Abstract
Objective
This study was performed to assess the efficacy of GKS in patients with ten or more brain metastases.
Methods
From Aug 2002 to Dec 2007, twenty-six patients (13 men and 13 women) with ten or more cerebral metastatic lesions underwent GKS. The mean age was 55 years (32-80). All patients had Karnofsky performance status (KPS) score of 70 or better. According to recursive partitioning analysis (RPA) classification, 3 patients belonged to class I and 23 to class II. The location of primary tumor was lung (21), breast (3) and unknown (2). The mean number of the lesions per patient was 16.6 (10-37). The mean cumulated volume was 10.9 cc (1.0-42.2). The median marginal dose was 15 Gy (9-23). Overall survival and the prognostic factors for the survival were retrospectively analyzed by using Kaplan Meier method and univariate analysis.
Results
Overall median survival from GKS was 34 weeks (8-199). Local control was possible for 79.5% of the lesions and control of all the lesions was possible in at least 14 patients (53.8%) until 6 months after GKS. New lesions appeared in 7 (26.9%) patients during the same period. At the last follow-up, 18 patients died; 6 (33.3%) from systemic causes, 10 (55.6%) from neurological causes, and 2 (11.1%) from unknown causes. Synchronous onset in non-small cell lung cancer (p=0.007), high KPS score (≥80, p=0.029), and controlled primary disease (p=0.020) were favorable prognostic factors in univariate analysis.
Conclusion
In carefully selected patients, GKS may be a treatment option for ten or more brain metastases.
Keywords: Multiple, Brain metastases, Gamma knife radiosurgery, Prognostic factor
INTRODUCTION
Brain metastasis is the most common neurologic complication of cancer that occurs in 30% of cancer patients13). The incidence of brain metastasis is increasing with improving systemic care of cancer patients and longer survival. In general, the prognosis in this population is poor; the median survival time is only a month without treatment, 1 to 2 months with medical treatment for relief of increased intracranial pressure4,14,22), and 4 to 6 months after whole brain radiotherapy (WBRT)4,6,22). Stereotactic radiosurgery has been employed in patients with brain metastasis either alone or in combination with WBRT. Recently reported results of a prospective randomized trial demonstrated beneficial effects of stereotactic radiosurgery added to WBRT on survival or quality of life in patients with one to three metastatic lesions9). Also, it was reported that stereotactic radiosurgery alone in the patients with 1-4 lesions was comparable to stereotactic radiosurgery combined with WBRT in survival2). Though there is no level one evidence, benefit of gamma knife radiosurgery (GKS) in the patients with 4 or more lesions was suggested in retrospective analysis data3,10). Recursive partitioning analysis (RPA) class10) or the total volume of intracranial lesions3) were suggested as more important prognostic factors than the number of lesions. There are even less number of reports that suggest benefit of GKS in the patients with ten or more cerebral lesions15,18) and radiosurgery is not commonly recommended to the patients with such large number of lesions in usual clinical practice. However, there are patients who have favorable prognostic factors, among most of the clinical parameters, other than the great number of brain lesions. In this report, we retrospectively analyzed the outcome after GKS for ten or more brain metastases.
MATERIALS AND METHODS
Patient population
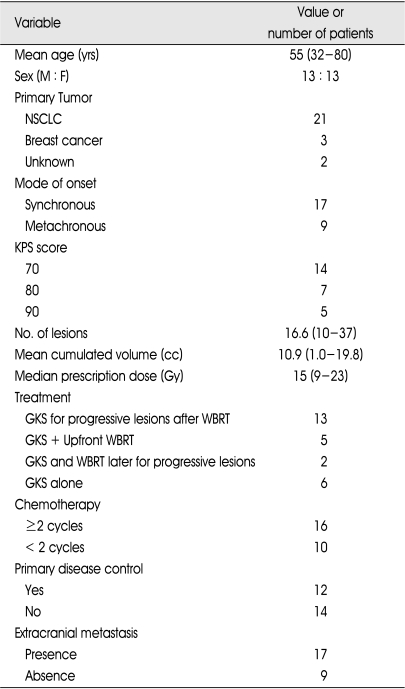
Twenty-six patients underwent GKS for a total of 410 lesions from Aug 2002 to Dec 2007 in our institute. All patients harbored ten or more brain metastases at the time of initial GKS (mean 16.6, range 10-37). The mean patient's age was 55 years (range 32-80 years). There were 13 men and 13 women. The mean KPS score in this series was 77 (range 70-90). According to RPA classification7), there were three patients of class I and twenty-three of class II (class I : KPS>70, age≤65, no extracranial metastasis, controlled primary tumor; class III : KPS<70; class II : others). The primary sites of origin for the brain metastases are shown in Table 1; non-small cell lung cancer (NSCLC) represented the most common sources of brain metastases in this series. The mode of onset was synchronous (i.e. diagnosis of brain lesion not later than 2 months from diagnosis of primary tumor) in 17 patients and metachronous in 9.
