Abstract
Wear simulation is an essential pre-clinical method to predict the mid- and long-term clinical wear behavior of newly introduced devices for total disc arthroplasty. The main requirement of a suitable method for spinal wear simulation has to be the ability to distinguish between design concepts and allow for a direct comparison of predicate devices. The objective of our study was to investigate the influence of loading and kinematic patterns based on two different protocols for spinal wear simulation (ISO/FDIS 18192-1 (2006) and ASTM F2423-05). In vitro wear simulation was performed with six activ® L lumbar artificial disc devices (Aesculap Tuttlingen, Germany). The applied kinematic pattern of movement was multidirectional for ISO (elliptic track) and unidirectional with a curvilinear shape for ASTM. Testing was done for 10 million cycles in the ISO loading mode and afterwards with the same specimens for 5 million cycles according to the ASTM protocol with a customized six-station servohydraulic spinal wear simulator (EndoLab Thansau, Germany). Gravimetrical and geometrical wear assessment, a slide track analysis correlated to an optical surface characterization, and an estimation of particle size and morphology were performed. The gravimetric wear rate for the first 10 million cycles was ISOinitial = 2.7 ± 0.3 mg/million cycles. During the ASTM test period (10–15 million cycles) a gravimetric wear rate of 0.14 ± 0.06 mg/million cycles was estimated. The wear rates between the ISO and ASTM driven simulations differ substantially (approximately 20-fold) and statistical analysis demonstrates a significant difference (p < 0.001) between the test groups. The main explanation of divergency between ISO and ASTM driven wear simulations is the multidirectional pattern of movement described in the ISO document resulting in a cross-shear stress on the polyethylene material. Due to previous retrieval observations, it seems to be very unlikely that a lumbar artificial disc is loaded with a linear wear path.Testing according to ASTM F2423-05 with pure unidirectional motion does not reflect the kinematics of TDA patients‘ daily activities. Based on our findings it seems to be more reliable to predict the clinical wear behavior of an artificial disc replacement using the ISO/FDIS 18192-1 method.
Keywords: Lumbar total disc arthroplasty, Wear simulation, Pattern of movement, Particle analysis
Introduction
The aim of total disc arthroplasty (TDA) is to restore the function of the intervertebral disc, provide long-term pain relief and avoid adjacent segment disease [8]. Based on the short and midterm clinical observations total disc arthroplasty may be a viable option to lumbar fusion in patients with symptomatic disc degeneration [5, 19, 27–29, 42, 50]. The outcome of total disc arthroplasty depends to a significant extent on the pre-operative degree of painful disintegration of the intervertebral disc and the facet joints and after TDA on the long-term properties of the artificial disc device in mostly young and active patients [4, 25, 26, 49].
The intervertebral disc is a complex anatomic and functional structure, which makes the development of an efficient artificial disc a challenge [28, 43]. Today the joint articulation of most of the clinically introduced TDA devices is based on the materials combination CoCr29Mo6 for the highly polished metallic endplates and ultra high molecular weight polyethylene (UHMWPE) for the intermediate gliding inlay [17].
From total joint replacements it is known that the generation of polyethylene particles caused by wear activates a biological cascade reaction in the periprosthetic tissue [20, 40]. Initiated by the phagocytosis of the particles a release of inflammatory cytokines takes place which changes the metabolism of the periprosthetic tissue [18, 31]. A possible effect is the shift of the equilibrium for bone formation and bone resorption which leads to osteolysis, the formation of fibrous tissue and consequently aseptic loosening of the implant components [2, 7, 15, 20, 37].
In comparison to hip and knee replacements, few if any, long term clinical outcome, retrieval studies or in vitro wear simulator analyses exist for TDA prostheses. Furthermore, little is known about the possible adverse effects of wear particles on inflammation, osteolysis, and potential impairment of neurological structures [9, 10, 13, 35, 44]. Based on the complexity of the anatomical structures and the nearly unknown loading and environmental conditions [28, 48] little knowledge exists about the biotribological behavior of TDA devices and the release of polyethylene wear debris in the human lumbar spine [3, 18, 22, 23, 30, 36, 41].
At the moment two different testing protocols (ISO/FDIS 18192-1 (2006) and ASTM F2423-05) are described for spinal wear simulation. Since uniform and comparable testing conditions are the goal for standardized pre-clinical testing, the existence of two different testing standards makes a direct comparison impossible.
Objectives
The objective of our study was to investigate the influence of required loading and kinematic patterns of two different protocols (ISO and ASTM documents), on the in vitro wear behavior of TDA prostheses.
Materials and methods
A comparative wear simulation was performed with activ® L lumbar artificial discs (Aesculap AG Tuttlingen, Germany). The activ® L design consists of a convex shaped mobile inlay made out of UHMWPE which articulates with an inferior and superior metallic endplate in the form of a spherical joint (Fig. 1). The radii of the inferior and superior bearings are 50 and 14 mm, respectively.
Fig. 1.
Total disc arthroplasty device for the lumbar spine (activ® L), with inferior and superior endplate made from CoCr29Mo6 alloy with a pure titanium coating and a thin layer of calcium phosphate at the vertebral interfaces and an inlay made out of UHMWPE with a ball in socket joint and additional mobility in the A/P direction
The mobile polyethylene inlay is able to move in the anterior posterior direction relative to the inferior endplate to avoid in clinical use facet joint degeneration due to overloading. The UHMWPE core was machined from GUR 1020 with a surface roughness of less than 1 μm (Ra value), packaged under nitrogen atmosphere and sterilized by electron beam (β-irradiation 25–40 kGy). The endplates of the disc were made from CoCr29Mo6 alloy with highly polished bearing surfaces and interfaces foreseen for bone ingrowth with a pure titanium porous coating and an additive thin layer of dicalcium phosphate dehydrate (Plasmapore® μCaP Aesculap, Tuttlingen). For the wear test the intermediate disc size M was used in the implant configuration with inferior and superior endplate and a lordotic angle of 0 and 6°, respectively. The nominal total height of the two endplates in combination with the polyethylene inlay was 14 mm.
