Abstract
Background information. At fertilization in mammalian eggs, the sperm induces a series of Ca2+ oscillations via the production of inositol 1,4,5-trisphosphate. Increased inositol 1,4,5-trisphosphate production appears to be triggered by a sperm-derived PLCζ (phospholipase C-ζ) that enters the egg after gamete fusion. The specific phosphatidylinositol 4,5-bisphosphate hydrolytic activity of PLCζ implies that DAG (diacylglycerol) production, and hence PKC (protein kinase C) stimulation, also occurs during mammalian egg fertilization. Fertilization-mediated increase in PKC activity has been demonstrated; however, its precise role is unclear.
Results. We investigated PLCζ- and fertilization-mediated generation of DAG in mouse eggs by monitoring plasma-membrane translocation of a fluorescent DAG-specific reporter. Consistent plasma-membrane DAG formation at fertilization, or after injection of physiological concentrations of PLCζ, was barely detectable. However, when PLCζ is overexpressed in eggs, significant plasma-membrane DAG production occurs in concert with a series of unexpected secondary high-frequency Ca2+ oscillations. We show that these secondary Ca2+ oscillations can be mimicked in a variety of situations by the stimulation of PKC and that they can be prevented by PKC inhibition. The way PKC leads to secondary Ca2+ oscillations appears to involve Ca2+ influx and the loading of thapsigargin-sensitive Ca2+ stores.
Conclusions. Our results suggest that overproduction of DAG in PLCζ-injected eggs can lead to PKC-mediated Ca2+ influx and subsequent overloading of Ca2+ stores. These results suggest that DAG generation in the plasma membrane of fertilizing mouse eggs is minimized since it can perturb egg Ca2+ homoeostasis via excessive Ca2+ influx.
Keywords: Ca2+ store, diacylglycerol, egg activation, fertilization, protein kinase C, phospholipase C-ζ (PLCζ)
Abbreviations: BAPTA, bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid; CHE, chelerythrine chloride; cps, counts per seconds; DAG, diacylglycerol; fura 2/AM, fura 2 acetoxymethyl ester; GFP, green florescent protein; hCG, human chorionic gonadotropin; CCD camera, charge-coupled-device camera; IVF, in vitro fertilization; ICCD camera, intensified CCD camera; PKC, protein kinase C; (m)PLC, (mouse) phospholipase C; PM/C ratio, plasma membrane/cytosol ratio
Introduction
During fertilization in mammalian eggs, the sperm stimulates a series of intracellular Ca2+ oscillations that is responsible for activating the egg (Stricker 1999; Miyazaki and Ito, 2006). These Ca2+ oscillations stimulate calmodulin-dependent protein kinase II, which can trigger meiotic resumption and early development in mouse eggs (Madgwick et al., 2005; Ducibella et al., 2006; Knott et al., 2006). The Ca2+ oscillations at fertilization are triggered by the production of inositol 1,4,5-trisphosphate, which causes release of Ca2+ from intracellular stores (Miyazaki et al., 1993; Lee et al., 2006). The sperm appears to trigger the increase in inositol 1,4,5-trisphosphate production at fertilization by introducing a sperm-specific PLC (phospholipase C) (PLCζ) into the egg cytoplasm following sperm–egg fusion (Saunders et al., 2002; Knott et al., 2005; Lee et al., 2006; Miyazaki and Ito, 2006; Swann et al., 2006). Since PLCζ hydrolyses [PIP2 (phosphatidylinositol 4,5-bisphosphate)], it generates both inositol 1,4,5-trisphosphate and DAG (diacylglycerol) in eggs (Kouchi et al., 2004; Nomikos et al., 2005). While many studies have reported Ca2+ oscillations in PLCζ-injected eggs, there have been no investigations of PLCζ-stimulated generation of DAG.
The main signalling target for DAG in cells is PKC (protein kinase C) (Nishizuka, 1992). It has been shown that PKC activity increases at fertilization in mouse eggs (Gallicano et al., 1997; Tatone et al., 2003). Furthermore, mouse and rat eggs have been shown to express a number of different isoforms of PKC including the conventional α, β and γ isoforms that are activated by Ca2+ and DAG, the novel isoform δ, which is stimulated by DAG, and the atypical isoforms ζ and λ, which are not stimulated by either Ca2+ or DAG (Jones, 1998; Pauken and Capco, 2000; Baluch et al., 2004; Halet, 2004). The translocation of α, β and γ isoforms to the egg cortex has been demonstrated after sperm–egg interaction or after Ca2+ ionophore-induced egg activation (Luria et al., 2000; Halet et al., 2004). PKCδ and PKCζ are localized to the meiotic spindle and may play a role in spindle stability and progression through meiosis (Tatone et al., 2003; Viveiros et al., 2003; Baluch et al., 2004). A role for PKC at fertilization is suggested by the finding that the potent PKC activator, PMA, can stimulate the initial stages of second polar body emission and trigger a degree of cortical granule exocytosis in mouse eggs (Gallicano et al., 1997; Eliyahu and Shalgi, 2002). PKC may also regulate Ca2+ signals in eggs since some studies have shown that PMA causes small Ca2+ oscillations (Cuthbertson and Cobbold, 1985) and, more recently, it was shown that PMA can stimulate store-operated Ca2+ influx (Halet et al., 2004). However, the role of PKC activation at fertilization is unclear because while some inhibitors of PKC block events such as meiotic resumption, cortical granule exocytosis and transcription at the one-cell stage (Gallicano et al., 1997; Yu et al., 2007), other inhibitors are without any effect (Ducibella and Lefevre, 1997).