Table 1.
Demographic and clinical characteristics in 26 patients with 10 or more metastatic brain lesions
NSCLC : non-small cell lung cancer, KPS : Karnofsky performance status, GKS : gamma knife radiosurgery, WBRT : whole brain radiotherapy
Radiosurgical treatment
All patients underwent application of a Leksell stereotactic frame G (Elekta Instrument AB, Stockholm, Sweden) after infiltration of local anesthetics. MR imaging for radiosurgery planning was performed. T2-weighted axial images were obtained with a 1.5- to 2-mm slice thickness and no gap, and a double dose of contrast agent was administered followed by the acquisition of three-dimensional SPGR images with 1- to 1.5-mm slice thickness. Leksell Gamma Plan versions 5.32 and 5.34 (Elekta Instruments AB) were used to create the radiosurgery plans. Radiosurgery was performed with Leksell Gamma Knife type B or C (Elekta Instruments AB). A total of 42 GKS procedures were performed in 26 patients including 9 patients who were treated more than once for local or remote recurrent lesions. The number of lesions treated at the initial GKS session was 410 and the mean number of lesions per patients was 16.6 (range 10-37). The mean cumulated volume at initial GKS was 10.9 cc (range 1.0-19.8 cc). The marginal dose covering the tumor varied between 9 Gy and 23 Gy (median 15 Gy), which was prescribed from 33 to 88% isodose line (median 50%).
Additional treatment before and after GKS
GKS for the progressive lesions after WBRT was performed in 13 patients (65.0%). GKS with upfront WBRT (simultaneously conducted within 1 month of interval) was performed in 5 patients (19.2%). The other 8 patients (30.8%) were treated with GKS alone as an initial management and 2 among them were given additional WBRT later for progressive lesions. Therefore, 6 patients were finally treated with GKS alone. Nine patients (34.6%) received GKS more than once and systemic chemotherapy was continued in eight patients (30.8%) after initial GKS.
Follow-up evaluation
Follow-up evaluation Patients were examined clinically at 1 and 3 months posttreatment and then every 3 months thereafter. Neurological status was evaluated, and all complications were recorded. MRI scan was obtained every 3 months following radiosurgery.
Statistical analysis
With statistical software program (SPSS, version 15.0, SPSS Inc., Chicago, IL), the Kaplan-Meier method was used to calculate overall survival rates. The prognostic factors for survival were analyzed for significance by using univariate analysis with log-rank tests. It was considered significant when p-value was less than 0.05.
RESULTS
Overall survival and tumor control
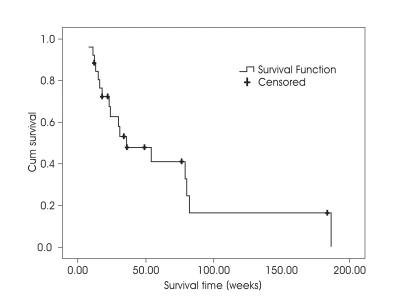
Overall median survival from first GKS was 34 weeks (8-199 weeks) (Fig. 1). At the last follow-up 18 (69.2%) had died from the disease. The causes of death were systemic in 6 patients (33.3%), neurological in 10 (55.6%), and unknown in 2 (11.1%). The earliest death from systemic cause was observed in a 48 year-old female patient who underwent GKS for 10 lesions (total volume of 5.89 cc) and died from respiratory failure due to progression of lung lesion and pleural effusion at 13 weeks after initial GKS. Meanwhile, the earliest death from neurologic cause was in a 46 yearold female patient treated with GKS for 18 lesions (total volume of 3.87 cc) who died from aggravation of brain edema and seizure at 11 weeks after GKS.
