Abstract
Previous reports have emphasized the importance of neural decompression through either an anterior or posterior approach when reconstruction surgery is performed for neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine. However, the contribution of these decompression procedures to neurological recovery has not been fully established. In the present study, we investigated 14 consecutive patients who had incomplete neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine and underwent posterior instrumented fusion without neural decompression. They were radiographically and neurologically assessed during an average follow-up period of 25 months. The mean local kyphosis angle was 14.6° at flexion and 4.1° at extension preoperatively, indicating marked instability at the collapsed vertebrae. The mean spinal canal occupation by bone fragments was 21%. After surgery, solid bony fusion was obtained in all patients. The mean local kyphosis angle became 5.8° immediately after surgery and 9.9° at the final follow-up. There was no implant dislodgement, and no additional surgery was required. In all patients, back pain was relieved, and neurological improvement was obtained by at least one modified Frankel grade. The present series demonstrate that the posterior instrumented fusion without neural decompression for incomplete neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine can provide neurological improvement and relief of back pain without major complications. We suggest that neural decompression is not essential for the treatment of neurological impairment due to osteoporotic vertebral collapse with dynamic mobility.
Keywords: Neurological deficit, Osteoporosis, Vertebral collapse, Thoracolumbar spine, Posterior fusion
Introduction
Previous reports have shown that incomplete neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine are caused by neural compression due to retropulsed bone fragments in the spinal canal, progression of kyphosis, and instability at the fracture site [1, 4, 7, 13, 17, 18]. Of the several types of surgical procedures reported for the treatment of this condition, most have emphasized the importance of decompressing the spinal cord and/or cauda equina through either an anterior or posterior approach [4, 7, 8, 13–16, 19]. However, some authors have reported that conservative treatment provides reliable neurological improvement, even without neural decompression [2, 11]. Furthermore, several studies have shown that remodeling of fractured vertebrae progresses toward recovering the normal structure of the spinal canal [5, 6]. Thus, contribution of those neural decompression procedures to neurological recovery has not been fully established.
We hypothesized that instability at the fracture site rather than neural compression is the major factor causing neurological disorders in patients with osteoporotic thoracolumbar vertebral collapse, and consequently have performed posterior instrumented fusion without neural decompression for this condition. In the present study, we investigate the clinical outcomes of this procedure.
Materials and methods
Patient population
From August 2001 to December 2006, 14 consecutive patients with delayed neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine underwent posterior instrumented fusion without neural decompression at our institute (Table 1). The patients included ten females and four males (average age at surgery, 73.2 years; range, 46–86 years). Their average follow-up period was 25 months (range, 12–50 months). The mean duration from the onset of symptoms to surgery was 3.8 months (range, 1–12 months). Causes of osteoporosis were classified as senility-related in nine patients, rheumatoid arthritis in four, and alcoholism in one. The affected level was T8 in two patients, T10 in one, T11 in three, T12 in five, and L1 in three. Eight patients had a total of 16 old thoracolumbar compression fractures at other levels: 4 in adjacent vertebrae, 6 in vertebrae two levels above or below the affected level, and 6 in vertebrae three or more levels above or below the affected level. Their neurological functions were assessed with a modified Frankel grading system (Table 2) [3]. Five patients had deficits of grade C, six had grade D1 deficits, two grade D2 deficits, and one grade D3 deficits. Six patients had neurogenic bladder dysfunction.
Table 1.