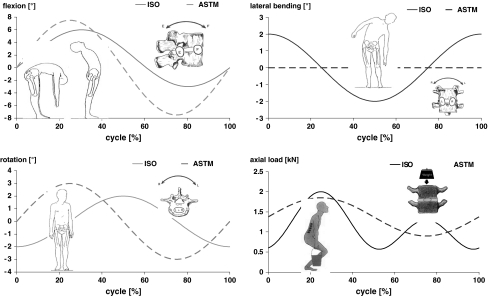
The in vitro wear simulation was performed in a direct comparison of two different test methods according to ISO/FDIS 18192-1:2006 and ASTM F 2423-05. For the ISO protocol the applied kinematic pattern is based on a displacement of 6° in flexion and 3° in extension. Left and right lateral bending is simulated 108° out of phase to flexion/extension with a movement of ±2° and bilateral axial rotation is given 180° out of phase in respect to lateral bending with an amplitude of ±2°. The corresponding axial force is applied in a double peak loading mode with 2,000 N in full flexion, 600 N in neutral position and 1,300 N for full extension1 (Fig. 2). In contrast to the applied load profile all movements are realized in a sinusoidal waveform.
Fig. 2.
Load and displacement parameters for the wear test according to ISO/FDIS 18192-1 (2006) and ASTM F2423-05 (dotted lines)
According to the ASTM document flexion/extension (amplitude ±7.5°) is coupled in phase with axial rotation (±3°) and an axial loading mode of 1,850 N for flexion and 900 N for extension is applied (Table 1). All displacements and forces are based on a sinusoidal waveform (Fig. 2).
Table 1.
Comparison of the applied load and displacement parameters for wear simulation according to ISO/FDIS 18192-1 (2006) and ASTM F 2423-05
| Parameter | Flexion/extension | Lateral Bending | Axial rotation | Loading | Coupling |
|---|---|---|---|---|---|
| ISO/FDIS 18192–1 | 6°/3° | ±2° | ±2° | Flexion: 2,000 N, neutral: 600 N, extension: 1,300 N | All motions out of phase |
| ASTM F 2423-05 | ±7.5° | – | ±3° | Flexion: 1,850 N, extension: 900 N | Fe/Ext and AR in phase |
For the ISO and ASTM accomplished tests the loads and displacements were realized with a frequency of 1 Hz. In reference position the specimen had an inclination of 10° in the sagittal plane due to the anatomical situation in the lower lumbar spine resulting in additional anterior-posterior shear loading on the polyethylene inlay.
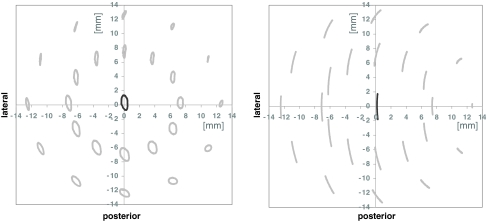
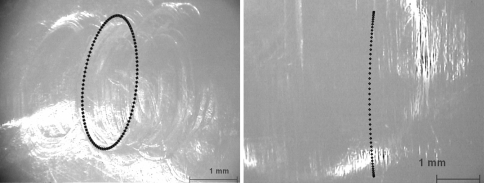
For the superior articulation a slide track analysis was undertaken based on a previously described method by Saikko and Calonius [38, 39] for the characterization of hip wear simulators. For the estimation of the wear path the resultant track drawn by the point of its application was considered, so that the sliding increment is integrated over one motion cycle. Due to these calculations and consequently during wear simulation the applied kinematic pattern of movement was multidirectional for ISO/FDIS 18192-1 (elliptic track) and unidirectional with a curvilinear shape for ASTM F 2423-05 (Fig. 3).
Fig. 3.
Pattern of movement of the superior bearing of the polyethylene inlay relative to the superior endplate in a planar view perpendicular to the pole of the sphere (generated by simulator data acquisition) according to ISO/FDIS 18192-1 (left) and ASTM F 2423-05 (right)
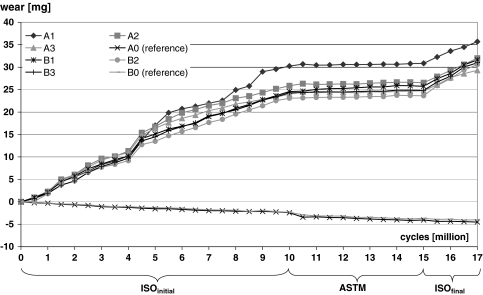
Testing was done for 10 million cycles according to ISO/FDIS 18192–1:2006 (ISOinitial) and afterwards with the same specimen for 5 million cycles following the ASTM F 2423-05 protocol (ASTM) with a customized 6 station servo-hydraulic spinal wear simulator (EndoLab Mechanical Engineering GmbH, Thansau, Germany). Finally the identical disc components were tested again for 2 million cycles according to the ISO protocol (ISOfinal) to investigate if the wear behavior of the disc replacements has changed due to the sequential testing procedure. All polyethylene inlays were soaked prior to simulation in serum based test medium for 30 days to allow for saturated fluid absorption. Six disc assemblies (specimens A1–A3 and B1–B3) were fixed with epoxy resin and mounted in the wear test stations and two references (specimens A0 and B0) were loaded axially only for loaded soak controls. Testing was performed at 37° in a lubricant of newborn calf serum from a single source lot (Biochrom AG Berlin, Germany) diluted with deionized water to install a defined protein content of 30 g/l. The lubricant was pH-stabilized by ethylene diamine tetraacetic acid (EDTA) and amphotericine was added for fungal decay preservation. The test chambers were sealed with an elastic membrane to avoid contamination of the lubricant during the test.
