-bodied, the observation and pre-brace groups. Compared to the able-bodied group, the mean position of the COPAP (Fig. 2a) for the observation and pre-brace groups were significantly closer to the heels by 7.9 mm (P = 0.04) and 12 mm (P = 0.001), respectively. The mean position of the COPML was not statistically significant between all three groups as shown in Fig. 2b. Nonetheless, the observation group displayed a 1.8 mm displacement more to the right than the able-bodied subjects and 2.8 mm than the pre-brace girls. The absence of difference may be due to the intra-group variability.
Fig. 2.
Mean COP position in the a antero-posterior and b medio-lateral directions for the able-bodied, the observation and pre-brace groups. Asterisks denote significant differences for P < 0.05
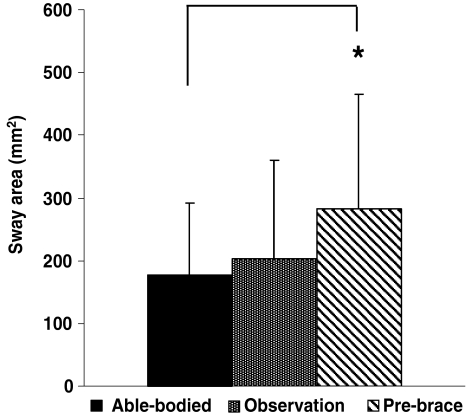
Sway area is reported in Fig. 3 for the able-bodied girls, and the AIS (observation and pre-brace) groups. The observation group had 15% more sway area than the able-bodied group, and 14% less sway area than the pre-brace group. However, these differences were not statistically significant. The pre-brace group displayed a 58% statistically larger (P = 0.008) sway area than the able-bodied group.
Fig. 3.
Sway area for the able-bodied, the observation and pre-brace groups. Asterisk denote significant differences for P < 0.05
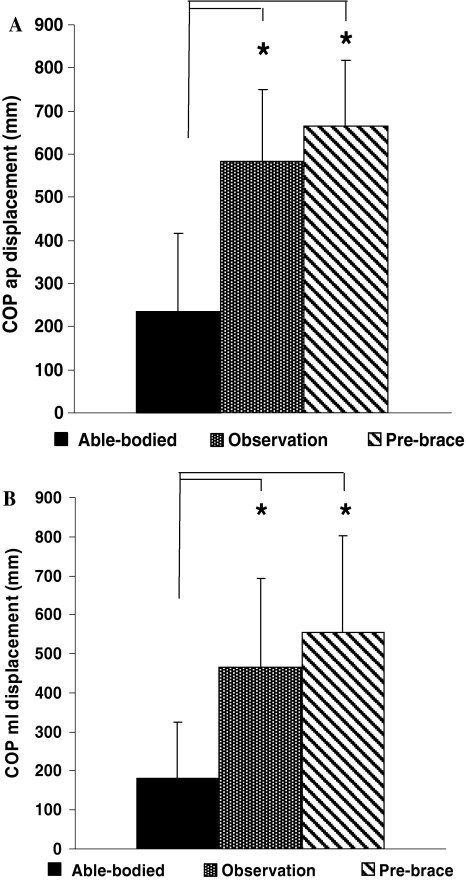
Figure 4 represents the mean sum of the displacement of the COPAP (LAP; Fig. 4a) and the COPML (LML; Fig. 4b) for all groups. The mean sum of the displacement of the COPAP, was 256.3 mm (P < 0.001) longer for the observation group than in the able-bodied group and statistically greater by 374.2 mm (P < 0.001) in the pre-brace group compared to the able-bodied group. The mean sum of the displacement of the COPML (LML; Fig. 4b) was also significantly larger in the observation (P < 0.001) and pre-brace (P < 0.001) groups than in the able-bodied group.
Fig. 4.
