Abstract
Purpose
Renal cell carcinoma (RCC) is rare in patients <40 years old and conflicting data regarding presentation and outcome are present in the literature. We reviewed our experience with young RCC patients comparing them to older counterparts.
Methods
We identified 1,720 patients 18-79 years old managed with partial or radical nephrectomy for RCC between 1989 and 2005. Patients were grouped according to age and outcome analyses were conducted.
Results
Among the 1,720 RCC patients, there were 89 (5%), 672 (39%), and 959 (56%) patients aged <40, 40-59, and 60-79 years old, respectively. There were no significant differences in sex, tumor size, TNM stage, or multifocality by age group. However, patients <40 years old were significantly more likely to present with symptomatic tumors (p=0.028). Additionally, there were significant differences in histology by age (p<0.001); chromophobe histology decreased while papillary histology increased with age. Despite similar tumor sizes in each age group, the percentage of patients treated with partial nephrectomy declined with age; 49% of patients <40 years old were treated with partial nephrectomy compared with 35% and 30% of patients aged 40-59 and 60-79 years old, respectively (p<0.001). With a median follow-up of 2.6 years (range 0-14.5), we did not observe a significant difference in cancer-specific survival according to age (p=0.17).
Conclusions
Younger RCC patients are more likely to have symptomatic tumors with chromophobe histology although prognosis appears similar across age groups. Older patients are more likely to be treated with radical nephrectomy and this requires careful scrutiny for current clinical practice.
Keywords: Carcinoma, renal cell; Kidney neoplasms; Age factors; Adenoma, chromophobe
INTRODUCTION
The vast majority of patients treated surgically for suspected renal cell carcinoma (RCC) are over the age of 40. In fact, only 5% of all RCC patients present before the age of 40 while median age at diagnosis remains between 60 and 65 years old.1, 2 Thus, observations of younger patients with RCC are limited with approximately 700 patients <40 years old reported in the current literature.
Among studies that included >50 patients under the age of 40 and compared pathologic features and outcome with older counterparts, the older comparison group consisted of patients aged 58-61,3 60-70,1 and 40-90 years old.2 Despite the various older age groups used for comparison, collectively these previous observations suggest that younger patients (<40 years old) are more likely to harbor tumors with a lower primary tumor classification (T stage) and tended to have an improved progression-free or cancer-specific survival compared with the aforementioned older patients.1-3 However, there remains conflicting data on a number of important characteristics with regards to presentation and outcome. First, younger patients were significantly more likely to have node-positive disease according to MD Anderson data,3 whereas the Mayo Clinic reported no significant difference.1 In contrast, the group from France reported that younger patients were significantly less likely to have node-positive or metastatic disease at presentation.2 Additionally, the group from Mayo Clinic reported that younger patients are significantly more likely to have chromophobe histology,1 while the group from France suggested that younger patients were significantly more likely to have papillary histology,2 and the group from MD Anderson reported no overall difference in histology between young and old patients, although younger patients were more likely to have sarcomatoid features.3 Collectively, these discordant findings suggest that further data is warranted. In this report, we evaluate our surgical management of younger patients with renal cell carcinoma (RCC) compared with older patients. As an older comparison group, we did not see a strong rationale for choosing one of the previously reported age ranges (i.e. 58-61, 60-70, 41-90) over another, so we elected to compare patients <40 years old to groups of patients aged 40-60 and 60-80.
MATERIALS AND METHODS
Patient Identification
After obtaining Institutional Board Review, we accessed the Memorial Sloan-Kettering kidney cancer database. This database is prospectively maintained and includes all patients treated surgically for a renal mass at our institution beginning in 1989. From this database, we identified 2,006 patients >18 and <80 years old without a known syndrome who underwent partial or radical nephrectomy at our institution between 1989 and 2005. Additionally, 29 patients with missing pathologic features were excluded leaving 1,977 patients available for analysis.
Clinical and Pathologic Features
The clinical features studied included age at surgery, sex, and symptoms at presentation. Patients with palpable abdominal mass, flank discomfort, gross hematuria, or acute onset varicocele were considered to have local symptoms at presentation. Patients with constitutional symptoms fatigue, fevers, night sweats, or unintentional weight loss were considered to have systemic symptoms at presentation. Additionally, type of surgery (radical vs partial nephrectomy) was also studied.
The pathologic features studied included histology, tumor size, primary tumor classification, regional lymph node status, presence of metastasis, ipsilateral multifocality, and the presence of bilateral synchronous tumors at presentation.
Statistical Analysis
Clinical and pathologic features were compared between the age groups using Chi-square or Fisher's exact test when appropriate, and Kruskal-Wallis test for continuous variables. Cancer-specific survival was estimated using the Kaplan-Meier method. For patients who had documented metastatic disease following nephrectomy and died of unknown causes were considered to have died from RCC. When evaluating cancer-specific survival, patients who died from unknown causes and did not have documented metastatic disease (n=36, 2%) were censored. Follow-up duration was calculated from the date of surgery to the date of death or last follow-up. The association between age as a categorical variable and cancer-specific survival were evaluated via the log-rank test, while age was also assessed as a continuous variable using Cox proportional hazards regression models and summarized with a hazard ratio (HR) and 95% confidence interval (CI). The associations of age with cancer-specific survival was evaluated univariately and in a multivariate analysis adjusting for symptoms at presentation, pathologic stage, histology, and type of surgery (radical vs. partial). Clinical Outcome analyses were conducted only on patients with RCC (i.e. patients with benign histology were excluded).