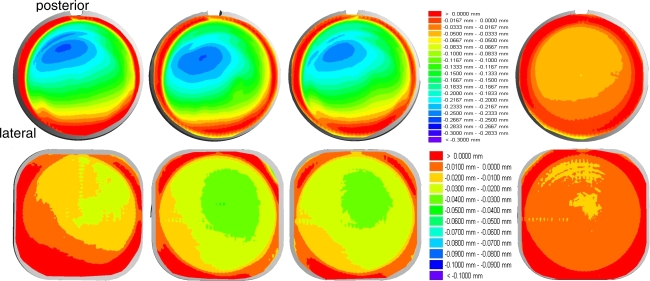
At each measurement interval (0.5 million cycles) cleaning was performed following a procedure described in ISO 14242-2:2002 (E) for wear assessment of hip joint articulations. The wear of the polyethylene inlays was estimated gravimetrically with an analytical balance (Mettler-Toledo Type AG 204 Balingen, Germany) to a precision of 0.1 mg under consideration of the air buoyancy and the bearing surfaces were inspected with a stereo microscope (Wilde M3Z Herrenbrugg, Switzerland). The mean and standard deviation of the gravimetrically measured weight loss was calculated. To adjust the effect of fluid absorption the loaded soak controls were used to estimate the linear association between cycle count (time) and mass change. A geometrical assessment of plastic deformation (creep) and wear was performed after 10 million cycles (ISOinitial) with a three-dimensional measuring machine (UMM850, Zeiss Oberkochen, Germany) in a tactile measuring mode (points per scan 3,500). The geometrical changes were displayed vertically to the transversal plane of the polyethylene inlay with a pseudocolor mode, whereas the colors were spread between red = 0.0 mm and dark blue = −0.3 mm for the superior surface of the polyethylene inlay and red = 0.0 mm and dark blue = −0.1 mm for the inferior surface, respectively.
The lubricant was replaced at each station after each inspection interval and stored for wear particle isolation and analysis following a procedure described by Affatato et al. [1] and Niedzwiecki et al. [34]. The particles were digested in 37% hydrochloric acid, diluted in methyl alcohol and filtered through a cascade of polycarbonate filters with a pore size of 0.1, 1.0 and 10 μm, beginning with the largest filter size. Subsequently a SEM micrograph analysis was performed with at least five images per filter pore size for the software assisted particle count (size and morphology) at each measurement point to obtain a representative particle size distribution. The serum of the six tested specimens (A1–A3, B1–B3) and the loaded references (A0,B0) was analyzed for size and shape of the wear particles every 0.5 million cycles up to 3 million cycles and after 5, 7.5, and 10 million cycles (ISOinitial). For a direct comparison the serum of the six tested specimens and the loaded references was also analyzed after wear simulation according to ASTM F2423-05 at 12.5 million cycles (2.5 Mio cycles in ASTM wear mode). The mean particle diameter was used to describe the size of the particles and the roundness was used to describe the shape of the particles.
Finally a statistical analysis was performed to verify the Gaussian distribution (Kolmogorov–Smirnov-test) and to discriminate the wear rates between the groups ISOinitial, ASTM and ISOfinal (paired Student‘s t test).
Results
For the six polyethylene inlays under wear simulation the mean gravimetric wear rate of the initial test period (0–10 million cycles) was ISOinitial = 2.7 ± 0.3 mg/million cycles (Fig. 4). During the second period (10–15 million cycles), the simulation was performed according to the ASTM protocol, a mean gravimetric wear rate of 0.14 ± 0.06 mg/million cycles was calculated.
Fig. 4.
Average mass change of the polyethylene inlays under wear testing conditions (specimens A1–A3, B1–B3) and loaded soak controls (specimens A0,B0) resulting in the gravimetric wear behavior of the activ® L spinal disc system according to ISO/FDIS 18192-1 (2006) and ASTM F 2423-05 test protocols
For the final test period (15–17 million cycles),—again according to the ISO protocol,—an amount of wear of ISOfinal = 2.85 ± 0.44 mg/million cycles was estimated.
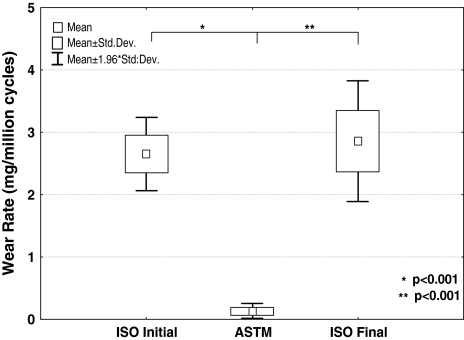
The wear rates between the ISO and ASTM performed simulations differ substantially (approximately 20-fold) and statistical analysis demonstrates a significant difference between the test groups ISOinitial versus ASTM (p < 0.001) and ASTM versus ISOfinal (p < 0.001) (Fig. 5).
Fig. 5.
Box-Wisker-plot for statistical analysis between the groups ISOinitial versus ASTM and ASTM versus ISOfinal (paired Student-t test)
In a direct comparison between ISOinitial and ISOfinal the wear rates differ only by a factor of 1.1, and it is obvious that there is a high level of repeatability and no statistical difference of these two groups could be observed (p = 0.563).
Due to a combination of creep and wear after 10 million cycles (ISOinitial) a mean height loss of less than 0.28 mm for the superior articulation and less than 0.05 mm for the inferior articulation was detected in respect to the initial state prior wear testing (Fig. 6). After completion of the ASTM test period no geometrical wear assessment was performed. But due to the very low wear rate (ASTM) no substantial geometrical changes could be expected.
Fig. 6.