Sum of the COP displacements in the a COPAP and b COPML between for the able-bodied, the observation and pre-brace groups. Asterisks denote significant differences for P < 0.05
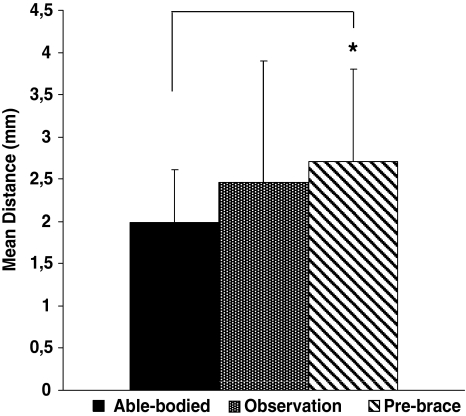
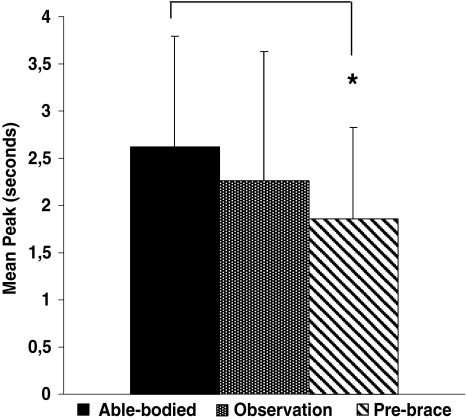
Figures 5 and 6 present the structural parameters (MP and MD) of the COP excursion for all groups. The MD (Fig. 5) and the MP (Fig. 6) were statistically different for the pre-brace group compared to the able-bodied group. The mean distance was 36% longer (MD P = 0.009) and the time spent in the stability region was 29% smaller (MP P = 0.02) for the pre-brace group compared to the able-bodied group. No statistical difference was found between the observation and the able-bodied groups, neither between the observation and pre-brace groups.
Fig. 5.
Mean distance of the COP between the able-bodied, the observation and pre-brace groups. Asterisk denote significant differences for P < 0.05
Fig. 6.
Mean peak of the COP between the able-bodied, the observation and pre-brace groups. Asterisk denote significant differences for P < 0.05
Discussion
The objective of this study was to determine whether a sensory integration disorder is related to scoliosis’ severity in non-treated AIS girls. The time parameters in quiet standing, (increase of sway area and COP displacements) were statistically increased in the observation and pre-brace groups compared to the able-bodied group. It suggests the presence of a balance control problem although these measures do not indicate the cause. The present study not only confirms the findings of Gregorič et al. [14] and Byl et al. [5], but it also associates the ability to recalibrate the COP position according to COM (i.e. to find a different equilibrium point) with a sensory integration disorder.
The results of the sway density plots help in identifying the mechanisms that cause greater balance instability in AIS. The increase in the MD and a decrease in the MP values obtained from sway density plots observed in the pre-brace group suggest the existence of a sensory integration disorder. This reflects a poor balance and an increase of oscillations during quiet standing. A longer distance between each stabilization center for the pre-brace girls and less time spent on each stabilization center is due to more frequent COP shifting. Zatsiorsky and Duarte [24, 34] reported that this type of pattern represents different strategies of the CNS to produce permanent changes on the COP. An inappropriate feedback or a deficiency of CNS programming demonstrated by the increase of MD and a decrease of MP in pre-brace group may be due to the alteration in visual [15] and vestibular inferences [24, 25] as well as spinal deformity [16].
The irregularity of sensory integration in the pre-brace group could be related to a reduction in the accuracy or a deterioration of the motor control [2]. Herman et al. [16] has demonstrated that the AIS adopt a new motor strategy based upon the recalibration or reinterpretation of the proprioceptive inferences. The postural strategy adopted by the pre-brace girls may have a profound effect on the structural deformity of the scoliotic curve [16]. The imbalance in the observation group was documented by increase of the sway area and COP displacement in time domain. Therefore, the observation group could constitute an intermediate sensorimotor condition between the able-bodied and the pre-brace group.
The originality of our study confirms a sensory integration disorder for the pre-brace group and demonstrates that the observation group has a sensorimotor system similar to the able-bodied group. If the progression of the AIS is related to a neurological dysfunction [31], we can assume that the progression of the spinal deformity could evolve in two stages. During the initial stage, the development of a small curvature might be due to a defect in the neuromuscular and in the sensorimotor system. This stage shows a statistically significant difference between the observation and the able-bodied groups in the time domain. In the second stage, the increase in scoliotic curve and the neurological dysfunction seem to perturb the ability to recalibrate the position of the COP in relation to the COM due to a sensory integration disorder. Hence, time domain parameters highlight a postural imbalance while the structural parameters (sway density plots) emphasize sensory integration disorders as distinguished in the pre-brace group.
Conclusion
Our study has demonstrated that the adolescent idiopathic scoliotic girls are less stables and have more oscillations than the able-bodied group. The severity of scoliosis in pre-brace girls might be related to an increase in the sensory integration disorder as indicated by an increase of mean distance and a decrease of MP of the COP excursion which showed postural instability.
Acknowledgments
The authors wish to thank Manon S. Allard and Jelémis Soulacroupe, who helped collect and process the clinical data. Financial support was given in part by the Natural Science and Engineering Council of Canada.