RESULTS
All Renal Mass Patients
Among the 1,977 patients included in this study, 102 were <40, 765 were 40-59, and 1,110 were 60-79 years old. After pathologic review, 244 (12.3%) patients had benign tumors (Table 1). The frequency of benign histology was not significantly different among the age groups as benign tumors were noted in 9%, 12%, and 13% of patients <40, 40-59, and 60-79, respectively (p=0.44). However, younger patients were significantly less likely to have oncocytoma which was present in 44%, 60%, and 85% of patients <40, 40-59, and 60-79 years old with benign histology (p<0.001). Malignant histology was present in 1,733 patients including 1,720 (99%) patients who had either clear cell, papillary, chromophobe, collecting duct, multilocular cystic RCC, or RCC not-otherwise specified.
Table 1.
Histology for patients with benign renal tumors treated surgically. Data are given as No. (%)
| Histology | <40 yrs old (n=9) |
40 - 59 yrs old (n=88) |
60 - 79 yrs old (n=147) |
|---|---|---|---|
| Oncocytoma | 4 (44) | 53 (60) | 125 (85) |
| Angiomyolipoma | 1 (11) | 13 (15) | 10 (7) |
| Benign cystic tumor | 2 (22) | 9 (10) | 6 (4) |
| Metanephric adenoma | 2 (22) | 5 (6) | 1 (1) |
| Mixed epithelial-stromal tumor | 0 | 3 (3) | 1 (1) |
| Other | 0 | 5 (6) | 4 (3) |
Patients with RCC
Among the 1,720 patients with RCC, 89 (5%), 672 (39%), and 959 (56%) were <40, 40-59, and 60-79 years old. Clinical and pathologic features for these age groups are detailed in Table 2. There were no statistically significant differences in sex, tumor size, primary tumor classification, lymph node involvement, distant metastases at nephrectomy, ipsilateral multifocality, or bilateral synchronous tumors between the three age groups (Table 2). However, patients <40 years old were significantly more likely to present with symptomatic tumors compared with patients aged 40-59 or patients aged 60-79 (p=0.028). Symptoms related to the tumor (i.e. local symptoms) were present in 42%, 26%, and 26% of patients <40, 40-59, and 60-79 years old, respectively; however, systemic/constitutional symptoms at presentation were similar across the age groups and noted in 6%, 5%, and 6% of patients <40, 40-59, and 60-79 years old, respectively. Additionally, there were significant differences in histology among the RCC patients by age (p<0.001). Specifically, patients <40 years old (17%) were more likely to have chromophobe histology compared with patients aged 40-59 years old (13%) and patients aged 60-79 years old (8%). In contrast, the frequency of papillary histology increased with age, present in 7%, 13%, and 16% of patients aged <40, 40-59, and 60-79 years old, respectively. The presence of clear cell RCC was similar across the age groups; 69%, 70%, and 72% of patients aged <40, 40-59, and 60-79 years old, respectively.
Table 2.
Clinical and pathologic features by age group for patients treated surgically for renal cell carcinoma. Data are given as No. (%) unless otherwise noted.
| Feature | <40 yrs old (n=89) |
40 - 59 yrs old (n=672) |
60 - 79 yrs old (n=959) |
p-value |
|---|---|---|---|---|
| Median Age (Range) | 35 (22 - 39) | 53 (40 - 59) | 69 (60 - 79) | - |
| Sex: | 0.222 | |||
| Female | 39 (44) | 238 (35) | 332 (35) | |
| Male | 50 (56) | 434 (65) | 627 (65) | |
| Local Symptoms at presentation | 37 (42) | 175 (26) | 248 (26) | <0.001 |
| Systemic symptoms at presentation | 5 (6) | 38 (6) | 52 (5) | <0.001 |
| Surgery type: | <0.001 | |||
| Radical nephrectomy | 45 (51) | 439 (65) | 672 (70) | |
| Partial nephrectomy | 44 (49) | 233 (35) | 287 (30) | |
| Median tumor size cm (IQ Range) |
4.3 (2.5, 7.1) | 4.5 (3.0, 7.5) | 4.5 (2.9, 7.2) | 0.744 |
| 2002 primary tumor classification: | 0.668 | |||
| pT1 | 57 (64) | 419 (62) | 589 (61) | |
| pT2 | 11 (12) | 75 (11) | 92 (10) | |
| pT3 | 20 (23) | 172 (26) | 263 (27) | |
| pT4 | 1 (1) | 6 (1) | 15 (2) | |
