Abstract
Objective
Many vascular neurosurgeons tend to remove bone flap in patients with large aneurysmal intracerebral hematomas (ICH). However, relatively little work has been done regarding the effectiveness of prophylactic decompressive craniectomy in a patient with a large aneurysmal ICH.
Methods
Large ICH was defined as hematoma when its volume exceeded 25 mL, ipsilateral to aneurysms. The patients were divided into two groups; aneurysmal subarachnoid hemorrhage (SAH) associated with large ICH, January, 1994 - December, 1999 (Group A, 41 patients), aneurysmal SAH associated with large ICH, January, 2000 - May, 2005 (Group B, 27 patients). Demographic and clinical variables including age, sex, hypertension, vasospasm, rebleeding, Hunt-Hess grade, aneurysm location, aneurysm size, and outcome were compared between two groups, and also compared between craniotomy and craniectomy patients in Group A.
Results
In Group A, 21 of 41 patients underwent prophylactic decompressive craniectomy. In Group B, only two patients underwent craniectomy. Surgical outcome in Group A (good 23, poor 18) was statistically not different from Group B (good 15, poor 12). Surgical outcomes between craniectomy (good 12, poor 9) and craniotomy cases (good 11, poor 9) in Group A were also comparable.
Conclusion
We recommend that a craniotomy can be carried out safely without prophylactic craniectomy in patients with a large aneurysmal ICH if intracranial pressure is controllable with hematoma evacuation.
Keywords: Clipping, Craniectomy, Aneurysm, Intracerebral hematoma
INTRODUCTION
Decompressive craniectomy has the potential benefit of both local and global intracranial pressure (ICP) control due to improved brain perfusion. Recently, the role of decompressive craniectomy in the setting of cerebral infarction2,3), trauma17), and subarachnoid hemorrhage (SAH)5) has been investigated. Decompressive craniectomy allows significant parenchymal swelling in the posthemorrhagic period without the occurrence of increased ICP or herniation syndrome. Prompt removal of the hematoma with simultaneous clipping of the aneurysm can lead to a better outcome and allows aggressive treatment of vasospasm and postoperative complications.
Many vascular neurosurgeons tend to remove bone flap unnecessarily in patients with large aneurysmal intracerebral hematomas (ICH). In this study, the authors report recent personal experience that craniotomy can be performed safely in a subcategory of patients with large aneurysmal ICH.
MATERIALS AND METHODS
One-hundred-and-seven of 701 consecutive patients showed an ICH from ruptured aneurysm and were urgently operated on from January 1994 to May 2005. Large ICH was defined as hematoma when its volume exceeded 25 mLl4), ipsilateral to aneurysms. Hematoma volume was measured with an image analyzer on brain CT (Siemens, Munich, Germany). Patients were assigned a Hunt and Hess (H/H) grade at admission. The surgical approach planned to allow simultaneous clipping of the aneurysm and complete clot evacuation. An enlarged pterional craniotomy was performed for exposing the hematoma and aneurysm adequately. After craniotomy and opening of the dura, the hematoma distant from the aneurysm dome was partially removed to provide brain relaxation. Hematoma evacuation was completed after proximal control and definitive obliteration of the aneurysm.
Before December of 1999, prophylactic decompressive craniectomy was performed in patients with large ICH and difficult aneurysms to clip such as large sized or complex aneurysms that required more brain manipulation. Since January 2000, patients were treated with craniotomy instead of prophylactic craniectomy, if ICP was intraoperatively controllable, in patients with the similar features. The patients were divided into two groups; aneurysmal SAH associated with large ICH, January, 1994 - December, 1999 (Group A, 41 patients), aneurysmal SAH associated with large ICH, January, 2000 - May, 2005 (Group B, 27 patients). Two groups were analyzed for comparability of demographic and clinical variables including age, sex, hypertension, vasospasm, rebleeding, Hunt-Hess grade, aneurysm location, and aneurysm size. Outcomes were also compared between two groups.