The dynamic translocation of PKCs to the plasma membrane of fertilizing mouse eggs was previously measured using GFP (green florescent protein)-tagged PKCα or PKCγ (Halet et al., 2004). It was shown that these PKC isoforms translocate to the plasma membrane with similar kinetics to the sperm-induced Ca2+ increases (Halet et al., 2004). The translocation is transient and largely dependent on the amplitude of the initial Ca2+ increase, and was mainly driven by the Ca2+-dependent C2 domain of these conventional PKC isoforms. Here, we investigate DAG production and PKC activation in fertilizing, and PLCζ-injected, mouse eggs using a fluorescent probe for DAG based on the tandem C1 domain of PKCδ (C12δ–GFP; Codazzi et al., 2001; Dries et al., 2007). We find that minimal DAG production occurs in the plasma membrane at fertilization, or after physiological levels of PLCζ injection, in mouse eggs. However, overproduction of DAG by high levels of PLCζ can lead to excessive Ca2+ influx and an unusual pattern of high-frequency Ca2+ oscillations.
Results
C12δ–GFP detects plasma-membrane DAG in mouse eggs
In a previous study, the C1 domain of conventional PKCα was used as a DAG biosensor in mouse eggs, but it failed to detect any DAG in the plasma membrane of fertilized eggs (Halet et al., 2004). We re-investigated DAG synthesis in fertilized eggs using tandem C1 domain of PKCδ (C12δ–GFP), which is reported to have an affinity for DAG binding two orders of magnitude higher than the binding affinity of the C1 domain from PKCα (Dries et al., 2007). Figure 1(A) shows that ionomycin treatment of unfertilized mouse eggs caused a large Ca2+ increase and a delayed translocation of C12δ–GFP to the plasma membrane, indicating DAG production. In contrast, the addition of 100 ng/ml PMA to eggs caused an immediate increase in C12δ–GFP plasma-membrane translocation without any increase in Ca2+ levels (Figure 1B). These results show that C12δ–GFP can effectively report DAG production at the plasma membrane in mouse egg, which is presumably due to the activity of endogenous Ca2+-sensitive PLCs (Halet et al., 2004). When we examined eggs at fertilization, we found that translocation of C12δ–GFP to the plasma membrane was only just detectable in two eggs (Figure 1C), whereas in most of the eggs at fertilization (12/14) there was no evidence for translocation to the plasma membrane (Figure 1D). In the two eggs that showed evidence of C12δ–GFP translocation, this was restricted only to the initial Ca2+ increase at fertilization and tended to be evident in a restricted portion of the cortex. C12δ–GFP did not show any clear localization in the meiotic spindle either before or after fertilization. These results suggest that there is very little DAG production in the plasma membrane of fertilizing mouse eggs in comparison with that seen with ionomycin.
Figure 1. GFP-tagged PKCδ C1 domain (C12δ–GFP) translocation in mouse eggs.
The translocation of C12δ–GFP, indicating DAG accumulation, was measured as the plasma-membrane fluorescence divided by the bulk cytoplasmic fluorescence (PM/C ratio) (dotted line, labelled ‘DAG’). The fluorescence of Fura Red was monitored to indicate changes in Ca2+ (solid line, labelled ‘Ca2+’). Fura Red fluorescence decreases on Ca2+ binding, so we inverted the fluorescence signal of Fura Red (at 488 nm excitation) to represent Ca2+ levels. The slight drift up in the trace with time was due to some loss of dye from the egg. The insets show example images of eggs taken at the time indicated by the arrows. In (A), the egg was exposed to 2 μM ionomycin, which caused an immediate Ca2+ increase and a delayed C12δ–GFP translocation from cytosol to the plasma membrane (n=10). In (B), eggs treated with 100 ng/ml PMA showed an immediate C12δ–GFP translocation without a Ca2+ increase (n=11). In (C), a recording is shown for 1 of 2 eggs that showed a small C12δ–GFP translocation during the initial Ca2+ increase at fertilization. In (D), a typical trace is shown from the remaining 12 eggs where there was no detectable increase in C12δ–GFP translocation during Ca2+ oscillations at fertilization.
High levels of PLCζ promote DAG synthesis in mouse eggs
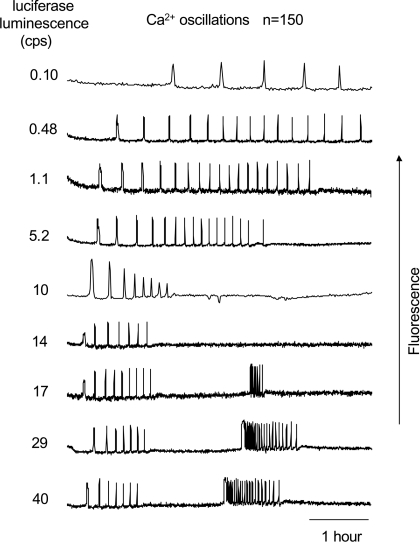
PLCζ triggers Ca2+ oscillations similar to those seen at fertilization, but unlike fertilization it can be expressed in eggs at a range of different concentrations that can be quantified with a luciferase tag (Nomikos et al., 2005; Yu et al., 2008). Figure 2 shows the patterns of Ca2+ oscillations triggered by injection of luciferase-tagged PLCζ RNA (PLCζ–luc) at various concentrations. Increasing levels of mouse PLCζ expression led to an increase in Ca2+ oscillation frequency. However, when expression was higher than 10 cps (counts per seconds), Ca2+ oscillations ceased prematurely, and the highest expression levels (20–40 cps) were associated with an unexpected secondary series of high-frequency Ca2+ oscillations that occurred with a delay of 3–4 h (Figure 2). When we examined C12δ–GFP translocation in PLCζ-injected eggs, we found that the initial series of Ca2+ oscillations was not associated with any evidence of translocation (Figure 3). However, with high PLCζ expression levels, and when secondary Ca2+ oscillations were initiated, C12δ–GFP translocation to the plasma membrane was clearly detectable in all eggs in synchrony with the initial Ca2+ transient of the secondary series. These results suggest that DAG production can occur in the plasma membrane of mouse eggs when PLCζ is overexpressed for inducing a secondary series of Ca2+ oscillations.