References
- 1.Allard P, Nault ML, Hinse S, LeBlanc R, Labelle H (2001) Relationship between morphologic somatotypes and standing posture equilibrium. Ann Hum Biol 6:624–633. doi:10.1080/03014460110047946 [DOI] [PubMed]
- 2.Baratto L, Morasso PG, Re C, Spada G (2002) A new look at posturographic analysis in the clinical context: sway-density versus other parameterization techniques. Motor Control 6:246–270 [DOI] [PubMed]
- 3.Barrack R, Whitecloud TIII, Burcke S, Cook S, Harding A (1984) Proprioception in idiopathic scoliosis. Spine 9:681–685. doi:10.1097/00007632-198410000-00005 [DOI] [PubMed]
- 4.Bunnell WP (1986) The natural history of idiopathic scoliosis before skeletal maturity. Spine 11:773–776. doi:10.1097/00007632-198610000-00003 [DOI] [PubMed]
- 5.Byl NN, Holland S, Jurek A (1997) Postural imbalance and vibratory sensitivity in patients with idiopathic scoliosis: implications for treatment. J Orthop Sports Phys Ther 26:60–68 [DOI] [PubMed]
- 6.Carpenter M, Frank J, Winter D, Peysor G (2001) Sampling duration effects on centre of pressure summary measures. Gait Posture 13:35–40. doi:10.1016/S0966-6362(00)00093-X [DOI] [PubMed]
- 7.Chen PQ, Wang JL, Tsuang YH, Liao TL, Huang PI, Hang YS (1998) The postural stability control and gait pattern of idiopathic scoliosis adolescents. Clin Biomech (Bristol, Avon) 13:S52–S58. doi:10.1016/S0268-0033(97)00075-2 [DOI] [PubMed]
- 8.Dalleau G, Allard MS, Beaulieu M, Rivard CH, Allard P (2007) Free moment contribution to quiet standing in able-bodied and scoliotic girls. Eur Spine J 16:1593–1599. doi:10.1007/s00586-007-0404-0 [DOI] [PMC free article] [PubMed]
- 9.Ford DM, Bagnall KM, Mc Fadden KD, Greenhill BJ, Rasco VJ (1984) Paraspinal muscle imbalance in adolescent idiopathic scoliosis. Spine 9:373–376. doi:10.1097/00007632-198405000-00008 [DOI] [PubMed]
- 10.Ford DM, Bagnall KM, Clements CA, McFadden KD (1988) Muscle spindles in the paraspinal musculature of patients with adolescent idiopathic scoliosis. Spine 13:461–465. doi:10.1097/00007632-198805000-00004 [DOI] [PubMed]
- 11.Gauchard G, Lascombes P, Kuhnast M, Prrin P (2001) Influence of different types of progressive idiopathic scoliosis on static and dynamic postural control. Spine 26:1052–1058. doi:10.1097/00007632-200105010-00014 [DOI] [PubMed]
- 12.Goldberg CJ, Fogaty E, Moore DP, Dowling FE (1997) Scoliosis and developmental theory. Spine 22:2228–2238. doi:10.1097/00007632-199710010-00006 [DOI] [PubMed]
- 13.Goldberg CJ, Kaliszer M, Moore DP, Dowling FE (2001) Surface topography, Cobb angles, and cosmetic change in scoliosis. Spine 26:E55–E63. doi:10.1097/00007632-200102150-00005 [DOI] [PubMed]
- 14.Gregorič M, Pečak F, Trontelj J, Dimitrijevič M (1981) Postural control in scoliosis. Acta Orthop Scand 52:59–63 [DOI] [PubMed]
- 15.Herman R, MacEwan GD (1979) Idiopathic scoliosis: a visuovestibular disorder of the central nervous system. In: Zorab PA, Siegler D (eds) Scoliosis. Academic Press, New York, pp 61–99
- 16.Herman R, Mixon J, Fisher A, Stuyck J, Maulucci R (1985) Idiopathic scoliosis and the central nervous system: a motor control problem. Spine 10:1–14. doi:10.1097/00007632-198501000-00001 [DOI] [PubMed]
- 17.LeBlanc R, Labelle H, Poitras B, Rivard CH (1997) Relation between adolescent idiopathic scoliosis and morphologic somatotypes. Spine 22:2532–2536. doi:10.1097/00007632-199711010-00013 [DOI] [PubMed]
- 18.Lindstrom J, Friberg S, Lindstrom L, Sahlstrand T (1988) Postural control in siblings to scoliosis patients and scoliosis patients. Spine 13:1070–1074. doi:10.1097/00007632-198809000-00017 [DOI] [PubMed]