Data of surgeries for 14 patients
| Case no. | Age (years)/gender | Cause of osteoporosis | Affected level | Levels of old VB fx | Fusion levels | Anchors of posterior instrumented fusion | ||
|---|---|---|---|---|---|---|---|---|
| Pedicle screw | Sublaminar wiring | Hook | ||||||
| 1 | 80/F | Senility | T12 | T11, L2 | T7–L3 | L3 | T8, T10, L2 | T7, T9, L1, L3 |
| 2 | 67/F | Senility | T10 | L1, L3, L4 | T7–L3 | T11, L2, L3 | – | T7, T8, T9 |
| 3 | 74/F | Senility | T11 | – | T7–L3 | T10, L2, L3 | – | T7, T8, L3 |
| 4 | 46/F | RA | T8 | T6, T7 | T4–T11 | T4, T5, T6, T9, T10, T11 | T4, T5, T6, T9, T10, T11 | – |
| 5 | 79/F | Senility | T11 | L4 | T9–L1 | T9, T10, T12, L1 | – | L1 |
| 6 | 83/F | Senility | T12 | – | T9–L2 | T9, T10, T11, T12, L1, L2 | T9, T10, T1, T12, L1, L2 | – |
| 7 | 66/F | RA | L1 | T12, L3 | T10–L4 | T10, T11, T12, L2, L3, L4 | T10, T11, T12, L2, L3, L4 | – |
| 8 | 71/M | Senility | L1 | – | T10–L2 | T10, T11, T12, L2 | T10, T11 | L2 |
| 9 | 72/M | Alcoholism | T11 | T7, T9, L1 | T7–L2 | T7, T8, T9, T10, T12, L1, L2 | T7, T12, L1 | – |
| 10 | 72/F | RA | T12 | – | T9–L3 | T9, T10, T11, L1, L2, L3 | T9, T10, L1, L2, L3 | – |
| 11 | 74/F | RA | T8 | T7, T11 | T4–T12 | T4, T5, T6, T7, T9, T10, T1, T12 | T4, T5, T6, T10, T11, T12 | – |
| 12 | 72/M | Senility | T12 | – | T9–L2 | T9, T10, T11, L2, L3 | T9, T10, L2, L3 | – |
| 13 | 81/F | Senility | T12 | T10 | T9–L2 | T9, T10, T11, L2, L3 | T9, T10, L2, L3 | – |
| 14 | 86/M | Senility | L1 | – | T10–L3 | T10, T11, T12, L2, L3 | T10, T11, L2, L3 | – |
VB fx vertebral fracture, RA rheumatoid arthritis
Table 2.
The modified Frankel grading system
| Grade | Neurological status |
|---|---|
| A | Complete motor loss and sensory loss |
| B | Preserved sensation only, voluntary motor function absent |
| C | Preserved motor less than fair grade (nonfunctional for any useful purpose) |
| D1 | Preserved motor at lowest functional grade (3+/5+) and/or with bowel or bladder paralysis with normal or reduced voluntary motor function |
| D2 | Preserved motor at midfunctional grade (3+ to 4+/5+) and/or with neurogenic bowel or bladder dysfunction |
| D3 | Preserved motor at high-functional grade (4+ to 5+) and normal voluntary bowel or bladder function |
| E | Complete motor loss and sensory function normal (may still have abnormal reflexes) |
Surgical techniques
In our surgical method, no procedure for decompressing the spinal cord and/or cauda equina was performed, and all patients underwent in situ posterior fusion. At surgery, the patients were moved into a prone position on the operating table with the hip joint flexed in order to reduce the thoracolumbar kyphosis. Intraoperatively, we did not attempt to correct either kyphosis or vertebral height by applying force to the implant or manual corrective adjustment. Posterior and posterolateral fusion was performed using an autologous iliac crest bone graft and a pedicle screw and rod system. We principally used pedicle screws for anchors of the instrumented fusion, and augmentation with sublaminar cables and/or hooks at multi-levels was added to prevent the pullout of the screws (Table 1). The spine was fused from three levels above to two levels below the collapsed vertebra. In eight patients, a longer segment of the spine was fused, because of the presence of concomitant old vertebral compression fractures (Table 1). The average number of fusion levels was 6.2 (range 4–9). In five patients who had large bone defect in the fractured vertebral body, transpedicular impaction of hydroxyapatite block was added to fill the defect. However, we did not perform the correction of kyphosis at this impaction. Patients were allowed out of bed with spine protected by a plastic orthosis at 4 or 5 days after the operation. They remained in the plastic orthosis for 3 months, and then changed to a canvas brace for an additional 3 months.