All patients were managed according to a same perioperative policy including aggressive intensive care, intraventricular ICP monitoring, and the anesthetic technique. Immediate postoperative CT scans were obtained for all patients and compared with preoperative studies to assess the extent of clot removal. Serial transcranial doppler studies and CT scans supplemented the clinical evaluation. Outcome was assessed at the last follow-up intervals according to the Glasgow Outcome Scale (GOS) with "good" or "moderate disability" classified as a good outcome (GOS 1-2) and "severe disability", "vegetative" or "death" classified as a poor outcome (GOS 3-5). The statistical significance of observed differences between the variables were assessed by a t-test or χ2 test. A p value 0.05 or less was considered significant.
RESULTS
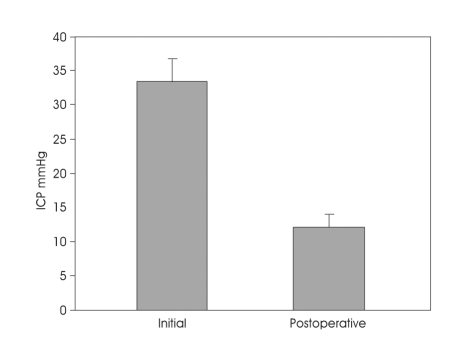
In Group A, 21 of 41 patients underwent prophylactic decompressive craniectomy. In Group B, only two patients underwent craniectomy; one patient with vasospasm and rebleeding with ICH (50 cc, H/H grade IV) (Fig. 1), the other patient of H/H grade V and ICH (40 cc). Patients data are summarized in Table 1. Subjects in two treatment groups were similar with regard to age, sex, hypertension, vasospasm, rebleeding, Hunt-Hess grade, aneurysm location, and aneurysm size. Postoperatively, all patients with ICH experienced an immediate decrease in ICP to 20 mmHg or lower (initial mean ICP 33.4±4.3 mmHg, postoperative mean ICP 12.1±3.2 mmHg) (Fig. 2). None of patients required reoperation after craniotomy due to uncontrollable increased ICP. Surgical outcomes in Group A (good 23, poor 18) were statistically not different from Group B (good 15, poor 12). Surgical outcomes bet-ween craniectomy (good 12, poor 9) and craniotomy cases (good 11, poor 9) in Group A were also comparable.
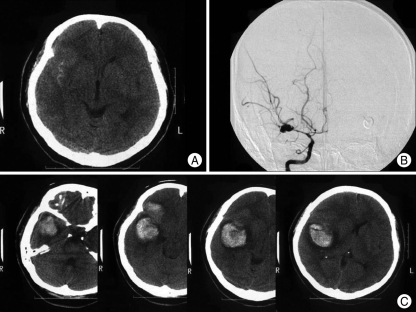
Fig. 1.
A 51-year-old woman presenting with Hunt and Hess (H/H) grade III. Initial CT (computed tomography) shows subarachnoid hemorrhage in the right sylvian cistern (A), and carotid angiogram demonstrates severe vasospasm and a right middle cerebral artery bifurcation aneurysm (B). Brain CT after rebleeding (H/H grade IV, C) shows large intracerebral hematoma in the right fronto-temporal area.
Table 1.
Demographic data and clinical features of the patinets
Group A : aneurysmal subarachnoid hemorrhage (SAH) associated with large intracerebral hematoma (ICH), January, 1994-December, 1999. Group B : aneurysmal SAH associated with large ICH, January, 2000-May, 2005. All clinical variables are not significantly different between two groups (t-test for age, hematoma volume, and size of aneurysm, χ2 test for other variables). Data are expressed as the mean±the standard error. ACA : anterior cerbral artery, MCA : middle cerebral artery, ICA : internal carotid artery, Pcom : posterior communicating artery
Fig. 2.
Bar graph showing intracranial pressure (ICP) change between initial and immediate postoperative period in patients with intracerebral hematoma. Postoperatively, all patients experienced immediate decrease in ICP. Values are mean±standard error.
Illustrative case (Fig. 3)
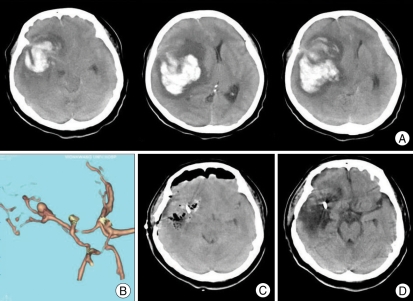
Fig. 3.