Fig. 1.
Kaplan-Meier survival curves for the patients with ten or more brain metastases after gamma knife radiosurgery.
Until 3 months after initial GKS, one patient died from systemic cause, one from neurological cause, and one from unknown cause. Follow up MR imaging at 3 months after treatment was available for 367 lesions of 23 patients. 63.7 % of the metastases decreased in size, 23.2% were stable and 13.1% increased in size resulting in a local tumor control rate of 86.9% at 3 months (Table 2). Until 6 months after radiosurgery, 9 patients with a total of 147 lesions died (3 patients from systemic causes, 4 from neurological causes, and 2 from unknown causes). Therefore, 263 lesions of 17 patients were available for analysis and local control till 6 month after GKS was possible for 209 lesions (79.5%). The control of all treated lesions was possible in at least 14 patients (53.8%) until 6 months after GKS. New metastatic tumors appeared in 7 (26.9%) among 26 patients until 6 months. In summary, the local tumor control rate at 3 and 6 months after radiosurgery were 86.9% and 79.4%, respectively and rate of freedom from development of new lesion was (73.1%) at 6 months.
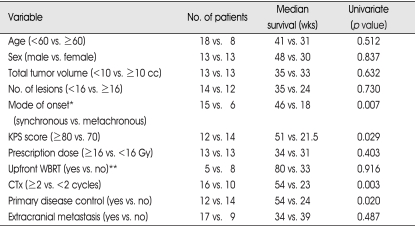
Table 2.
Univariate analysis for prognostic factors influencing survival of the patients with ten or more brain metastases
*analyzed in NSCLC patients, **analyzed in newly diagnosed patients
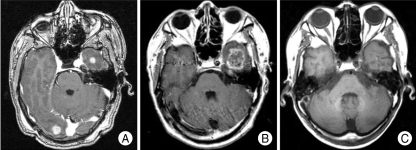
There was no serious acute toxicity related to GKS, however, overt radiation necrosis as a late complication was observed in a patient (Fig. 3). The patient was diagnosed as adenocarcinoma of the lung and was treated for 19 metastatic lesions of synchronous onset. GKS was performed with 15 Gy of 50% isodose line for a total volume of 8.77 cc. He did not receive WBRT before and after GKS. He complained aggravation of headache and follow up brain MRI at 7 months revealed improved or stable lesions except one lesion located in left temporal lobe showing increase in size and enhancement. MR spectroscopy and perfusion MRI finding was compatible with radiation necrosis and the lesion was not treated furthermore. Brain MRI at 38 months showed decrease in size and disappearance of enhancement. Therefore, the lesion was thought to be radiation necrosis and he died from respiratory failure due to progression of the lung lesion at 44 months after GKS.
Fig. 3.
Serial magnetic resonance image (MRI) scans show the development of radiation necrosis and resolution. A : 66 year-old male patient was treated gamma knife radiosurgery (GKS) for 19 lesions with (radiation necrosis) marginal dose of 15 Gy at 50% isodose. B : Brain MRI at 7 months after GKS reveals increase in size of rim-enhancing lesion on left temporal lobe. C : The lesion was not treated furthermore and brain MRI at 38 months after initial GKS shows decrease in size and no enhancement of this lesion.
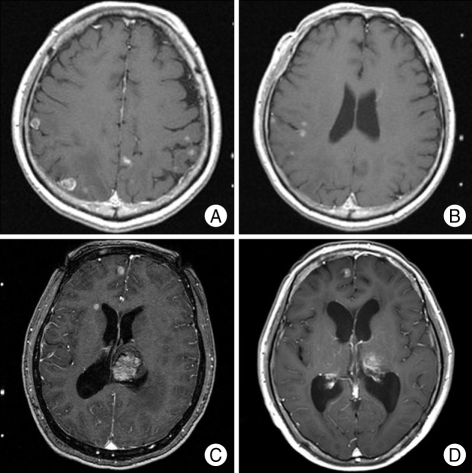
The representative case with the largest number of the lesions (37) and another one with the longest survival are shown in (Fig. 4).
Fig. 4.