Height loss in vertical direction to the transverse plane of the polyethylene inlays at the superior and inferior bearing after 10 million cycles (ISOinitial)—specimen A1–A3 under wear simulation (left) and loaded soak control specimen A0 (right)—superior scale: red = 0.0 mm and dark blue = −0.3 mm, inferior scale: red = 0.0 mm and dark blue = −0.1 mm
The height loss of the loaded soak controls (ISOinitial) is up to 0.067 mm on the superior surface and 0.02 mm at the inferior surface. The geometrical changes of the loaded reference specimens are due to plastic deformation (creep). Therefore, approximately 25% of the geometrical changes of the superior surface and 40% of the inferior surface are not related to a wear process with particle generation. The larger geometrical changes on the posterior part of the superior surface are due to the applied movement patterns and the anatomical inclination of the specimens according to the ISO/FDIS 18192-1. The slightly lateral position of the largest change in the geometry is directly correlated to the asymmetrical distribution of the axial force vector during the load cycle and corresponds well to the applied load and displacement pattern of the ISO protocol.
The slide track analysis for the superior articulation results in a multidirectional pattern of movement with oval shape for the ISO/FDIS 18192-1 and in a unidirectional displacement pattern with curvilinear shape for the ASTM F 2423-05 (Fig. 3). The calculated patterns of movement for ISO and ASTM are consistent with the observations of the wear traces on the polyethylene inlays (Fig. 7). A direct influence of the different pattern of load and displacement on the shape of the generated wear traces was detected.
Fig. 7.
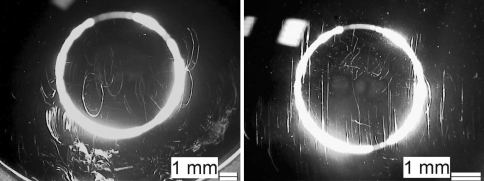
Characteristical wear traces on the superior bearing surfaces of the polyethylene inlays with multidirectional oval shape after wear simulation according to ISO/FDIS 18192-1 (ISOinitial, 10 million cycles) and with curvilinear wear traces resulting from the ASTM F 2423-05 kinematic pattern of movement (ASTM, 15 million cycles)
The images of the superior bearing of the polyethylene inlays illustrate apparently the close relationship of the resulting wear traces to the applied ISO or ASTM wear simulation protocol. All images of the optical wear surface analysis were undertaken in a planar view perpendicular to the pol of the sphere.
The initial test period (ISOinitial) provokes multidirectional and oval shaped wear traces, whereas the second test period according to ASTM removes the previously formed wear traces continuously and leads to linear wear traces (Fig. 7). After returning to the ISO test protocol (ISOfinal) the oval shaped wear traces appear again. These characteristic findings are consistent for all tested specimens. The microscopic observations of all polyethylene inlays indicate that abrasive/adhesive wear and plastic deformation are the main wear mechanisms. There is no observation of crack formation nor pitting or delamination on the UHMWPE inlay bearings after 17 million wear cycles.
The different wear traces and their changes between the first and second test period (ISOinitial and ASTM) are also obvious on the superior metallic endplates counterpart (Fig. 8).
Fig. 8.
Superior endplate of specimen B1 (magnification 10:1) after 10 million cycles (ISOinitial) with oval shaped wear traces on the metallic bearing surface and specimen B2 after 15 million cycles (ASTM) with mainly linear wear traces on the superior metallic bearing surface (magnification 16:1)
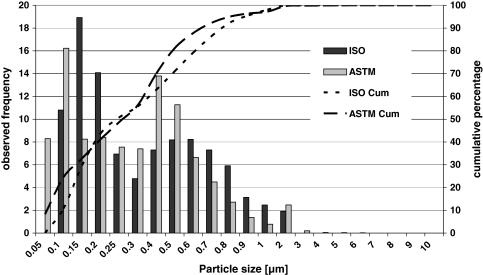
The particle size distribution and the shape of the particles is uniform for all six tested specimens in every test interval following the ISO wear simulation (ISOinitial). This indicates a similar steady wear behavior for all six tested specimens, which is consistent with the gravimetric wear results. After the running-in period (up to 1.0 million cycles) the particle size distribution demonstrates, in general, steady state characteristics. The average value of the mean particle diameter increases from 0.17 to 0.2 μm at the first 1.5 million cycles of the wear test to values between 0.2 and 0.4 μm for the complete ISOinitial test duration. The lower 95% confidence limit is located at 0.1 μm and the upper 95% confidence limit is in a range between 0.7 and 0.9 μm. A direct comparison of the observed frequency and cumulative percentage of the particle size distribution demonstrates a similar wear debris behavior between ISO and ASTM wear simulation (Fig. 9).
Fig. 9.
Mean particle diameter distribution after 2.5 million cycles in the ISO/FDIS 18192-1 (2006) and in the ASTM F 2423-05 wear simulation model
For ISOinitial and ASTM the largest particles are observed in a size range between 5 and 10 μm with a frequency very below 2%. The smallest particles which were detected are in a size range of approximately 0.05 μm for all tested lubricants. The particle size distribution resulting from the ASTM wear simulation is bimodal for all specimens, whereas for the ISOinitial a bimodal tendency is present. For both groups (ISOinitial and ASTM) most of the submicron particles are smooth and granular and there are only a few elongated particles and several larger flakes more than 1.0 μm in diameter.
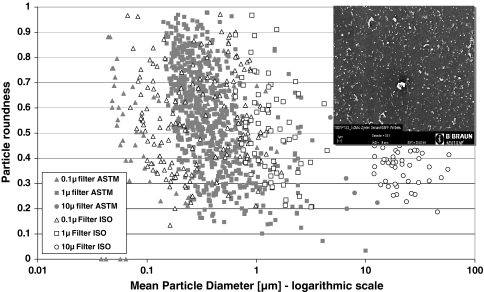
The morphology of the particles for the ISOinitial group is mainly granular and stable with a roundness of approximately 0.5 for all tested lubricants. Most of the particles of the ASTM group have a roundness above 0.5 indicating a more spherical shape rather than a fiber structure. The distribution of the particle roundness demonstrates a slightly more spherical shape for the particles in submicron size (Fig. 10).
Fig. 10.