Radiographic assessment
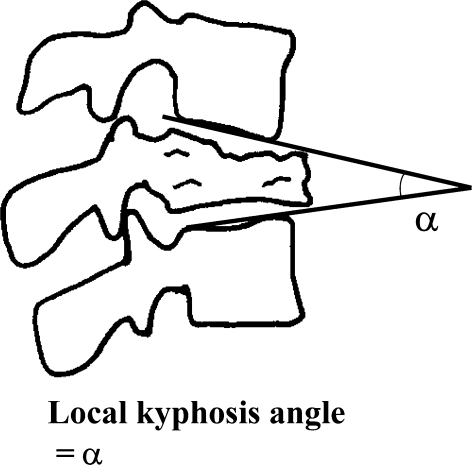
In preoperative radiographs, the local kyphosis angle was measured as the angle between the lower endplate of the uninvolved vertebra above the fractured level, and the upper endplate of the uninvolved vertebra below the fractured level (Fig. 1). The sagittal Cobb angle was also measured as the angle between the upper endplate of the uppermost vertebra and the lower endplate of the lowest vertebra at the instrumented fusion levels. Eleven patients underwent myelography preoperatively, and blockade of contrast medium was evaluated. In ten patients, the spinal canal occupation due to retropulsed bone fragments was measured on CT images before surgery and 6 months after surgery. Bone union of the collapsed vertebra and the instrumented fusion levels was assessed to be successful, when there was no change of the local kyphosis angle and the sagittal Cobb angle on flexion and extension radiographs. Postoperative subsequent vertebral fractures and complications related to the surgical instrumentation were also analyzed.
Fig. 1.
Schematic diagrams of radiographic measurements
Clinical assessment
Back pain was classified into four grades: none, mild, moderate, and severe. Mild pain was defined as intermittent pain during motion; moderate pain was defined as pain preventing the patient from sitting in a chair, but without pain while resting in bed; and severe pain was defined as pain even while resting in bed. Clinical outcomes for back pain were assessed using this classification. In addition, neurological function was evaluated using a modified Frankel grading system (Table 2) [3].
Results
Before surgery, the mean local kyphosis angle was 14.6° (range, 0–40°) on flexion radiographs and 4.1° (range, −18–27°) on extension radiographs (Table 3). Thus, the mean change in the local kyphosis angle in flexion and extension was 10.6° (range, 7–18°), demonstrating marked instability at the collapsed vertebrae. Intraoperatively, there was no finding of deficiency and attenuation in the supra- and inter-spinous ligaments in 14 patients, indicating little participation of the posterior elements of spinal column to the instability.
Table 3.
Alteration of the local kyphosis angle at the affected vertebra
| Case no. | Local kyphosis angle (°) | ||||
|---|---|---|---|---|---|
| Before surgery | Immediately after surgery | Six months after surgery | Final follow-up | ||
| Flexion | Extension | ||||
| 1 | 21 | 9 | 10 | 18 | 18 |
| 2 | 0 | −18 | −10 | −4 | −3 |
| 3 | 19 | 11 | 8 | 15 | 16 |
| 4 | 25 | 19 | 25 | 28 | 28 |
| 5 | 6 | −1 | 0 | 10 | 10 |
| 6 | 18 | 5 | 10 | 13 | 14 |
| 7 | 5 | −6 | −8 | −5 | −5 |
| 8 | 11 | 3 | 0 | 4 | 4 |
| 9 | 0 | −7 | −7 | −5 | −5 |
| 10 | 28 | 18 | 18 | 18 | 17 |
| 11 | 40 | 27 | 29 | 30 | 30 |
| 12 | 11 | 3 | 5 | 5 | 6 |
| 13 | 15 | 2 | 6 | 6 | 6 |