A 57-year-old woman presenting with semicomatose mental state and right side anisocoria without light reflex. A : Brain computed tomography scan shows huge right temporo-parietal intracerebral hematoma. B : CT (computed tomography) angiogram demonstrates a right middle cerebral artery bifurcation aneurysm measuring 12.0×5.7 mm in size. C : Immediate postoperative CT scan reveals evacuation of the hematoma. D : One month later, CT shows infarction in the right temporal lobe.
A 57-year-old woman was admitted to our department due to mental deterioration. She was semicomatose with right side anisocoria without light reflex. Brain CT scan showed a huge right temporo-parietal ICH and 10 mm of midline shift. An aneurysm of the right middle cerebral artery bifurcation measuring 12.0×5.7 mm in size was identified on the CT angiography. A right fronto-temporal craniotomy was performed. The brain was found to be extremely swollen when the dura was opened. After partial evacuation of the hematoma through the superior temporal gyrus, the sylvian fissure was split proximally and the M1 segment and middle cerebral artery bifurcation were delineated under the microscope and the aneurysm was clipped. An immediate postoperative CT scan revealed evacuation of the hematoma. One month later, a CT showed infarction in the right temporal lobe, and she was discharged with an alert mental state and left hemiparesis grade 2.
DISCUSSION
ICH constitutes a less frequent manifestation of aneurysm rupture than SAH. Its incidence in patients with ruptured aneurysm varies from 4% to 35%7,15,16). Certain patterns of collections of blood seem to be related to specific aneurysmal locations. It is well known that : 1) middle cerebral artery and posterior communicating artery aneurysms will predominantly cause intratemporal clots, with possible extension into the adjacent lobes; 2) anterior communicating artery aneurysms will most likely produce frontobasal hematomas; and 3) anterior cerebral artery aneurysms will mainly cause interhemispheric clots. Shimoda et al.20) found that favorable outcomes in patients with ICH and diffuse SAH was lower than for patients with ICH alone. They suggested that ICH caused by bleeding of aneurysm directly into the brain parenchyma from the sac adhered to the pia mater was fundamentally a subcortical hemorrhage. Patients with temporal or frontal hematoma without diffuse SAH from ruptured aneurysm were much better tolerated notwithstanding persisting ICP. Kang10) also reported that it was related to a grave prognostic significance when intraventricular hemorrhage associated with an ICH, compared with ICH only, IVH only, and SAH only group.
The comparative investigation for the effect of ICH caused by head injury or stroke on the clinical course was rarely reported. In evaluation of patients with supratentorial ICH, Andrews et al.1) noted that head injury was the cause of the hematoma in six of the seven patients who developed brain stem compression. They mentioned that this might simply reflect the predominance of head injuries in this group. Alternatively, they indicated that traumatic hematomas are more likely to enlarge and compress the brain stem, which is not commonly seen in spontaneous supratentorial hematomas associated with hypertension, tumor, aneurysm, or arteriovenous malformation. Traumatic hematoma were associated with twice the edema per unit volume of hematoma, and a doubling of median edema volume on second CT, compared with spontaneous or aneurysmal hematoma21). Rengachary et al.18) presented that two younger patients of three cases of multilobar stroke were associated with severe focal cerebral edema, and mentioned that atrophic older brains and a dilated ventricular system might accommodate localized edema with relatively less shift of midline structures. Also, the edematous response to ischemia might be more severe in younger brains. In patients whose stroke developed at a relatively older age, the mass effect was relatively focal. In contrast, traumatic brain swelling tends to be diffuse and to have associated with brain stem injuries from severe acceleration/deceleration injuries. With head injuries, the impediment of venous drainage and contusion of the cortex further aggravate brain edema.
It is generally accepted that cerebral ischemia caused by incresed ICP following SAH compromises the BBB, resulting in early cytotoxic edema and later vasogenic edema. The rapid formation of mass effect is a prominent feature of cerebral contusion11,12), which is not seen in other pathologic conditions associated with brain edema. The cellular elements in the central areas of contusion uniformly undergo disintegration and homogenization as the primary consequence of mechanical injury, even early after injury. This can create a pathophysiological condition in which tissue osmolality increases rapidly in the core of the contusion and attracts a large amount of water within the necrotic tissue6). Nowadays, burr hole aspiration of localized hematoma in hemorrhagic stroke is indicated for all patients with moderate, severe and fulminant types of hemorrhage instead of open surgery but not in traumatic ICH usually associated with multiple contusion. This may berrelated to the inherent difference between a spontaneous and a traumatic ICH.