Morphology of the wear particles for specimen B3 (ISOinitial and ASTM mode at 2.5 million cycles each)—particle roundness in dependence of the mean particle diameter (logarithmic scale) and characteristical wear particles on the polycarbonate filter in a SEM image (scale 1 μm)
Discussion
Wear simulation is an essential pre-clinical method to predict the mid- and long-term clinical wear behavior of newly introduced devices for total joint arthroplasty. In contrast to hip and knee replacement, inadequate knowledge exists about the biotribological behavior of TDA devices. The reasons are that there is precious little clinical long-term data available and the in vivo aspects of spinal loading and the environmental conditions are nearly unknown. In addition only fragmentary retrieval data exists [13, 22, 23, 35].
In spite of these limitations, spinal wear simulator testing was introduced which is based on the experiences of hip and knee replacements, the well investigated range of motion kinematics of the native spine and rudimentary retrieval analysis. One of the main requirements of a suitable method for spinal wear simulation has to be the ability to distinguish between design optimizations arising during the development process and to allow for a direct comparison to predicate devices with clinical history.
Therefore, the objective of our study was to investigate the influence of loading and kinematic patterns based on two different protocols for spinal wear simulation (ISO/FDIS 18192-1 (2006) and ASTM F2423-05). The applied kinematic pattern of movement was multidirectional (ISO) and unidirectional with a curvi-linear shape (ASTM). In a previous study Nechtow et al. [32] analyzed the effect of kinematics on TDA wear and found that cross-shear loading increased significantly the wear rate compared to curvi-linear motion. During knee wear testing Kang et al. [21] quantified the effect and found that cross-shear increases the apparent wear factor of polyethylene by more than five-fold in comparison to unidirectional sliding. In basic tribological analyses performed by Wang [47], the experimental wear factor of polyethylene increased by an order of magnitude as the cross-shear angle changed from 0 to 7.5°. Due to these previous findings the results of our study are not unexpected but to our knowledge it is the first published data with a direct comparison of the two existing methods based on one and the same TDA design which guarantees the elimination of design, material and manufacturing influences.
Anyone can argue that from the sequential testing procedure a limitation of the study can arise and a logical approach would have been to test the ISO and ASTM protocols separately in two independent groups of devices. In contrast to that we decided to apply the ISO and ASTM protocols in sequence on the same devices to avoid any potential influence from different wear testing machines, orientation of the cranial and caudal endplates during embedding and manufacturing tolerances.
Our results demonstrate that the wear rates of ISOinitial and ISOfinal are profoundly similar without detectable running in phase. In addition to that there is obviously a seamless transition in regard to the substantial changes in wear rate between the ISO and ASTM protocols (10–10.5 million cycles) and also vice versa (15–15.5 million cycles).
Following the ISO protocol in our current study the mean gravimetric wear rate was ISOinitial = 2.7 ± 0.3 mg/million cycles. In comparison to previously reported results from Nechtow et al. [32] with wear rates of 16.59 ± 0.96 mg/million cycles for ProDisc® L and 19.35 ± 1.16 mg/million cycles for Charité the current wear results for activ® L are 6.1 and 7.2 times lower, respectively. Nechtow et al. [32] used an identical simulator type and applied an earlier draft version of the ISO protocol with identical kinematic patterns but a slightly different loading mode. Nechtow et al. [32] applied a sinusoidal loading of 1,750 N for flexion and 150 N for extension whereas in our study a double peak loading of 2,000 N for flexion, 600 N in neutral position and 1,300 N for extension was used. This difference in loading does not lead to a reasonable explanation for the observed discrepancy between our study and the predicate designs. One major source of difference may be the fact that Nechtow et al. [32] applied the load and all motions at a different frequency. The effect of these frequency shift on the TDA devices wear rate is not known. Also the chosen sterilization method for the polyethylene inlay (gamma or electron beam), the sterilization dosage and the size of the disc replacement (not specified by Nechtow et al. [32]) could influence the wear characteristics of the implant.
Furthermore the lubricant could be a source of high influence on wear rates, but the used lubricant of Nechtow et al. [32] and the one used in our testing is completely comparable in regard to xenolog origin, protein concentration and the content of EDTA. The only difference is the laboratory praxis for prevention of micro-fungal contamination were we use Amphotericine and Nechtow et al. [32] added Patricin, but this should not noticeable effect the wear test conditions. One main reason for these markable discrepancies in wear behavior may be also the degree of constraint of the different TDA designs.
Also surface roughness plays a major role under the aspect of wear generation in artificial joint replacements. An increased surface quality of the CoCr29Mo6 endplates has been established based on a machine-made polishing process, which leads to a homogeneous structure of the articulating metallic surfaces. These substantial manufacturing optimizations could be an explanation for the favorable low wear rates found in our study. This could be also an explanation for the findings in a recent study of Nechtow et al. [33] were they performed wear testing on ProDisc® L under the ISO protocol and reported in good consistency to our results wear rates of 4.64 and 5.3 mg/million cycles. Based on our findings in comparison to the literature it can be assumed that the ISO method is able to distinguish between the wear behavior of different TDA designs. By wear simulation according to ASTM F2423-05 a mean wear rate of 0.14 ± 0.06 mg/million cycles has been estimated, which corresponds well to published wear data from Serhan et al. [41] with 0.13 mg/million cycles measured under similar test conditions for the Charité. For a unidirectional loading pattern with lower amplitudes than given in the ASTM protocol also Nechtow et al. [32] reported wear rates of less than 0.1 mg/million cycles.
Our direct comparison of the two alternative wear simulations results in a difference of the gravimetric wear amount of approximately 20-fold between the ISO and ASTM method. This high degree of difference between ISO and ASTM described wear simulations (p < 0.001) cannot be reasonably explained by pure amplitude differences of the given loading and displacement parameters. For example, the calculated wear path based on the ASTM protocol is even larger as the wear path defined in the ISO document.