| 14 | 9 | −8 | −5 | 0 | 2 |
| Mean ± SD | 14.6 ± 11.2 | 4.1 ± 12.1 | 5.8 ± 12.0 | 9.5 ± 11.5 | 9.9 ± 11.3 |
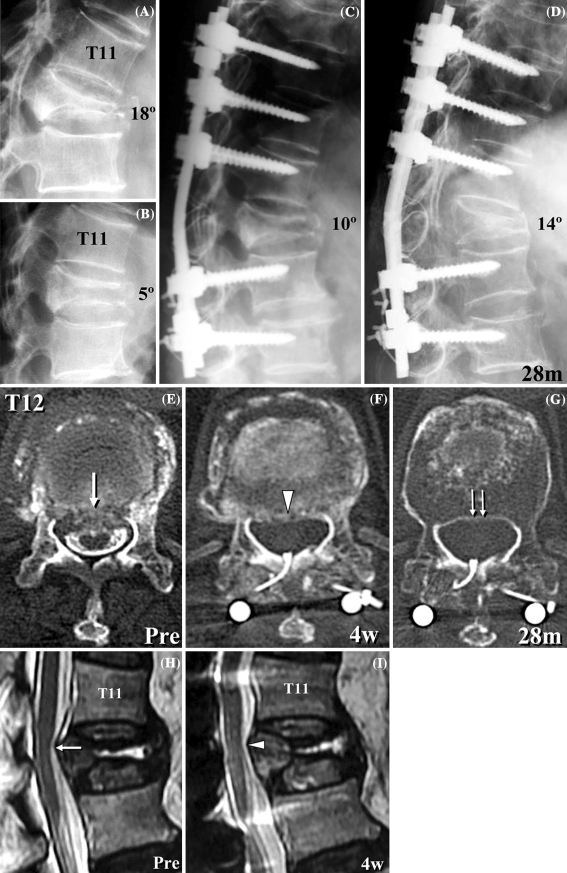
Myelogram showed no obstruction of the contrast medium in all 11 patients examined. Before surgery, the mean spinal canal occupation by retropulsed bone fragments was 21.1% (range, 0–37.4%). Among ten patients examined, the spinal canal compromise was 0% before surgery in two patients. In the remaining eight patients, remodeling of the spinal canal was observed (Fig. 4e, f, g). The average spinal canal compromise was decreased from 26.3% before surgery to 18.1% at 6 months after surgery. Anterior spinal canal encroachment due to factors other than bone fragments was shown on MRI; granulation tissue was found in the vertebral body of one patient with pseudoarthrosis and hematoma in another patient, both conditions resolved spontaneously after surgery.
Fig. 4.
Preoperative flexion (a) and extension (b) radiographs of an 83-year-old woman with T12 osteoporotic vertebral collapse (case 6) demonstrates marked instability at T12. c Immediately after surgery, the local kyphosis was corrected to 10°. d At the final follow-up of 28 months after surgery, bone union of T12 vertebral body was achieved and local kyphosis was 14°. Preoperative CT-myelogram (e) shows retropulsed bone fragments (arrow). A CT image at 4 weeks after surgery shows the resorption of the bone fragments (f, arrowhead). A CT image at 28 months after surgery shows that remodeling of the spinal canal has progressed (g, double arrow). T2-weighted midsagittal MRI images show that the anterior impingement of the spinal cord was present before surgery (h, arrow), but it was reduced at 4 weeks after surgery (i, arrowhead)
The mean local kyphotic angle in flexion before surgery (14.6°; Table 3) was significantly reduced to 5.8° immediately after surgery, 9.5° at 6 months after surgery, and 9.9° at the final follow-up (Table 3). The mean correction of the local kyphosis was 8.9° immediately after surgery and 4.7° at the final follow-up; thus the mean loss of correction was 4.1°. There was no implant dislodgement, and no additional surgery was required. Solid union of both parts of the collapsed vertebral body as well as posterior and posterolateral spine fusion was successfully achieved in all patients.
In seven patients, subsequent vertebral compression fractures developed after surgery within the fusion level and/or at adjacent or nearby vertebrae above or below the fusion level (Table 4). All the subsequent fractures were well managed conservatively, and no patients complained residual back pain at the final follow-up. Increase of local kyphotic angle after the onset of each subsequent fracture was within 5° at the final follow-up (Table 4), indicating no development of the junctional kyphosis [9].
Table 4.