Figure 2. The patterns of Ca2+ oscillations triggered by PLCζ–luc.
Different concentrations of mPLCζ–luc RNA (0.05–1 μg/μl) were injected into mouse eggs. The oscillations in Ca2+ were indicated by changes in fluorescence intensity (in arbitrary units) of Oregon Green BAPTA. The relative expression levels of PLCζ–luc protein are indicated by luminescence in photon cps along the left-hand side of each fluorescence trace. The traces are representative and were selected from 150 eggs (in the range of 0.1–40 cps) and the expression level on the left-hand side is that determined for each sample egg (rounded to two significant figures). Low PLCζ–luc expression was associated with long-lasting low-frequency Ca2+ oscillations, whereas oscillations similar to fertilization were associated with expression levels of 1–5 cps. High levels of PLCζ–luc expression were associated with two distinct series of Ca2+ oscillations.
Figure 3. C12δ–GFP translocation initiated in PLCζ–lucinjected eggs.
The conditions were otherwise the same as in Figure 1. High level of mPLCζ–luc (1 μg/μl) caused rapid Ca2+ oscillations. A typical trace is shown where there was no C12δ–GFP translocation during the first series of Ca2+ oscillations (n=8). However, a clear translocation of C12δ–GFP occurred during the series of secondary Ca2+ oscillations (n=8).
PKC activation and Ca2+ influx promote secondary Ca2+ oscillations
The premature cessation, and the subsequent generation of a delayed secondary series of Ca2+ oscillations, is a novel feature of high levels of PLCζ expression. Previous studies have suggested that pronuclear formation can regulate Ca2+ oscillations by sequestering PLCζ (Larman et al., 2004). However, pronuclear formation or dissolution did not play any role in the pattern of Ca2+ changes in eggs overexpressing PLCζ, because the basic phenomena of premature cessation and secondary oscillations were still seen in eggs that had been enucleated (results not shown). The cessation of Ca2+ oscillations could occur because of inositol 1,4,5-trisphosphate receptor down-regulation, since this can be induced by injection of agents such as the inositol 1,4,5-trisphosphate receptor agonist, adenophostin, which causes a single series of Ca2+ oscillations (Brind et al., 2000; Jellerette et al., 2000). The occurrence of secondary Ca2+ oscillations, however, is not obviously explained by this down-regulation mechanism.
We then considered the possibility that DAG production and hence PKC stimulation could play a role in causing the secondary oscillations. Figure 4 shows eggs that had been injected either with adenophostin, or with PLCζ (corresponding to low levels of expression), or else fertilized in vitro by sperm. With each of these stimuli, there is normally only a singular series of Ca2+ oscillations. However, when PMA was applied after the cessation of Ca2+ oscillations, we found that most of the eggs underwent an extra series of Ca2+ oscillations (Figures 4A–4C). These secondary series of Ca2+ oscillations were of high frequency and resembled the pattern of secondary Ca2+ oscillations seen with high PLCζ expression.
Figure 4. Secondary Ca2+ oscillations triggered by PMA and raising extracellular Ca2+.
Eggs were injected with adenophostin (Ad), or with PLCζ to cause low levels of expression (luciferase expression indicated in parentheses), or they were fertilized (IVF). In each case, a single series of Ca2+ oscillations was seen and then PMA (1 μg/ml, at the time indicated by the arrows) was added (in A–C). The addition of PMA led to another set of Ca2+ oscillations in most of the eggs (the number of eggs responding is indicated above each trace). In a similar set of experiments (D–F), the extracellular Ca2+ was increased to 10 mM after the cessation of the initial Ca2+ oscillations. In these cases, all the eggs responded to the raising of extracellular Ca2+ by showing a second set of Ca2+ oscillations (the numbers of eggs are indicated above each sample trace).
Previous studies in mouse eggs have suggested that PMA can increase the rate of Ca2+ influx (Halet et al., 2004). If the above effects of PMA are mediated via Ca2+ influx, then it should be possible to stimulate secondary Ca2+ oscillations by increasing extracellular Ca2+ levels. Figure 4 shows eggs that had again been injected either with adenosphostin, or with PLCζ (low level of expression), or that had been fertilized in vitro. After the normal primary series of Ca2+ oscillations had ceased, raising extracellular Ca2+ to 10 mM led to a secondary series of Ca2+ oscillations that again resembled the high-frequency response observed with high PLCζ expression (Figures 4D–4F). These results suggest that stimulation of PKC and subsequent Ca2+ influx can produce secondary Ca2+ oscillations in mouse eggs.
If PKC plays a role in triggering the secondary Ca2+ oscillations, we should be able to inhibit PLCζ-induced secondary oscillations by blocking PKC activity. The PKC inhibitor, CHE (chelerythrine chloride), has previously been shown to be an effective inhibitor of PKC activity in mouse eggs (Ducibella and Lefevre, 1997; Gallicano et al., 1997). Figure 5 shows the effects of adding CHE to mouse eggs that were injected with PLCζ. We found that the occurrence of PLCζ-induced secondary Ca2+ oscillations was virtually abolished by treatment with CHE (0.8 μM). The results collected from almost 300 eggs showed that incubation with CHE reduced the occurrence of secondary Ca2+ oscillations from 37.8% (8/23) to 10.6% (5/47), and 85.7% (12/14) to 16.7% (3/18) in the PLCζ expression ranges of 15–30 and 30–45 cps respectively. These results support the hypothesis that increased PKC activity plays a role in triggering secondary Ca2+ oscillations in mouse eggs.
Figure 5. PLCζ–luc-induced Ca2+ oscillations in eggs treated with the PKC inhibitor CHE.