A and B : Initial brain magnetic resonance image (MRI) scans from the 53 year-old male patient who had the largest number of lesions (37) among the patients included in this study. He underwent gamma knife radiosurgery (GKS) and survived 18 weeks after initial GKS and die from unknown origin. C and D : Brain MRI scans from the 55 year-old female patient who is surviving 199 weeks after initial GKS for 10 lesions. MRI at initial GKS (C) shows multiple lesions and then she underwent GKS 2 times more. Brain lesions are still stable at 199 weeks after GKS (D).
Prognostic factors for survival
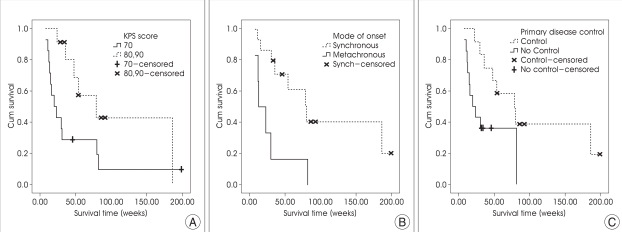
Patient's age, sex, mode of onset (synchronous vs. metachronous in NSCLC), KPS score (≥80 vs. 70), cumulated tumor volume (<10 vs. ≥10cc), number of lesions (<16 vs. ≥16), prescribed dose to the tumor margin (≥16 vs. <16 Gy), whole brain radiotherapy (upfront GKS plus WBRT vs. GKS alone in newly diagnosed patients), chemotherapy after GKS (≥2 vs. <2 cycles), the presence of extracranial metastasis, and the control of primary disease were included in univariate analysis. The results are summarized in Table 2. The synchronous onset in NSCLC (p=0.007), KPS score ≥80 (p=0.029), and chemotherapy after GKS (p=0.003) were related to longer survival (Fig. 2). The median survival for NSCLC patients of synchronous onset was 46 weeks; whereas it was 18 weeks for those of metachronous onset. The median survival for patients with KPS score ≥80 was 51 weeks; whereas it was 21.5 weeks for those with 70. In the patients with chemotherapy after GKS, the patients who received 2 or more cycle of chemotherapy survived longer than those who did not. Controlled primary disease at the time of radiosurgery improved survival (p=0.020; 54 weeks vs. 24 weeks), but the absence of extracranial metastases (p=0.487) did not reach statistical significance. Upfront WBRT in newly diagnosed brain metastases, cumulated tumor volume, number of lesions, and the prescribed dose to the tumor margin were analyzed and none of them had an impact on survival.
Fig. 2.
Kaplan-Meier survival curves for the patients with ten or more brain metastases after gamma knife radiosurgery stratified by (A) Karnofsky performance status score, (B) mode of onset in NSCLC, and (C) the primary disease control.
DISCUSSION
Several study results demonstrated benefit of radiosurgery in patients with one to three metastatic lesions5,8,9,12). A prospective randomized trial of WBRT plus radiosurgery compared with WBRT alone17) showed statistically significant prolongation of survival for the following patients : those with solitary brain metastasis, RPA class I, age younger than 50 years, and patients with NSCLC. In the other patients with one to three metastatic lesions, the patients in the stereotactic surgery group were more likely to have a stable or improved KPS score than those who were allocated to WBRT alone (43% and 27% at 6 months, respectively)1). In another randomized trial, it was reported that radiosurgery alone was not statistically different from radiosurgery combined with WBRT in freedom from progression (71% and 79% at 1 year, respectively) and median survival (11.3 and 11.1 months, respectively) 16). Therefore, there is a general consensus that stereotactic radiosurgery is helpful in patients with good KPS scores and a relatively small number of metastatic lesions, and omission of WBRT in this patients group became a more important issue. However, it is still unknown how many lesions can be reasonably treated with GKS. Several authors reported that radiosurgery prolonged survival in patients with ten or more brain metastases15,18-20). According to Satoshi, et al.18), metastasis-related neurological symptoms improved or were stabilized after GKS in 92% of patients with ten or more lesions. Because of the limited expected survival time, it was strongly recommended that the inpatient period be shortened and that the patient's quality of life be maintained. In a recent study for 10 or more brain metastases from NSCLC15), neurological survival and qualitative survival of the GKS group were longer than those of the WBRT group. Significant poor prognostic factors were systematically uncontrolled patients, WBRT group, and poor initial KPS score. In conclusion, it was suggested that WBRT was probably unnecessary for patients with as many as 10 multiple cerebral metastases from NSCLC15).