Although decompression does not reverse the primary brain injury associated with ICH, it can ameliorate secondary damage caused by elevation of ICP. However, craniectomy is not universally successful, and there are several experimental evidences that it enhances the formation of brain edema. Cooper et al.4) reported that craniectomy causes an increase in formation of cold-induced edema. Hatashita and Hoff8) showed decompressive craniectomy after experimental brain injury produced an increase in the volumetric compensatory capacity of the intracranial cavity and at the same time brain edema formation is facilitated.
Drawbacks of craniectomy are cosmetic disfigurement, the need for postoperative protection of the craniectomy area, cost (the need for a second operation to repair the defect), and the risk of infection. Although this procedure may decrease mortality2,17,19), the long-term quality of life experienced by many survivors is quite poor9,13,22). Because of the varied functional outcomes after the procedure, further study needs to assess the most appropriate candidates for the procedure.
Decompressive craniectomy may be effective in selected patients with aneurysmal ICH, but its widespread use as a standard management of such patients is not supported because the data is too small to have statistical impact and give guidelines concerning indications for craniectomy. Various factors such as surgical manipulation, retraction, and disturbed venous drainage may be responsible for the development of intraoperative cerebral swelling. Although the relationship between surgical techniques and outcome in both groups could not be compared directly, we believe that less brain manipulation reflecting advances in surgical experiences may be associated with comparable outcomes in later periods without prophylactic decompressive craniectomy based on our results, unfortunately, the ability to predict which patients might potentially benefit from craniectomy was not answered in this study because of the small sample size. In patients with craniectomy in Group B, the decision was made by intraoperative aspect of brain swelling (angry-looking brain). Only a large prospective, randomized, controlled study could definitively establish a role for craniectomy in the setting of large aneurysmal ICH.
CONCLUSION
The long-standing ICP elevation depends on the hematoma and/or disturbances of autoregulation. Additionally, traumatic hematomas are more likely to enlarge and compress the brain stem, which has not been commonly reported in patients with aneurysmal intracerebral hematoma. We therefore recommend that a craniotomy can be carried out safely without an unnecessary prophylactic craniectomy in patients with a large aneurysmal intracerebral hematoma if ICP is controllable with hematoma evacuation.
Acknowledgements
This paper was supported by Wonkwang University in 2008.
References
- 1.Andrews BT, Chiles BW, 3rd, Olsen WL, Pitts LH. The effects of intracerebral hematoma location on the risk of brain-stem compression and on clinical outcome. J Neurosurg. 1988;69:518–522. doi: 10.3171/jns.1988.69.4.0518. [DOI] [PubMed] [Google Scholar]
- 2.Carter BS, Ogilvy CS, Candia GJ, Rosas HD, Buonanno F. One-year outcome after decompressive surgery for massive nondominant hemispheric infarction. Neurosurgery. 1997;40:1168–1175. doi: 10.1097/00006123-199706000-00010. discussion 1175-1176. [DOI] [PubMed] [Google Scholar]
- 3.Cho DY, Chen TC, Lee HC. Ultra-early decompressive craniectomy for malignant middle cerebral artery infarction. Surg Neurol. 2003;60:227–232. doi: 10.1016/s0090-3019(03)00266-0. discussion 232-233. [DOI] [PubMed] [Google Scholar]
- 4.Cooper PR, Hagler H, Clark WK, Barnett P. Enhancement of experimental cerebral edema after decompressive craniectomy : implications for the management of severe head injuries. Neurosurgery. 1979;4:296–300. doi: 10.1227/00006123-197904000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Fisher CM, Ojemann RG. Bilateral decompressive craniectomy for worsening coma in acute subarachnoid hemorrhage : observations in support of the procedure. Surg Neurol. 1994;41:65–74. doi: 10.1016/0090-3019(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 6.Freytag E, Lindenberg R. Morphology of cortical contusions. AMA Arch Pathol. 1957;63:23–42. [PubMed] [Google Scholar]