In (A), two example traces are shown where eggs were expressing high relative levels of PLCζ–luc expression (32 cps). The top traces show the usual pattern of initial and secondary Ca2+ oscillations, whereas the bottom trace shows that secondary Ca2+ oscillations did not occur in egg incubated with 0.8 μM CHE. In (B), the histogram summarizes data on eggs treated with CHE (open bars) or control eggs without CHE (filled bars). The expression levels are placed into groups according to the expression levels and the numbers of eggs for each group are indicated above columns bars. The asterisks indicate statistically significant differences between groups (P<0.05).
PKC activation promotes Ca2+ influx and overloading of the Ca2+ stores
Since PKC-driven Ca2+ influx could cause the secondary Ca2+ oscillations, we sought to assay Ca2+ influx in eggs. The Mn2+-induced quenching of fura 2 fluorescence has previously been used as a surrogate for monitoring the rate of Ca2+ influx into mouse eggs (McGuiness et al., 1996; Mohri et al., 2001). To establish whether PKC activity stimulates bivalent cation influx, eggs were treated with 1 μg/ml PMA for 1 h before the addition of Mn2+. The trace in Figure 6(A) shows the quenching of fura 2 and indicates that PMA significantly stimulated the Mn2+ influx rate (4.93±0.54 relative fluorescence units/s decrease, n=25 compared with the control eggs; 1.5±0.1 fluorescence units, n=19, P<0.01). In order to investigate whether Ca2+ influx was related to the occurrence of secondary Ca2+ oscillations, we used the same Mn2+ quenching protocol at different time points in eggs injected with either adenophostin or high PLCζ concentrations. We compared overexpression of PLCζ with the effects of adenophostin injected, since adenophostin only causes a single set of Ca2+ oscillations and it is not expected to cause significant DAG production since it generates moderate Ca2+ increase compared with ionomycin (Brind et al., 2000; Jellerette et al., 2000). Eggs at 2 and 4 h after mPLCζ (mouse PLCζ) or adenophostin injection were loaded with fura 2 and then exposed to Mn2+ for inducing fura 2 quenching (Figures 6B and 6C). We chose the 2 h time point because all of the eggs should be completing the primary oscillations phase, whereas at 4 h the PLCζ–luc-injected eggs should be commencing the secondary Ca2+ oscillations. At 2 h post-injection, the quenching rate (measured over 150 s) in both mPLCζ (2.7±0.30, n=9) and adenophostin A (2.7±0.15, n=21) injected eggs were similar, but higher than that in control eggs (1.8±0.12, n=23). However, at 4 h after injection, the quenching rate became higher (3.54±0.39, n=11) in mPLCζ–luc-injected eggs, while it returned to control levels in adenophostin-injected eggs (2.1±0.2, n=15). These data support previous data on Ca2+ influx in fertilizing mouse eggs (Halet et al., 2004) and suggest that high PLCζ expression differs from adenophostin in that there is a prolonged phase of increased Ca2+ influx.
Figure 6. Bivalent cation influx in mouse eggs in response to adenophostin, PLCζ and PMA.
Bivalent cation influx was measured by the quenching of fura 2 fluorescence by Mn2+ (0.1 mM) that was added at the arrows for each sample trace. In each case, the dotted line represents a control (uninjected or untreated) egg that was treated at the same time in the same experiment with the numbers of eggs for each experiment indicated. In (A), Mn2+ was added to eggs after 1 h of PMA treatment. In (B), eggs were injected with 10 μM adenophostin A and monitored 2 and 4 h after injection. In (C), eggs were injected with PLCζ–luc and Mn2+ quenching was monitored for 2 and 4 h after injection. The straight lines indicate the slopes used for estimating the initial Mn2+ influx rate.
Increased Ca2+ influx could promote intracellular Ca2+ release via the overloading of Ca2+ stores. To investigate this possibility, we measured the relative amount of Ca2+ in the thapsigargin-releasable Ca2+ store 4 h after injection of adenophostin, or PLCζ, when maximal differences might be expected. Thapsigargin and BAPTA [bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid] were added to the Ca2+-free medium containing the eggs to trigger Ca2+ release that was detected with fura 2 dextran fluorescence at 380 nm wavelength [(Ft−F0)/Ft]. This phasic Ca2+ increase is used as a measure of the size of the Ca2+ store (El-Jouni et al., 2005). Figure 7 shows that 4 h after injection, the Ca2+ increase elicited by thapsigargin was larger in eggs overexpressing PLCζ–luc compared with eggs injected with adenophostin (Figure 7A). The size of the Ca2+ stores was assessed either by the amplitude of the thapsigargin-induced Ca2+ increase or by the integral of the phasic Ca2+ increase (area under the curve). With either metric, there was a significant increase in Ca2+ store content in eggs overexpressing PLCζ–luc (Figures 7C and 7D). The increase in Ca2+ store content was also present in uninjected eggs that had been treated with PMA for 1 h (Figure 7B). These results suggest that Ca2+ stores are substantially more loaded than normal by overexpression of PLCζ and by PKC stimulation.
Figure 7. Thapsigargin-releasable Ca2+ store content in eggs.
Eggs were exposed to 20 μM thapsigargin (at the arrow) in Ca2+-free medium. Fura 2 dextran fluorescence (at 380 nm) [(Ft−F0)/Ft] is plotted to gain a measure of the amount of Ca2+ released. In (A), the fluorescence change is plotted for eggs injected 4 h previously with either adenophostin (dotted line) or PLCζ–luc (solid line). In (B), the fluorescence change is plotted for an egg treated with PMA (solid line) compared with a control untreated egg (dotted line). To quantify the changes in store content, we plotted the amplitude of the fluorescence change (C) or else the integral of the elevated phase of Ca2+ (area under the curve) in (D). All joint sets of bars show a significant difference between each other (P<0.02) with the numbers of eggs in each group shown above each bar.