The two most important issues in radiosurgery of numerous brain lesions are safety and efficacy. Yamamoto, et al.21), reported that the cumulative doses for whole brain with numerous radiosurgical targets were not considered to exceed the threshold level of normal brain necrosis. In our series, there was no incidence of serious acute toxicity related to GKS. We identified overt late toxicity, the radiation necrosis, in only one patient who survived 186 weeks. Because most of the patients died within a year due to progression of disease, late toxicity might be masked and missed to be recognized. Therefore, the safety issue is not clearly resolved, however, at the same time it seems that potential risk from radiation injury is not a factor precluding application of GKS in this population with short expectancy of survival. The more crucial question should be whether radiosurgery in this population with numerous lesions truly improve survival or quality of life. Survival of the patients in our series is not poor as presumed according to traditional concept of multiple metastatic lesions. In a report of 4 or more lesions treated with WBRT alone, the median survival of RPA class II was only 3.6 months11). Though survival benefit with GKS cannot be clearly defined because our study is not a randomized trial, the median survival of 34 weeks in our series is a promising result. Meanwhile our results show relatively higher rate of death from neurological causes compared with data from radiosurgery for oligo-metastatic lesions. It is not an unpredictable result. Higher percentage of the patients would have probability of local progression because probability of failure to local control in at least one among numerous lesions may be very high. Also, predisposing condition that might already result in numerous brain metastases is likely to work after GKS and the patients initially presenting with numerous metastases may be more vulnerable to remote recurrence after treatment. Anyway, the relatively higher rate of neurological death suggests that overall benefit of GKS for numerous lesions is not exactly similar to that for small number of lesions. Though GKS improves local control, still more effective measures to control brain lesions are necessary to improve survival further. Concerning the quality of life, we cannot get an answer from this study because quality of life was not surveyed systematically and there are not comparable data from supportive care only or WBRT either. It is necessary that these issues can only be clarified through randomized controlled study in the future.
In our study, the significant factors influencing survival were mode of onset (synchronous) in NSCLC, KPS score (≥80), controlled primary disease, and systemic chemotherapy after GKS. Better KPS score and control of primary disease are well known favorable prognostic factors. However, mode of onset was not proven as a significant prognostic factor in the most of the literatures. It is speculated that the better prognosis of patients with synchronous brain metastases may reflect recent advance of systemic treatment and supportive care. The patients with synchronous brain metastases may have more available treatment modalities such as palliative chemotherapy for extracranial lesions than those with metachronous brain metastases that frequently exhausted therapeutic armamentarium before development of brain lesion. As a result, progression of systemic disease in the patients with synchronous brain lesion may be halted more effectively and they survive longer. Meanwhile, it is very much likely that longer survival of the patients who received systemic chemotherapy after GKS results from selection bias. Only the patients who maintain favorable performance status can continue to be treated with chemotherapy and it is easily predictable that they survive longer.
As noted above, our study has several limitations because it is not randomized controlled trial. There is no control group to be compared and this study includes a limited number of patients with incomplete follow-up and heterogenous combination of treatment modalities. Thus, the impact of other prognostic factor such as WBRT, total tumor volume, and marginal dose might be underestimated. Whatever these limitations may be, the results of this study indicate that outcome after GKS for ten or even more brain metastases is not necessarily be poor. In reality, it is much better in a certain subgroup of the patients than presumed with traditional concept. Aggressive approach including GKS does not need to be withheld in these patients, particularly in the group with predominantly favorable prognostic factors.
CONCLUSION
The outcome of GKS for numerous brain metastases is not as poor as generally predicted. GKS may be recommended for selected patients with favorable performance status, radiosensitive pathology, small tumor burden, possibility of systemic treatment, and NSCLC of synchronous onset. In conclusion, GKS may be a treatment option for ten or more brain metastases in carefully-selected patients.