- 7.Gruss P. Therapeutic procedures in aneurysms with intracerebral hematoma. In: Schiefer W, Klinger M, Brock M, editors. Brain abscess and meningitis. Subarachnoid hemorrhage timing problems. vol 9. New York: Springer Verlag; 1981. pp. 271–280. Advances in neurosurgery. [Google Scholar]
- 8.Hatashita S, Hoff JT. Biomechanics of brain edema in acute cerebral ischemia in cats. Stroke. 1988;19:91–97. doi: 10.1161/01.str.19.1.91. [DOI] [PubMed] [Google Scholar]
- 9.Holtkamp M, Buchheim K, Unterberg A, Hoffmann O, Schielke E, Weber JR, et al. Hemicraniectomy in elderly patients with space occupying media infarction : improved survival but poor functional outcome. J Neurol Neurosurg Psychiatry. 2001;70:226–228. doi: 10.1136/jnnp.70.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang SD. Clinical significance of hemorrhage location in poor grade aneurysm patients. J Korean Neurosurg Soc. 1996;25:714–719. [Google Scholar]
- 11.Katayama Y, Tsubokawa T, Miyazaki S, Kawamata T, Yoshino A. Oedema fluid formation within contused brain tissue as a cause of medically uncontrollable elevation of intracranial pressure : the role of surgical therapy. Acta Neurochir Suppl (Wien) 1990;51:308–310. doi: 10.1007/978-3-7091-9115-6_104. [DOI] [PubMed] [Google Scholar]
- 12.Kushi H, Katayama Y, Shibuya T, Tsubokawa T, Kuroha T. Gadolinium DTPA-enhanced magnetic resonance imaging of cerebral contusions. Acta Neurochir Suppl (Wien) 1994;60:472–474. doi: 10.1007/978-3-7091-9334-1_129. [DOI] [PubMed] [Google Scholar]
- 13.Le Roux PD, Elliott JP, Newell DW, Grady MS, Winn HR. Predicting outcome in poor-grade patients with subarachnoid hemorrhage : a retrospective review of 159 aggressively managed cases. J Neurosurg. 1996;85:39–49. doi: 10.3171/jns.1996.85.1.0039. [DOI] [PubMed] [Google Scholar]
- 14.Mayer SA, Thomas CE, Diamond BE. Asymmetry of intracranial hemodynamics as an indicator of mass effect in acute intracerebral hemorrhage : a transcranial doppler study. Stroke. 1996;27:1788–1792. doi: 10.1161/01.str.27.10.1788. [DOI] [PubMed] [Google Scholar]
- 15.Modesti LM, Binet EF. Value of computed tomography in the diagnosis and management of subarachnoid hemorrhage. Neurosurgery. 1978;3:151–156. doi: 10.1227/00006123-197809000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Pia HW. The surgical treatment of intracerebral and intraventricular hematomas. Acta Neurochir (Wien) 1972;27:149–164. doi: 10.1007/BF01401878. [DOI] [PubMed] [Google Scholar]
- 17.Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84–92. doi: 10.1097/00006123-199707000-00018. discussion 92-94. [DOI] [PubMed] [Google Scholar]
- 18.Rengachary SS, Batnitzky S, Morantz RA, Arjunan K, Jeffries B. Hemicraniectomy for acute massive cerebral infarction. Neurosurgery. 1981;8:321–328. doi: 10.1227/00006123-198103000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998;29:1888–1893. doi: 10.1161/01.str.29.9.1888. [DOI] [PubMed] [Google Scholar]
- 20.Shimoda M, Oda S, Mamata Y, Tsugane R, Sato O. Surgical indications in patients with an intracerebral hemorrhage due to ruptured middle cerebral artery aneurysm. J Neurosurg. 1997;87:170–175. doi: 10.3171/jns.1997.87.2.0170. [DOI] [PubMed] [Google Scholar]
- 21.Statham PF, Todd NV. Intracerebral hematoma : aetiology and hematoma volume determine the amount and progression of brain edema. Acta Neurochir Suppl (Wien) 1990;51:289–291. doi: 10.1007/978-3-7091-9115-6_98. [DOI] [PubMed] [Google Scholar]
- 22.Walz B, Zimmermann C, Bottger S, Haberl RL. Prognosis of patients after hemicraniectomy in malignant middle cerebral artery infarction. J Neurol. 2002;249:1183–1190. doi: 10.1007/s00415-002-0798-x. [DOI] [PubMed] [Google Scholar]