Discussion
Most studies on signalling at fertilization in mouse eggs have concentrated on inositol 1,4,5-trisphosphate-induced Ca2+ release (Miyazaki et al., 1993; Kurakawa et al., 2004; Miyazaki and Ito, 2006). However, the involvement of PLCζ or any other PLCs will inevitably generate DAG and this has been less extensively studied for technical reasons. Here, we have used a GFP-linked probe with a high sensitivity and specificity for DAG. At fertilization, DAG synthesis in the plasma membrane was not consistently observed, and the signal detected was very small compared with the DAG increase elicited by ionomycin. Moreover, the transient increase in plasma-membrane DAG occurred solely during the first Ca2+ transient, which is consistent with a previous report showing that conventional PKC translocation to the plasma membrane of fertilized eggs is driven essentially by the Ca2+-binding C2 domain (Halet et al., 2004). Our results suggest that DAG accumulation in the plasma membrane of fertilized eggs is both relatively modest and short-lived. Since a very large Ca2+ increase itself can be a stimulus for DAG production, some egg-derived plasma-membrane PLCs may be stimulated in eggs and this could be the PLCβ isoforms (Igirashi et al., 2007).
As with fertilization, the expression of physiological levels of PLCζ did not lead to any measurable increase in DAG in the plasma membrane. However, overexpression of PLCζ resulted in the appearance of a delayed, second series of high-frequency Ca2+ oscillations that was consistently associated with plasma-membrane DAG production. Since a gap of several hours separates the two series of oscillations, the eggs would have expressed a significant level of PLCζ by that time, which may contribute to the increased DAG synthesis as a result of Ca2+-induced PLCζ stimulation since PLCζ is highly sensitive to cytosolic Ca2+ (Kouchi et al., 2004; Nomikos et al., 2005). This may resemble the situation where inositol 1,4,5-trisphosphate was monitored in mouse eggs using a fluorescence indicator that could only detect inositol 1,4,5-trisphosphate after a period that allowed for increased PLCζ expression (Shirakawa et al., 2006).
The secondary Ca2+ oscillations we describe in the present study have not been previously reported, because earlier studies injected PLCζ RNA corresponding approximately to the amount contained in a single sperm (Saunders et al., 2002; Miayzaki and Ito, 2006). The overexpression of PLCζ is likely to lead to the down-regulation of inositol 1,4,5-trisphosphate receptors, which could then explain the early cessation of oscillations (Brind et al., 2000; Jellerette et al., 2000). However, inositol 1,4,5-trisphosphate receptor down-regulation does not preclude further Ca2+ oscillations, because it has been shown that some sufficiently strong stimuli can still generate Ca2+ oscillations after 70–80% down-regulation of inositol 1,4,5-trisphosphate receptors (Malcuit et al., 2005). Our results suggest that PKC is important in providing the extra stimulus that re-stimulates Ca2+ oscillations, since PMA triggers a series of secondary Ca2+ oscillations in eggs that would otherwise only generate a single series of Ca2+ oscillations. Furthermore, the PKC inhibitor, CHE, significantly inhibits the occurrence of secondary Ca2+ oscillations in eggs injected with high concentrations of PLCζ. This shows that PKC-stimulation is both necessary and sufficient to trigger the secondary Ca2+ release that occurs with high levels of PLCζ.
It is remarkable that these dramatic effects of PKC can be inferred in the absence of any detectable DAG production in the plasma membrane of eggs during the time between the first and secondary Ca2+ oscillations. It should be noted that in astrocytes the same C12δ–GFP domain that we used in mouse eggs is very effective at reporting plasma-membrane DAG generation in response to metabotropic glutamate receptor-mediated stimulation that activates PLCβ (Codazzi et al., 2001). Our inability to observe translocation of the C12δ–GFP domain at fertilization, or during the period leading up to the secondary Ca2+ oscillations caused by excessive PLCζ expression, is therefore unlikely to represent any deficiency in the affinity of the DAG probe. It is possible that any DAG increase is too gradual to be readily detected with a translocation-based indicator, or else DAG is localized differently in eggs compared with astrocytes.
Our results suggest that the mechanism by which PKC causes secondary Ca2+ oscillations appears to involve Ca2+ influx. We also found evidence for enhanced filling of Ca2+ stores after PKC stimulation or overexpression of PLCζ. It is possible that Ca2+ influx and the subsequent overloading of Ca2+ stores could trigger the secondary Ca2+ oscillations, since the overloading of Ca2+ stores can lead to a ‘spontaneous’ release in sea-urchin egg homogenates (Galione et al., 1993). However, we cannot exclude the possibility that PKC increases could also alter the sensitivity of the inositol 1,4,5-trisphosphate receptor to inositol 1,4,5-trisphosphate such that Ca2+ release is enhanced (Vermassen et al., 2004). Whatever the mechanisms, our current findings imply that PKC overstimulation can have major negative effects on eggs when it causes an abnormal series of secondary Ca2+ oscillations. Similar to studies with human PLCζ, we found that none of the eggs that expressed high levels of mouse PLCζ developed to blastocyst stages even though they formed pronuclei (Yu et al., 2008). This suggests that the stimulation of PKC in the plasma membrane has to be minimal at fertilization. Given the evidence that calmodulin-dependent protein kinase II can trigger meiotic resumption and early development, the role of PKC in egg activation has to be questioned (Jones 1998; Madgwick et al., 2005; Knott et al., 2006). It is possible that most of the increase in PKC activity that has been reported at fertilization is due to non-conventional and novel isoforms such as PKCζ and PKCδ, which are localized to the meiotic spindle in mature mouse eggs where they may play a permissive role in meiotic resumption (Tatone et al., 2003; Viveiros et al., 2003).