Data of seven patients with the subsequent fracture after surgery
| Case no. | Fusion levels | Levels of subsequent fx | Surgery to subsequent fx (mo) | Local kyphosis angle at subsequent fx (°) | |
|---|---|---|---|---|---|
| Immediately after surgery | Final follow-up | ||||
| 1 | T7–L3 | T8 | 1 | 1 | 5 |
| T10 | 6 | 3 | 3 | ||
| L1 | 12 | 16 | 20 | ||
| 2 | T7–L3 | T7 | 4 | 6 | 11 |
| T5 | 36 | 2 | 7 | ||
| 3 | T7–L3 | T6 | 3 | 7 | 9 |
| L4 | 36 | −15 | −12 | ||
| 8 | T10–L2 | L4 | 3 | −16 | −13 |
| L2 | 6 | −3 | 1 | ||
| 10 | T9–L3 | T9 | 1 | 4 | 7 |
| L5 | 12 | −34 | −31 | ||
| 11 | T4–T12 | T4 | 1.5 | 14 | 15 |
| 12 | T9–L2 | T8 | 17 | 2 | 7 |
Subsequent fx = subsequent fracture
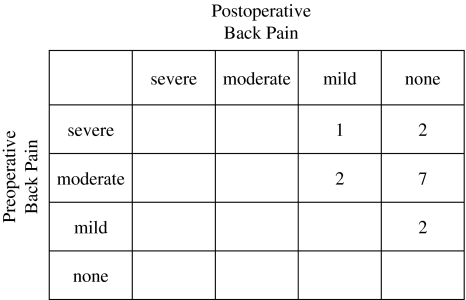
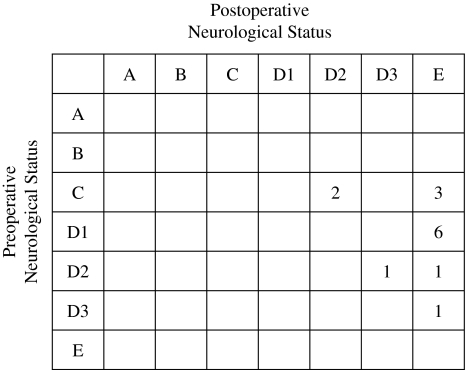
All patients had back pain before surgery. The pain grades in 12 patients were moderate or severe. In all 14 patients, back pain was relieved after surgery. At the latest follow-up assessment, 11 of 14 patients reported complete relief of pain (Fig. 2). In all patients, neurological function improved by at least one modified Frankel grade (Fig. 3). Of the five patients with Frankel grade C, two improved to grade D2 and 3 to grade E. Of the two patients with Frankel grade D2, one improved to grade D3 and the other to grade E. In seven patients with Frankel grade D1 and D3, all improved to grade E. Although all patients preoperatively had gait disturbance, all of them could walk with or without a cane at the latest follow-up. In all six patients with bladder dysfunction, their urinary symptoms were relieved after surgery.
Fig. 2.
Preoperative and postoperative back pain grade
Fig. 3.
Preoperative and postoperative neurological status using the modified Frankel grading system
Case presentation
Case 6
An 83-year-old woman suffered back pain after falling down at home, and then muscle weakness of her legs and bladder dysfunction gradually developed. When she was admitted to our institute 2 months after the onset of her symptoms, she was unable to walk independently. She had moderate back pain, and neurological deficits of modified Frankel grade C. Flexion and extension radiographs showed dynamic mobility of T12 vertebral collapse (Fig. 4a, b). CT-myelogram and MRI images revealed retropulsed bone fragments in the spinal canal, which impinged the spinal cord anteriorly (Fig. 4e, h, arrow). The spinal canal occupation was 27%. Posterior instrumented fusion was performed from T9 to L2 without decompression of the spinal cord. Local kyphosis was corrected to 10° just after surgery and 14° at the final follow-up (28 months after surgery; Fig. 4c, d). Four weeks after surgery, resorption of the bone fragments had already begun and anterior compression of the spinal cord was reduced (Fig. 4f, i, arrowhead). Further remodeling of the spinal canal had occurred, and no residual spinal canal stenosis was noted at the latest follow-up of 28 months after surgery (Fig. 4g, double arrow). At this stage, solid union of T12 vertebral body was achieved, without any implant-related complications. At the final follow-up, neurological deficit was completely recovered to grade E.
Discussion
The present results demonstrate that the main factor causing delayed neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine is instability of the spinal column at the fracture site rather than mechanical compression of the spinal cord by the bone fragments. In this series, all 14 patients possessed marked instability at the fractured vertebra, and the posterior spinal fusion obtained bony fusion and spinal stability that resulted in apparent neurological improvement even without any decompressive procedures.