- 19.Lowe T, Chir M, Margulies J, Miller N, Raso J, Reinker K et al (2000) Etiology of idiopathic scoliosis: current trends in research. J Bone Joint Surg 82:1157–1168 [DOI] [PubMed]
- 20.McIlroy WE, Maki BE (1997) Preferred placement of the feet during quiet stance development of standardized foot placement for balance testing. Clin Biomech (Bristol, Avon) 12:66–70. doi:10.1016/S0268-0033(96)00040-X [DOI] [PubMed]
- 21.Nault ML, Allard P, Hinse S, LeBlanc R, Caron O, Labelle H et al (2002) Relationships between standing stability and body posture parameters in adolescent idiopathic scoliosis. Spine 27:1911–1917. doi:10.1097/00007632-200209010-00018 [DOI] [PubMed]
- 22.Ólafsson Y, Odergren T, Persson HE, Saraste H (2002) Somatosensory testing in idiopathic scoliosis. Dev Med Child Neurol 44:130–132. doi:10.1017/S0012162201001797 [DOI] [PubMed]
- 23.Popa T, Bonifazi M, Della Volpe R, Rossi A, Mazzocchio R (2007) Adaptive changes in postural strategy selection in chronic low back pain. Exp Brain Res 177:411–418. doi:10.1007/s00221-006-0683-4 [DOI] [PubMed]
- 24.Sahlstrand T, Petruson B (1979) A study of labyrinthine function in patients with adolescent idiopathic scoliosis. Acta Orthop Scand 50:759–769 [DOI] [PubMed]
- 25.Sahlstrand T, Lidström J (1980) Equilibrium factors as predictors of the prognosis in adolescent idiopathic scoliosis. Clin Orthop Relat Res 52:232–236 [PubMed]
- 26.Simoneau M, Mercier P, Blouin J, Allard P, Teasdale N (2006) Altered sensory-weighting mechanisms is observed in adolescents with idiopathic scoliosis. BMC Neurosci 7:68. doi:10.1186/1471-2202-7-68 [DOI] [PMC free article] [PubMed]
- 27.Simoneau M, Richer N, Mercier P, Allard P, Teasdale N (2006) Sensory deprivation and balance control in idiopathic scoliosis adolescent. Exp Brain Res 170:576–582. doi:10.1007/s00221-005-0246-0 [DOI] [PubMed]
- 28.Stokes IA, Dansereau J (1989) Moreland MS. Rib cage asymmetry in idiopathic scoliosis. J Orthop Res 7:599–606. doi:10.1002/jor.1100070419 [DOI] [PubMed]
- 29.Teasdale N, Stelmach GE, Breunig A (1991) Postural sway characteristics of the elderly under normal and altered visual and support surface conditions. J Gerontol 46:B238–B244 [DOI] [PubMed]
- 30.Teasdale N, Bard C, LaRue J, Fleury M (1993) On the cognitive penetrability of posture control. Exp Aging Res 19:1–13. doi:10.1080/03610739308253919 [DOI] [PubMed]
- 31.Veldhuizen A, Weber D, Webb P (2000) The aetiology of idiopathic scoliosis: biomechanical and neuromuscular factors. Eur Spine J 9:178–184. doi:10.1007/s005860000142 [DOI] [PMC free article] [PubMed]
- 32.Weinstein SL (1994) Adolescent idiopathic scoliosis: prevalence and nature history. In: Weinstein SL (ed) The paediatric spine: principle and practice. New York, NY, pp 463–478
- 33.Yamada K, Yamamoto H, Nakagawa Y, Tezuka A, Tamura T, Kawata S (1984) Etiology of idiopathic scoliosis. Clin Orthop Relat Res 184:560–566 [PubMed]
- 34.Yamamoto H, Yamada K (1976) Equilibrial approach to scoliotic posture. Agressologie 17:61–66 [PubMed]
- 35.Yamamoto H, Tani T, MacEwen GD, Herman R (1982) An evaluation of brainstem functions as a prognostication of early idiopathic scoliosis. J Pediatr Orthop 2:521–527 [DOI] [PubMed]
- 36.Zatsiorsky VM, Duarte M (1999) Patterns of center of pressure migration during prolonged unconstrained standing. Motor Control 4:12–27 [DOI] [PubMed]
- 37.Zatsiorsky VM, Duarte M (1999) Instant equilibrium point and its migration in standing tasks: rambling and trembling components of the stabilogram. Motor Control 3:28–38 [DOI] [PubMed]