Materials and methods
Gamete collection, RNA preparation and microinjection
MF1 female mice were superovulated by intraperitoneal injection of 7.5 i.u. of PMSG (pregnant mare's serum gonadotrophin; Folligon) followed 48 h later by 10 i.u. of hCG (human chorionic gonadotropin; Folligon) (Saunders et al., 2002; Larman et al., 2004). Eggs (13–16 h post-hCG) were released from the oviduct into warmed M2 medium (Sigma). Cumulus cells were removed by a brief exposure to hyaluronidase and the zona pellucida removed by exposure to acid Tyrode's solution (Gibco). Oocytes were held in drops of M2 medium under paraffin oil in Falcon tissue culture dishes. Spermatozoa were expelled from cauda epididymis of male CBA/C57 mice into 1 ml of T6 medium containing 16 mg/ml BSA, and incubated under oil for 2–3 h at 37°C and 5% CO2 to capacitate. For IVF (in vitro fertilization) experiments, approx. 10 μl of sperm suspension was added to the dish containing the eggs. Complementary RNA encoding mouse PLCζ fused via the C-terminus to firefly luciferase (mPLCζ–luc), and PKCδ tandem C1 domain, tagged with GFP (C12δ–GFP in pcDNA3.1), were prepared with an mMessage mMachine T7 Ultra kit (Ambion), followed by polyadenylation (Halet et al., 2004; Nomikos et al., 2005). Microinjection procedures were carried out as previously described (Saunders et al., 2002). Eggs were injected to 3–5% egg volume with a solution containing different concentrations of mPLCζ–luc cRNA (0.05–1 μg/μl) mixed with 1 mM (pipette concentration) Oregon Green BAPTA dextran (Molecular Probes, http://www.probes.com) and/or C12δ–GFP cRNA (∼0.5 μg/μl). Unless otherwise stated, media and reagents were purchased from Sigma (Poole, Dorset, U.K.).
Confocal imaging
At 2–3 h after C12δ–GFP cRNA injection, eggs were loaded with 10 μM Fura Red/AM (Fura Red acetoxymethyl ester) (Molecular Probes) for 20 min and subsequently the zona pellucida was removed by incubation in acidic Tyrode's medium at 37°C. Zona-free eggs were placed in a chamber seated in a heated stage, and containing 1 ml BSA-free hKSOM medium. The changes in the distribution of GFP-tagged proteins and Ca2+ induced by mPLCζ–luc or other reagents were monitored simultaneously at the equator of the cells using a confocal microscope (TCS SP5; Leica), under a ×20 (0.75 NA) lens. Excitation was provided by the 488-nm line of an argon laser and time series were acquired at a rate of 1 frame every 10 s. Confocal data were analysed as described previously (Halet et al., 2004), using ImageJ (http://rsb.info.nih.gov/ij/). In brief, regions of interest were drawn in the cytosol or around the plasma membrane, and changes in fluorescence intensity were measured during confocal time series. The value of the PM/C ratio (plasma membrane/cytosol ratio) was used as an index of membrane translocation.
Measurement of intracellular Ca2+ and luciferase expression
The imaging of Ca2+ oscillations induced by mPLCζ–luc and the quantification of mPLCζ–luc expression were carried out as previously described (Yu et al., 2008). Briefly, injected oocytes were placed in a chamber with hKSOM medium containing 100 μM luciferin, on the temperature-controlled stage of an inverted microscope. In some cases, injected eggs were zona-free and attached to the polylysine-coated glass bottom of the chamber in calcium-free hKSOM medium (Larman et al., 2004). Ca2+ oscillations were monitored by measuring the fluorescence of Oregon Green BAPTA dextran, and luciferase expression was monitored by the luminescence. These measurements were both carried out on the same sets of eggs using a Zeiss Axiovert S100 microscope with light from the stage directed towards a cooled ICCD camera [intensified CCD camera (charge-coupled-device camera)]. The microscope and ICCD camera were inside a custom-made dark box, and the data collection and analysis was carried out using software supplied with the camera, which was from Photek (http://www.photek.co.uk). In most experiments, the fluorescence was recorded first by exposing oocytes to excitation light (450–490 nm) and reducing the sensitivity of the ICCD camera to 10%, and then the luminescence was recorded by removing the excitation light and switching the ICCD camera to maximum sensitivity.
Ca2+ influx measurement
The Ca2+ influx rate in eggs at 2 or 4 h after injection of mPLCζ–luc or adenophostin was estimated by using Mn2+ as a surrogate for Ca2+ and measuring Mn2+ quenching as described by McGuinness et al. (1996) and Mohri et al. (2001). Briefly, eggs were loaded with fura 2/AM (fura 2 acetoxymethyl ester; Molecular Probes; 1 μM in M2 medium) supplemented with 0.02% pluronic acid (Molecular Probes) at 37°C for 30 min. After the zona pellucida was removed, these eggs were transferred to BSA-free hKSOM medium on a coverslip that had been precoated with polylysine (1 mg/ml) in a chamber. The chamber was mounted on the microscope stage and kept at ∼37°C with a heated plate. Eggs were imaged using a CCD camera (CoolSNAP-HQ2) and collected using Image Q software both supplied by Photometrics (http://www.photomet.co.uk). After the addition of 0.1 mM MnCl2, Mn2+ entry was monitored in parallel with changes in Ca2+ by imaging the resulting quench in fura 2 fluorescence emission (>520 nm) as it was excited alternately at 340, 360 and 380 nm. At 360 nm, fura 2 fluorescence is independent of Ca2+ and any decrease in fluorescence is due only to Mn2+ entry. A Student's t test was used for comparing Mn2+ quenching rates among batches of eggs. The final values are expressed as a ratio (mean±S.E.M.) and the significance of any difference are indicated.