Various operative procedures have been reported to be successful in effecting neurological improvement in patients with neurological deficits due to osteoporotic vertebral collapse. Kaneda et al. [7] and Uchida et al. [19] emphasized the importance of anterior decompression and reconstruction with use of anterior instrumentation, but implant-related complications and pseudoarthrosis occurred in some patients because of the poor bone quality. Other investigators have insisted that anterior decompression and fusion should not be used alone, recommending additional posterior reinforcement to increase the rate of arthrodesis [10, 12]. An anterior approach is considered to increase the risk of injury to the chest or abdomen, which might be especially hazardous in elderly patients. In contrast, Shikata et al. [15] and Kim et al. [8] performed posterolateral decompression and posterior reconstruction using the posterior egg-shell procedure. Recently, posterior closing wedge osteotomy including posterior spinal shortening has been performed for both neural decompression and correction of kyphotic deformity [14, 16]. Although posterior procedures offer better kyphosis correction compared with anterior procedures, they have the risk of neural tissue damage, such as dural tear and spinal cord kinking due to shortening of the spinal column. Furthermore, a problem remains that the strength of the implant fixation in osteoporotic bone is not sufficient for reconstruction of a spine with marked instability. Laminectomy and resection of all posterolateral components including the pedicle may highly destabilize the spinal column in this unstable condition. Actually, previous reports on the spinal shortening described 15–58% rate of implant failure. In the present study, we first reported that posterior instrumented fusion alone allowed apparent neurological recovery of patients with neurological deficits following osteoporotic vertebral collapse. We believe that our procedure has a number of advantages. The simple posterior fixation without neural decompression is not only technically undemanding, but also much safer, because the risks of the anterior approach and potential damage to neural tissue from the posterolateral decompressive procedure can be avoided. In addition, the posterior elements of the spine important for maintaining spinal stability in this condition are preserved. This also provides enough space for bone grafting on the lamina of the affected vertebra. Thus, solid bony fusion and stabilization of the spinal column can be achieved.
Risks of implant failure are significant when instrumentation surgery is used to treat the osteoporotic spine. Several authors have suggested that correction of kyphotic deformity and restoration of anatomic alignment may reduce instrumentation failure [4, 14, 16]. However, the interface between the implant and osteoporotic bone may not be mechanically able to support the spinal column. In elderly patients whose thoracolumbar kyphosis was extensively corrected without any anterior support, flexion moment during standing or sitting may produce a posteriorly directed force that pulls out the implant. In this series, we did not apply any correction force to the implants. Thus no intraoperative procedure to correct kyphosis was conducted, and excessive kyphosis correction was thereby avoided. We performed fixation of the spinal column in the alignment achieved by posture reduction. Consequently, no implant dislodgement was found, and no additional operation was required. We found that local kyphosis angle was corrected to 5.8° just after surgery and correction loss was 9.9° at the final follow-up, which was comparable to the correction in the previous reports on anterior decompression and reconstruction [7, 19]. Although subsequent vertebral fractures were found after surgery in 50% of the present cases, they were well managed conservatively and no junctional kyphosis developed. No patients in the current study complained of residual back pain or any difficulty in activities of daily living at final follow-up. Thus, it is suggested that extensive correction of kyphotic deformity has few advantages in elderly patients with osteoporosis.
In conclusion, this study introduces the concept that incomplete neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine are mainly caused by instability of the fractured vertebra rather than neural compression. Although this series is relatively small, current results indicate that posterior instrumented fusion without neural decompression can provide significant neurological improvement and relief of back pain without major complications. We suggest that neural decompression of the spinal cord is not necessary for the treatment of neurological impairment in patients with osteoporotic vertebral collapse with dynamic mobility.