The main explanation of divergency is the multidirectional pattern of movement described in the ISO document resulting in a cross-shear stress on the polyethylene material. It has been previously demonstrated by Wang et al. [45–47], that cross-shear, caused by multidirectional movement patterns will be detrimental to the wear behavior of polyethylene components. In contrast to that the unidirectional linear movement pattern of the ASTM testing method provokes an orientation of the molecular chains in the polymer material which results in a strain hardening effect of the polyethylene [6, 21, 46]. However, the multidirectional wear path according to ISO prevents a strain hardening of the UHMWPE material due to continously changes of the direction of movement and leads to a significant higher wear rate. Therefore, a strong influence of load and movement patterns could be determined. Our findings are fully supported by the previous results of Nechtow et al. [32]. After completion of the wear simulation (17 million cycles) and optical analysis of the polyethylene inlays the bearing surfaces present burnishing, removal of machining marks and plastic deformation at the superior articulation, whereas at the inferior articulation machining marks are still visible. The main wear mechanism for the polyethylene inlays of the activ® L artificial discs are abrasive/adhesive wear and creep without any signs of pitting, delamination, crack formation or fatigue failure.
For the optical surface examination the high degree of agreement between the theoretical movement pattern predictions, the center of geometrically measured peak wear and the genuine wear traces is very impressive. Following the ISO kinematics the described oval-shaped multidirectional pattern of movement correspond very well to the left wear marks at the cranial surface of the polyethylene inlay and the superior metallic endplate. These findings are in agreement with the analysis of Kurtz et al. [23, 24] on retrieved Charité components. They observed evidence of burnishing, removed machining marks and multidirectional scratches. In contrast to that wear simulation according to the ASTM document leads to linear wear traces on the inlay and superior metallic endplate bearing surfaces as calculated, which do not harmonize with the known TDA retrieval findings [23, 24].
In regard to the release of polyethylene wear debris after TDA in the human lumbar spine the mean particle size found during ISOinitial and ASTM wear simulation was 0.2–0.4 μm with a range of 0.05–10 μm. Our current results without substantial differences in wear particle diameter and size distribution for multidirectional (ISO) versus unidirectional (ASTM) testing is in good agreement with the basic description of wear mechanisms by Wang et al. [45] that the morphology of polyethylene particles is not significantly influenced by the amount of cross-shear. The size distributions and morphologies of the generated particles in TDA wear simulation (mean size range 0.1–1 μm) are comparable to particles found in total hip and knee replacements [16, 20]. Therefore an osteolytic potential of the generated polyethylene wear debris has to be considered [16, 18, 20].
A comparison to the circumstances in total hip and knee replacement may not be directly applicable to total disc arthroplasty due to the absence of a surrounding joint capsule and non-synovial fluid boundary conditions. Cunningham et al. [11, 12] investigated in a small animal study with a high dosage deposit of UHMWPE particulates in the spinal cord region of new zealand white rabbits localized histiocytic reactions in the epidural tissues but without any acute neural or systemic histopathologic response.
The clinical outcomes of patients undergoing TDA with Charité or ProDisc® L artificial disc with clinical long term results [13, 14, 25, 26, 44] or well documented US IDE trial studies [5, 50] demonstrate that aseptic loosening following extensive osteolysis may not play the same role in TDA as in total hip replacements. However van Ooij et al. [35] reported one revision of a Charité prosthesis associated with osteolysis of the underlying sacrum due to severe wear and fracture of the polyethylene core accompagnied by presence of wear debris in inflammatory fibrous tissue layers. Since revision is a severe intervention, longterm survival of TDA devices is crucial, underlining the importance of spinal wear simulation studies.
Conclusion
Due to previous retrieval observations [22–24, 30, 35], it seems to be unlikely, that a lumbar artificial disc is loaded with a linear wear path in vivo. Thus, one can conclude that wear testing according to ASTM F2423-05 with pure unidirectional motion does obviously not reflect the kinematics of TDA patient’s daily activities, and therefore, a strain hardening leading to enhanced wear resistance of the polyethylene may not occur. Based on these findings it seems to be safer to predict the clinical wear behavior of an artificial disc replacement based on the multidirectional ISO/FDIS 18192-1 (2006) method.
Acknowledgments
The authors would like to thank Christoph Schilling, M.Sc. for the performance of the statistical analysis.
Footnotes
In the current final version of the ISO 18192-1:2008(E) the second peak for full extension has been changed to 2,000 N.