References
- 1.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases : phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 2.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases : a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar AK, Flickinger JC, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Cairncross JG, Kim JH, Posner JB. Radiation therapy for brain metastases. Ann Neurol. 1980;7:529–541. doi: 10.1002/ana.410070606. [DOI] [PubMed] [Google Scholar]
- 5.Chen JC, Petrovich Z, O'Day S, Morton D, Essner R, Giannotta SL, et al. Stereotactic radiosurgery in the treatment of metastatic disease to the brain. Neurosurgery. 2000;47:268–279. doi: 10.1097/00006123-200008000-00003. discussion 279-281. [DOI] [PubMed] [Google Scholar]
- 6.Coia LR, Aaronson N, Linggood R, Loeffler J, Priestman TJ. A report of the consensus workshop panel on the treatment of brain metastases. Int J Radiat Oncol Biol Phys. 1992;23:223–227. doi: 10.1016/0360-3016(92)90566-z. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 8.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 9.Lutterbach J, Cyron D, Henne K, Ostertag CB. Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery. 2003;52:1066–1073. discussion 1073-1064. [PubMed] [Google Scholar]
- 10.Nam TK, Lee JI, Jung YJ, Im YS, An HY, Nam DH, et al. Gamma knife surgery for brain metastases in patients harboring four or more lesions : survival and prognostic factors. J Neurosurg. 2005;(102 Suppl):147–150. doi: 10.3171/jns.2005.102.s_supplement.0147. [DOI] [PubMed] [Google Scholar]
- 11.Nieder C, Andratschke N, Grosu AL, Molls M. Recursive partitioning analysis (RPA) class does not predict survival in patients with four or more brain metastases. Strahlenther Onkol. 2003;179:16–20. doi: 10.1007/s00066-003-1028-x. [DOI] [PubMed] [Google Scholar]
- 12.Pirzkall A, Debus J, Lohr F, Fuss M, Rhein B, Engenhart-Cabillic R, et al. Radiosurgery alone or in combination with whole-brain radiotherapy for brain metastases. J Clin Oncol. 1998;16:3563–3569. doi: 10.1200/JCO.1998.16.11.3563. [DOI] [PubMed] [Google Scholar]
- 13.Posner JB. Management of brain metastases. Rev Neurol (Paris) 1992;148:477–487. [PubMed] [Google Scholar]
- 14.Posner JB. Management of central nervous system metastases. Semin Oncol. 1977;4:81–91. [PubMed] [Google Scholar]
- 15.Serizawa T, Iuchi T, Ono J, Saeki N, Osato K, Odaki M, et al. Gamma knife treatment for multiple metastatic brain tumors compared with whole-brain radiation therapy. J Neurosurg. 2000;93(Suppl 3):32–36. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 16.Sneed PK, Lamborn KR, Forstner JM, McDermott MW, Chang S, Park E, et al. Radiosurgery for brain metastases : is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43:549–558. doi: 10.1016/s0360-3016(98)00447-7. [DOI] [PubMed] [Google Scholar]
- 17.Sperduto PW, Scott C, Andrews D, Schell M, Werner-Wasik M, Demas W, et al. Preliminary report of RTOG 9508 : a phase III trial comparing whole brain irradiation alone versus whole brain irradiation plus stereotactic radiosurgery for patients with two or three unresected brain metastases [abstract] Int J Radiat Oncol Biol Phys. 2000;48:113. [Google Scholar]
- 18.Suzuki S, Omagari J, Nishio S, Nishiye E, Fukui M. Gamma knife radiosurgery for simultaneous multiple metastatic brain tumors. J Neurosurg. 2000;93(Suppl 3):30–31. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto H, Takagi M, Ushio Y, Ohno S. Gamma knife radiosurgery for multiple metastatic brain lesions. Neurosurg Lett. 1998;8:391–398. [Google Scholar]
- 20.Yamamoto M, Ide M, Jimbo M, Aiba M, Ito M, Hirai T, et al. Radiosurgery. in Kondziolka D. Vol. Basel: Karger; 1997. Gamma knife radiosurgery with numerous target points for intracranially disseminated metastases: early experience in 3 patients and experimental analysis of whole brain irradiation doses; pp. 94–109. [Google Scholar]
- 21.Yamamoto M, Ide M, Nishio S, Urakawa Y. Gamma Knife radiosurgery for numerous brain metastases : is this a safe treatment? Int J Radiat Oncol Biol Phys. 2002;53:1279–1283. doi: 10.1016/s0360-3016(02)02855-9. [DOI] [PubMed] [Google Scholar]
- 22.Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48:384–394. doi: 10.1002/1097-0142(19810715)48:2<384::aid-cncr2820480227>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]