References
- 1.Arciero RA, Leung KYK, Pierce JH (1989) Spontaneous unstable burst fracture of the thoracolumbar spine in osteoporosis. Spine 14:114–117. doi:10.1097/00007632-198901000-00024 [DOI] [PubMed]
- 2.Ataka H, Tanno T, Nemoto T et al (2004) Delayed neurologic deficit resulting from instability after osteoporotic vertebral fracture in the thoracolumbar spine: report of two cases. Rinsho Seikei Geka 39:851–856. Clinical Orthopaedic Surgery
- 3.Bradford DS, McBride GG (1987) Surgical management of thoracolumbar spine fractures with incomplete neurologic deficits. Clin Orthop Relat Res 218:201–216 [PubMed]
- 4.Chang KW, Chen YY, Lin CC et al (2005) Apical lordosating osteotomy and minimal segment fixation for the treatment of thoracic or thoracolumbar osteoporotic kyphosis. Spine 30:1674–1681. doi:10.1097/01.brs.0000170450.77554.bc [DOI] [PubMed]
- 5.Dai LY (2001) Remodeling of the spinal canal after thoracolumbar burst fractures. Clin Orthop Relat Res 382:119–123. doi:10.1097/00003086-200101000-00018 [DOI] [PubMed]
- 6.Fidler NW (1988) Remodeling of the spinal canal after burst fracture. J Bone Joint Surg Br 70:730–732 [DOI] [PubMed]
- 7.Kaneda K, Asano S, Hashimoto T et al (1992) The treatment of osteoporotic posttraumatic vertebral collapse using the Kaneda device and a bioactive ceramic vertebral prosthesis. Spine 17:S295–S303. doi:10.1097/00007632-199208001-00015 [DOI] [PubMed]
- 8.Kim KT, Suk KS, Kim JM et al (2003) Delayed vertebral collapse with neurological deficits secondary to osteoporosis. Int Orthop 27:65–69 [DOI] [PMC free article] [PubMed]
- 9.Kim YJ, Lenke LG, Bridwell KH et al (2007) Proximal junctional kyphosis in adolescent idiopathic scoliosis after 3 different types of posterior segmental spinal instrumentation and fusions: incidence and risk factor analysis of 410 cases. Spine 32:2731–2738. doi:10.1097/BRS.0b013e318074c3ce [DOI] [PubMed]
- 10.Malcom BW, Bradford DS, Winter RB et al (1981) Post-traumatic kyphosis: a review of forty-eight surgically treated patients. J Bone Joint Surg Am 63:891–899 [PubMed]
- 11.Mamada T, Iijima T (2005) Conservative treatment for paraplegia resulting from vertebral fractures in senile osteoporosis. Seikei Geka 56:1367–1371. Orthop Surg
- 12.McAfee PC, Bohlman HH, Yuan HA (1985) Anterior decompression of traumatic thoracolumbar fractures with incomplete neurological deficit using a retroperitoneal approach. J Bone Joint Surg Am 67:89–104 [PubMed]
- 13.Mochida J, Toh E, Chiba M et al (2001) Treatment of osteoporotic late collapse of a vertebral body of thoracic and lumbar spine. J Spinal Disord 14:393–398. doi:10.1097/00002517-200110000-00004 [DOI] [PubMed]
- 14.Saita K, Hoshino Y, Kikkawa I et al (2000) Posterior spinal shortening for paraplegia after vertebral collapse caused by osteoporosis. Spine 25:2832–2835 [DOI] [PubMed]
- 15.Shikata J, Yamamuro T, Iida H et al (1990) Surgical treatment for paraplegia resulting from vertebral fractures in senile osteoporosis. Spine 15:485–489. doi:10.1097/00007632-199006000-00010 [DOI] [PubMed]
- 16.Suk SI, Kim JH, Lee JH et al (2003) Anterior-posterior surgery versus posterior closing wedge osteotomy in posttraumatic kyphosis with neurologic compromised osteoporotic fracture. Spine 28:2170–2175. doi:10.1097/01.BRS.0000090889.45158.5A [DOI] [PubMed]
- 17.Sutherland CJ, Miller F, Wang GJ (1983) Early progressive kyphosis following compression fractures: two case reports from a series of “stable” thoracolumbar compression fractures. Clin Orthop Relat Res 173:216–220 [PubMed]
- 18.Tanaka S, Kubota M, Fujimoto Y et al (1993) Conus medullaris syndrome secondary to an L1 burst fracture in osteoporosis. Spine 18:2131–2134. doi:10.1097/00007632-199310001-00034 [DOI] [PubMed]
- 19.Uchida K, Kobayashi S, Nakajima H et al (2006) Anterior expandable strut cage replacement for osteoporotic thoracolumbar vertebral collapse. J Neurosurg Spine 4:454–462. doi:10.3171/spi.2006.4.6.454 [DOI] [PubMed]