References
- 1.Affatato S, Fernandes B, Tucci A, Esposito L, Toni A (2001) Isolation and morphological characterisation of UHMWPE wear debris generated in vitro. Biomaterials 22:2325–2331. doi:10.1016/S0142-9612(00)00403-8 [DOI] [PubMed]
- 2.Algan SM, Purdon M, Horowitz SM (1996) Role of tumor necrosis factor alpha in particulate-induced bone resorption. J Orthop Res 14(1):30–35. doi:10.1002/jor.1100140107 [DOI] [PubMed]
- 3.Anderson PA, Rouleau JP, Bryan VE, Carlson CS (2003) Wear analysis of the Bryan cervical disc prosthesis. Spine 28(20S):186–194. doi:10.1097/01.BRS.0000092212.42388.79 [DOI] [PubMed]
- 4.Bertagnoli R, Kumar S (2002) Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety of indications. Eur Spine J 11(Suppl. 2):131–136 [DOI] [PMC free article] [PubMed]
- 5.Blumenthal S, McAfee PC, Guyer RD, Hochschuler SH, Geisler FH, Holt RT, Garcia R, Regan JJ, Ohnmeiss DD (2005) A prospective, randomized, multicenter food and drug administration investigational device exemptions study of lumbar total disc replacement with the Charité artificial disc versus lumbar fusion. Spine 30(14):1565–1575. doi:10.1097/01.brs.0000170587.32676.0e [DOI] [PubMed]
- 6.Bragdon CR, O’Connor DO, Löwenstein JS, Jasty M, Syniuta WD (1996) The importance of multidirectional motion on the wear of polyethylene. Proc Instn Mech Engrs Part H 210:157–165. doi:10.1243/PIME_PROC_1996_210_408_02 [DOI] [PubMed]
- 7.Clohisy D (2003) Cellular mechanics of osteolysis. J Bone Joint Surg Am 80A(Suppl. 1):4–6 [DOI] [PubMed]
- 8.Cunningham BW, Gordon JD, Dmitriev AE, Hu N, McAfee PC (2003) Biomechanical evaluation of total disc replacement arthroplasty: an in vitro human cadaveric model. Spine 28(20S):110–117. doi:10.1097/01.BRS.0000092209.27573.90 [DOI] [PubMed]
- 9.Cunningham BW, Hallab NJ, Polly DW, Gaines R, Lubicky J, McAfee PC (2003) Basic science summary statement. Spine 28(20S):195. doi:10.1097/01.BRS.0000092213.71109.2C [DOI] [PubMed]
- 10.Cunningham BW, Dmitriev AE, Hu N, McAfee PC (2003) General principles of total disc replacement arthroplasty—seventeen cases in a nonhuman primate model. Spine 28(20S):118–124. doi:10.1097/00007632-200310151-00005 [DOI] [PubMed]
- 11.Cunningham BW (2004) Basic scientific considerations in total disc arthroplasty. Spine J 4(Suppl):219–230. doi:10.1016/j.spinee.2004.07.015 [DOI] [PubMed]
- 12.Cunningham BW, Hu N, Hallab NJ, McAfee PC (2007) Epidural application of particulate wear debris: a comprehensive analysis of ten different implant materials using an in vivo animal model. Global symposium on motion preservation technology 7th Annual Meeting 1–4 May Berlin, Abstract PA-WE-10, 2007
- 13.David T (2005) Revision of a Charité artificial disc 9.5 years in vivo to a new Charité artificial disc: case report and explant analysis. Eur Spine J 14:507–511. doi:10.1007/s00586-004-0842-x [DOI] [PMC free article] [PubMed]
- 14.David T (2007) Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the Charité artificial disc in 106 patients. Spine 32(6):661–666. doi:10.1097/01.brs.0000257554.67505.45 [DOI] [PubMed]
- 15.Goodman SB, Knoblich G, Song T, Hule P, Regia D, Aspenberg P, Lindgren LL (1995) Tissue ingrowth and differentiation in the bone harvest chamber in the presence of polyethylene particles. J Bone Joint Surg Am 77A:1025–1035 [DOI] [PubMed]
- 16.Green TR, Fisher J, Stone M, Wroblewski BM, Ingham E (1998) Polyethylene particles of a `critical size` are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 19:2297–2302. doi:10.1016/S0142-9612(98)00140-9 [DOI] [PubMed]
- 17.Hallab N, Link HD, McAfee PC (2003) Biomaterial optimization in total disc arthroplasty. Spine 28(20S):139–152. doi:10.1097/01.BRS.0000092214.87225.80 [DOI] [PubMed]
- 18.Hallab NJ, Cunningham BW, Jacobs JJ (2003) Spinal implant debris-induced osteolysis. Spine 28(20S):125–138. doi:10.1097/00007632-200310151-00006 [DOI] [PubMed]
- 19.Hochschuler SH, Ohnmeiss DD, Guyer RD, Blumenthal SL (2002) Artificial disc: preliminary results of a prospective study in the United States. Eur Spine J 11(Suppl. 2):106–110 [DOI] [PMC free article] [PubMed]
- 20.Ingham E, Fisher J (2000) Biological reactions to wear debris in total joint replacement. Proc Instn Mech Engrs vol. 214 Part H, pp 21–37 [DOI] [PubMed]
- 21.Kang L, Galvin AL, Brown TD, Jin Z, Fisher J (2008) Quantification of the effect of cross-shear on the wear of conventional and highly cross-linked UHMWPE. J Biomech 41(2):340–346. doi:10.1016/j.jbiomech.2007.09.005 [DOI] [PMC free article] [PubMed]
- 22.Kurtz S, van Ooij A, Peloza J, Villarrag M (2005) Standardized wear testing for total disc replacements: perspectives from retrieval analysis. ASTM symposium on wear of articulating surfaces, Committee F04, Hyatt Regency Dallas, Texas
- 23.Kurtz SM, von Ooij A, Ross ER, de Waal Malefijt J, Ciccarelli L, Villarraga ML (2007) Polyethylene wear and rim fracture in total disc arthroplasty. Spine J 7(1):12–21. doi:10.1016/j.spinee.2006.05.012 [DOI] [PubMed]
- 24.Kurtz SM, MacDonald D, Ciccarelli L, van Ooij A, Isaza J, Ross R, Bowden A, Patwardhan A (2007) Are one-sided wear patterns predictive of greater clinical wear in mobile bearing TDR’s. global symposium on motion preservation technology 7th annual meeting 1–4 May Berlin, Abstract PA-WE-09
- 25.Lemaire JP, Skalli W, Lavaste F, Templier A, Mendes F, Diop A, Sauty V, Laloux E (1997) Intervertebral disc prosthesis: results and prospects for the year 2000. Clin Orthop Relat Res (337):64–76. doi:10.1097/00003086-199704000-00009 [DOI] [PubMed]