Intracellular Ca2+ stores measurement
The relative content of the intracellular Ca2+ stores at 4 h after injection of mPLCζ–luc, or adenophostin, was measured as previously described (Jellerette et al., 2000; El-Jouni et al., 2005). Eggs were loaded with 1 μM fura 2/AM and placed in BSA and Ca2+-free hKSOM medium in a chamber as described above. Then, 20 μM thapsigargin plus 2 mM BAPTA was added to release Ca2+ from intracellular stores. Fura 2 fluorescence (380 nm) ratio [(Ft−F0)/Ft] across the whole imaging field was plotted over time. The amplitude of the Ca2+ rise elicited by thapsigargin, or the area under a line traced out by the elevated phase of the Ca2+ transient were taken as measures of relative thapsigargin-releasable Ca2+ store content. Data were collected from at least three different replicates. Values cited are the means and S.E.M. A Student's t test was used to compare the integral of Ca2+ rises between injected eggs and control eggs.
Acknowledgments
We are grateful to Tobias Meyer (Department of Chemical and Systems Biology, Stanford University, Stanford, CA, U.S.A.) for providing the plasmid encoding C12δ–GFP. This work was supported by BBSRC (Biotechnology and Biological Sciences Research Council) and Wellcome Trust grants awarded to K.S. and F.A.L.
References
- Baluch P.D., Koeneman B.A., Hatch K.R., McGuaghey R.W., Capco D.G. PKC isotypes in post-activated and fertilized mouse eggs: association with the meiotic spindle. Dev. Biol. 2004;274:45–55. doi: 10.1016/j.ydbio.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Brind S., Swann K., Carroll J. Inositol 1,4,5-trisphosphate receptors are downregulated in mouse oocytes in response to sperm and adenophostin A but not to an increase in intracellular Ca2+ or egg activation. Dev. Biol. 2000;223:251–265. doi: 10.1006/dbio.2000.9728. [DOI] [PubMed] [Google Scholar]
- Codazzi F., Teruel M.N., Meyer T. Control of astrocyte Ca2+ oscillations and waves by oscillating translocation and activation of protein kinase C. Curr. Biol. 2001;11:1089–1097. doi: 10.1016/s0960-9822(01)00326-8. [DOI] [PubMed] [Google Scholar]
- Cuthbertson K.S., Cobbold P.H. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+ Nature. 1985;316:541–542. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- Dries D.R., Gallegos L.L., Newton A.C. A single residue in the C1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. J. Biol. Chem. 2007;282:826–830. doi: 10.1074/jbc.C600268200. [DOI] [PubMed] [Google Scholar]
- Ducibella T., Lefevre L. Study of protein kinase C antogonists on cortical granule exocytosis and cell cycle resumption in fertilized mouse eggs. Mol. Reprod. Dev. 1997;46:216–226. doi: 10.1002/(SICI)1098-2795(199702)46:2<216::AID-MRD12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ducibella T., Schultz R.M., Ozil J.P. Role of calcium signals in early development. Semin. Cell Dev. Biol. 2006;17:324–332. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Eliyahu E., Shalgi R. A role for protein kinase C during rat egg activation. Biol. Reprod. 2002;67:189–195. doi: 10.1095/biolreprod67.1.189. [DOI] [PubMed] [Google Scholar]
- El-Jouni W., Jang B., Haun S., Machaca K. Calcium signaling differentiation Xenopus oocyte maturation. Dev. Biol. 2005;288:514–525. doi: 10.1016/j.ydbio.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Galione A., McDoughall A., Busa W.B., Willmott N., Gillot I., Whitaker M. Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea urchin eggs. Science. 1993;261:348–352. doi: 10.1126/science.8392748. [DOI] [PubMed] [Google Scholar]
- Gallicano G.I., McGaughey R.W., Capco D.G. Activation of protein kinase C after fertilization is required for remodeling the mouse egg into the zygote. Mol. Reprod. Dev. 1997;46:587–601. doi: 10.1002/(SICI)1098-2795(199704)46:4<587::AID-MRD16>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Halet G. PKC signalling at fertilization in mammalian eggs. Biochim. Biophys. Acta. 2004;1742:185–189. doi: 10.1016/j.bbamcr.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Halet G., Tunwell R., Parkinson S.J., Carroll J. Conventional PKCs regulate the temporal pattern of Ca2+ oscillations at fertilisation in mouse eggs. J. Cell Biol. 2004;164:1033–1044. doi: 10.1083/jcb.200311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igirashi H., Knott J.G., Schultz R.M., Williams C.J. Alterations of PLCβ1 in mouse eggs change calcium oscillatory behavior following fertilization. Dev. Biol. 2007;312:321–330. doi: 10.1016/j.ydbio.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellerette T., He C.L., Wu H., Parys J.B., Fissore R.A. Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev. Biol. 2000;223:238–250. doi: 10.1006/dbio.2000.9675. [DOI] [PubMed] [Google Scholar]
- Jones K.T. Protein kinase C action at fertilization: overstated or undervalued? Rev. Reprod. 1998;3:7–12. doi: 10.1530/ror.0.0030007. [DOI] [PubMed] [Google Scholar]
- Knott J.G., Kurokawa M., Fissore R.A., Schultz R.M., Williams C.J. Transgenic RNAi reveals role for mouse sperm phospholipase Cζ in triggering Ca2+ oscillations during fertilization. Biol. Reprod. 2005;72:992–996. doi: 10.1095/biolreprod.104.036244. [DOI] [PubMed] [Google Scholar]
- Knott J.G., Gardner A.J., Madgwick S., Jones K.T., Williams C.J., Schultz R. Calmodulin-dependent protein kinase II triggers mouse egg activation and embryo development in the absence of Ca2+ oscillations. Dev. Biol. 2006;296:388–395. doi: 10.1016/j.ydbio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Kouchi Z., Fukami K., Shikano T., Oda S., Nakamura Y., Takenawa T., Miyazaki S. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J. Biol. Chem. 2004;279:10408–10412. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- Kurakawa M., Sato K., Fissore R.A. Mammalian fertilization: from sperm factor to PLCzeta. Biol. Cell. 2004;96:37–45. doi: 10.1016/j.biolcel.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Larman M.G., Saunders C.M., Carroll J., Lai F.A., Swann K. Cell cycle-dependent Ca2+ oscillations in mouse embryos are regulated by nuclear targeting of PLCζ. J. Cell Sci. 2004;117:2513–2521. doi: 10.1242/jcs.01109. [DOI] [PubMed] [Google Scholar]
- Lee B., Yoon S.Y., Fissore R.A. Regulation of fertilization-initiated [Ca2+]i oscillations in mammalian eggs: a multi-pronged approach. Semin. Cell Dev. Biol. 2006;17:274–284. doi: 10.1016/j.semcdb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Luria A., Tennenbaum T., Sun Q.Y., Rubinstein S., Breitbart H. Differential localization of conventional protein kinase C isoforms during mouse oocyte development. Biol. Reprod. 2000;62:1564–1570. doi: 10.1095/biolreprod62.6.1564. [DOI] [PubMed] [Google Scholar]
- Madgwick S., Levasseur M., Jones K.T. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J. Cell Sci. 2005;118:3849–3859. doi: 10.1242/jcs.02506. [DOI] [PubMed] [Google Scholar]
- Malcuit C., Knott J.G., He C., Wainwright T., Parys J.B., Robl J.M., Fissore R.A. Fertilization and inositol 1,4,5-trisphosphate (IP3) induced calcium release in Type-1 inositol 1,4,5-trisphosphate receptor down regulated bovine eggs. Biol. Reprod. 2005;73:2–13. doi: 10.1095/biolreprod.104.037333. [DOI] [PubMed] [Google Scholar]
- McGuinness O.M., Moreton R.B., Johnson M.H., Berridge M.J. A direct measurement of increased divalent cation influx in fertilised mouse oocytes. Development. 1996;122:2199–2206. doi: 10.1242/dev.122.7.2199. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Ito M. Calcium signals for egg activation in mammals. J. Pharm. Sci. 2006;100:545–552. doi: 10.1254/jphs.cpj06003x. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Shirakawa H., Nakada K., Honda Y. Essential role of the inositol 1,4,5-trisphosphate/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev. Biol. 1993;58:62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- Mohri T., Shirakawa H., Oda S., Sato M.S., Mikoshiba K., Miyazaki S. Analysis of Mn2+/Ca2+ influx and release during Ca2+ oscillations in mouse eggs injected with sperm extracts. Cell Calcium. 2001;29:311–325. doi: 10.1054/ceca.2000.0196. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nomikos M., Blayney L.M., Larman M.G., Campbell K., Rossbach A., Saunders C.M., Swann K., Lai F.A. Role of phospholipase C-{zeta} domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J. Biol. Chem. 2005;280:31011–31018. doi: 10.1074/jbc.M500629200. [DOI] [PubMed] [Google Scholar]
- Pauken C.M., Capco D.G. The expression and stage specific localization of protein kinase C isotypes during mouse preimplantation development. Dev. Biol. 2000;223:411–421. doi: 10.1006/dbio.2000.9763. [DOI] [PubMed] [Google Scholar]
- Saunders C.M., Larman M.G., Parrington J., Cox L.J., Royse J., Blayney L.M., Swann K., Lai F.A. PLCζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Shirakawa H., Ito M., Sato M., Uezawa Y., Miyazaki S. Measurement of intracellular IP3 during Ca2+ oscillations in mouse eggs with GFP based FRET probe. Biochem. Biophys. Res. Commun. 2006;345:781–788. doi: 10.1016/j.bbrc.2006.04.133. [DOI] [PubMed] [Google Scholar]
- Stricker S.A. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- Swann K., Saunders C.M., Rogers N., Lai F.A. PLCζ(zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin. Cell Dev. Biol. 2006;17:264–273. doi: 10.1016/j.semcdb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Tatone C., Dell Monache S., Francione A., Gioia L., Barboni B., Colonna R. Ca2+-independent protein kinase C signaling in mouse eggs during the early phase of fertilization. Int. J. Dev. Biol. 2003;47:327–333. [PubMed] [Google Scholar]
- Vermassen E., Fissore R.A., Nadel Kasri N., Vanderheyden V., Callewart G., Missiaen L., Parys J.B., DeSmedt H. Regulation of the phosphorylation of the inositol 1,4,5-trisphosphate receptor by protein kinase C. Biochem. Biophys. Res. Commun. 2004;319:888–893. doi: 10.1016/j.bbrc.2004.05.071. [DOI] [PubMed] [Google Scholar]
- Viveiros M.M., O'Brien M., Wigglesworth K., Eppig J.J. Characterization of protein kinase C-δ in mouse oocytes throughout meiotic maturation and following egg activation. Biol. Reprod. 2003;69:1494–1499. doi: 10.1095/biolreprod.103.019018. [DOI] [PubMed] [Google Scholar]
- Yu B.Z., Fu W., Su W.H., Yu D.H., Zhang Z., Feng C. Effects of PKCzeta on early genome transcription activation in mouse 1-cell stage fertilized eggs. Cell Biochem. Funct. 2007;25:619–624. doi: 10.1002/cbf.1357. [DOI] [PubMed] [Google Scholar]
- Yu Y., Saunders C.M., Lai F.A., Swann K. Preimplantation development of mouse oocytes activated by different levels of human phospholipase Czeta. Human Reproduction. 2008;23:365–373. doi: 10.1093/humrep/dem350. [DOI] [PubMed] [Google Scholar]