- 26.Marnay T (2002) Lumbar disc replacement: 7 to 11-year results with ProDisc: proceedings of the NASS 17th annual meeting. Spine J 2:94. doi:10.1016/S1529-9430(02)00362-5 [DOI]
- 27.Mayer HM, Wiechert K, Korge A, Qose I (2002) Minimally invasive total disc replacement: surgical technique and preliminary clinical results. Eur Spine J 11(Suppl. 2):124–130 [DOI] [PMC free article] [PubMed]
- 28.Mayer HM, Korge A (2002) Non-fusion technology in degenerative lumbar spinal disorders: facts, questions, challenges. Eur Spine J 11(Suppl. 2):85–91 [DOI] [PMC free article] [PubMed]
- 29.McAfee PC, Fedder IL, Saiedy S, Shucosky EM, Cunningham BW (2003) Experimental design of total disc replacement—experience with a prospective randomized study of the SB Charité. Spine 28(20S):153–162. doi:10.1097/01.BRS.0000092217.34981.E1 [DOI] [PubMed]
- 30.Moore RJ, Fraser RD, Vernon-Roberts B, Finnie JW, Blumbergs PC, Haynes DR, Hutchens MJ, Walters RM, Kamat AS, Koszyca B (2002) The biologic response to particles from a lumbar disc prosthesis. Spine 27(19):2088–2094. doi:10.1097/00007632-200210010-00003 [DOI] [PubMed]
- 31.Murray DW, Rushton N (1990) Macrophages stimulate bone resorption when they phagocytose particles. J Bone Joint Surg Br 72B:988–992 [DOI] [PubMed]
- 32.Nechtow W, Hintner M, Bushelow M, Kaddick C (2006) Intervertebral disc replacement mechanical performance depends strongly on input parameters. 52nd Orthopaedic Research Society Chicago Abstract 0118
- 33.Nechtow W, Long W, Dana C, Bushelow M, Ochs A, Hintner M, Kaddick C (2008) Vertebral motion segment dynamics influence Prodisc-L wear performance. 54th Orthopaedic Research Society San Francisco poster no. 1928
- 34.Niedzwiecki S, Klapperich C, Short J, Jani S, Ries M, Pruitt L (2001) Comparison of three joint simulator wear debris isolation techniques: acid digestion, base digestion, and enzymatic cleavage. J Biomed Mater Res 56:245–249. doi:10.1002/1097-4636(200108)56:2<245::AID-JBM1091>3.0.CO;2-T [DOI] [PubMed]
- 35.van Ooij A, Kurtz SM, Stessels F, Noten H, van Rhijn L (2007) Polyethylene wear debris and long-term clinical failure of the Charité disc prosthesis. Spine 32(2):223–229. doi:10.1097/01.brs.0000251370.56327.c6 [DOI] [PubMed]
- 36.Pare P, Chan F, Buchholz P, Kurtz S (2005) McCombe P Is unidirectional wear testing clinical relevant for artificial disc implants? ASTM symposium on wear of articulating surfaces, committee F04. Hyatt Regency Dallas, Texas
- 37.Revell PA, Al-Saffar N, Kobayashi A (1997) Biological reaction to debris in relation to joint prostheses. Proc Instn Mech Engrs 211 Part H, 187–197 [DOI] [PubMed]
- 38.Saikko V, Calonius O (2002) Slide track analysis of the relative motion between femoral head and acetabular cup in walking and in hip simulators. J Biomech 35:455–464. doi:10.1016/S0021-9290(01)00224-X [DOI] [PubMed]
- 39.Saikko V, Calonius O (2003) An improved method of computing the wear factor for total hip prostheses involving the variation of relative motion and contact pressure with location on the bearing surface. J Biomech 36:1819–1827. doi:10.1016/S0021-9290(03)00228-8 [DOI] [PubMed]
- 40.Santavirta S, Holkka V, Eskola A, Kontinen YT, Paavilainen T, Tallroth K (1990) Aggressive granulomatous lesions in cementless total hip arthroplasty. J Bone Joint Surg Br 72B:980–985 [DOI] [PubMed]
- 41.Serhan HA, Dooris AP, Parsons ML, Ares PJ, Gabriel SM (2006) In vitro wear assessment of the Charité artificial disc according to ASTM recommendations. Spine 31(17):1900–1910. doi:10.1097/01.brs.0000228716.60863.ab [DOI] [PubMed]
- 42.Siepe CJ, Mayer HM, Wiechert K, Korge A (2006) Clinical results of total lumbar disc replacement with ProDisc II. Spine 31(17):1923–1932. doi:10.1097/01.brs.0000228780.06569.e8 [DOI] [PubMed]
- 43.Szpalski M, Gunzburg R, Mayer M (2002) Spine arthroplasty: a historical review. Eur Spine J 11(Suppl. 2):65–84 [DOI] [PMC free article] [PubMed]
- 44.Tropiano P, Huang RC, Girardi FP, Cammisa FP, Marnay T (2005) Lumbar total disc replacement—seven to eleven-year follow-up. J Bone Joint Surg Am 87A(3):490–496. doi:10.2106/JBJS.C.01345 [DOI] [PubMed]
- 45.Wang A, Stark C, Dumbleton JH (1996) Mechanistic and morphological origins of ultra-high molecular weight polyethylene wear debris in total joint replacement prostheses. Proc Inst Mech Engrs Part H 210:141–155. doi:10.1243/PIME_PROC_1996_210_407_02 [DOI] [PubMed]
- 46.Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH (1998) Lubrication and wear of ultrahigh molecular weight polyethylene in total joint replacements. Tribol Int 31(1–3):17–33. doi:10.1016/S0301-679X(98)00005-X [DOI]
- 47.Wang A (2001) A unified theory of wear for ultra-high molecular weight polyethylene in multi-directional sliding. Wear 248:38–47. doi:10.1016/S0043-1648(00)00522-6 [DOI]
- 48.Wilke HJ, Wenger K, Claes LE (1998) Testing criteria for spinal implants: recommendations for the standardization of in vitro testing of spinal implants. Eur Spine J 7:148–154. doi:10.1007/s005860050045 [DOI] [PMC free article] [PubMed]
- 49.Zeegers WS, Bohnen LMLJ, Laaper M, Verhaegen MJA (1999) Artificial disc replacement with the modular type SB Charité III: 2-year results in 50 prospectively studied patients. Eur Spine J 8:210–217. doi:10.1007/s005860050160 [DOI] [PMC free article] [PubMed]
- 50.Zigler JE (2003) Clinical results with ProDisc: European experience and US investigation device exemption study. Spine 28(20S):163–166. doi:10.1097/00007632-200310151-00009 [DOI] [PubMed]