| Positive lymph nodes | 6 (7) | 20 (3) | 26 (3) | 0.104 |
| Distant metastases | 3 (3) | 49 (7) | 69 (7) | 0.381 |
| Histology: | <0.001 | |||
| Clear cell | 61 (69) | 469 (70) | 689 (72) | |
| Papillary | 6 (7) | 90 (13) | 155 (16) | |
| Chromophobe | 15 (17) | 85 (13) | 75 (8) | |
| Collecting Duct | 0 | 2 (0.2) | 0 | |
| Multilocular cystic RCC | 3 (3) | 3 (0.4) | 6 (1) | |
| RCC, not otherwise specified | 4 (4) | 23 (3) | 34 (4) | |
| Multifocality | 4 (4) | 38 (6) | 69 (7) | 0.331 |
| Bilateral synchronous | 0 | 22 (3) | 29 (3) | 0.228 |
Interestingly, despite similar tumor sizes, the percentage of patients treated with partial nephrectomy declined with age (p<0.001); 49% of patients <40 years old received a partial nephrectomy compared with patients aged 40-59 years old (35%) and 60-79 years old (30%). However, the frequency of partial nephrectomy increased in each age group with time. For example, between the years 1990-1994, 1995-1999, and 2000-2005, the frequency of partial nephrectomy for patients <40 years old was 19%, 28%, and 76%, respectively. Similarily, the frequency of partial nephrectomy for patients 60-79 years old during the same time periods was 6%, 17%, and 46%, respectively. For the subset of patients found to have a benign renal mass, the frequency of partial nephrectomy also appeared to decline with age; 67% (6/9) of patients <40 years old received a partial nephrectomy compared with 51% (45/88) of patients 40-59 years old and 48% (71/47) of patients 60-79 years old.
Cancer-Specific Survival for RCC Patients
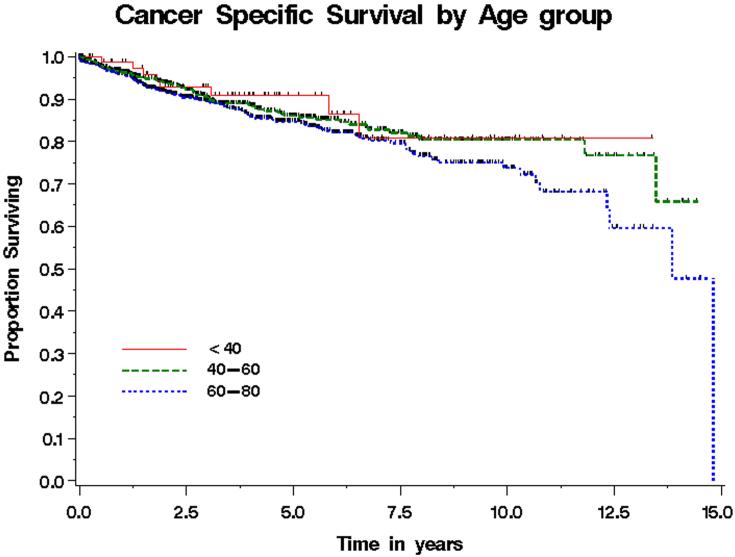
Among the RCC patients <40, 40-59, and 60-79 years old, 8 (9%), 69 (11 %), and 119 (13%) died from RCC, respectively. Median follow-up for patients still alive at last follow-up was 2.6 years (range 0 - 14) for all patients and was 3.6 years (range 0 - 13), 2.8 years (range 0 - 14), and 2.3 years (range 0 - 14) for patients <40, 40-59, and 60-79 years old, respectively. Cancer-specific survival for patients with RCC by age group is shown in Figure 1. Estimated cancer-specific survival rates at 5 years for patients <40 years old were 91% compared with 86% for patients 40-59 years old and 85% for patients 60-79 years old (p=0.17 per log-rank test). We also evaluated age as a continuous variable in a univariate Cox model, and age remained not statistically significantly associated with cancer-specific survival (HR 1.13, 95% CI (0.99-1.28) for 10 year increase in age, p=0.07). In a multivariate model adjusting for symptoms at presentation, histology, stage, and type of surgery, age as a continuous variable was not significantly associated with death from disease (HR 1.01, 95% CI .996 - 1.022, p=0.186).
Figure 1.
Cancer-specific survival by age for patients treated surgically for renal cell carcinoma. Estimated cancer-specific survival rates at 5 years were 91%, 86%, and 85% for patients <40, 40-59, and 60-79 years old, respectively (p=0.17).
DISCUSSION
Approximately 5% of RCC patients present before the age of 40 and the literature is limited to nearly 700 patients described with this entity. In this report, we describe our experience with surgical management of renal cortical tumors in 102 patients <40 years old including 89 patients with RCC. Consistent with previous observations from Gillett et al, our data suggest that younger RCC patients are more likely to have chromophobe histology and for patients with benign tumors, younger patients are less likely to have oncycytoma.1 We also demonstrate that younger patients are more likely to be symptomatic at presentation despite having similar size tumors compared with older counterparts, which is also supported by previous observations.1-3 Among the eight published retrospective comparisons between RCC patients <40 years old and older patients, five included all patients >40 years old,2, 4-7 while the remaining three studies included patients 58-61,3 60-70,1 and >79 years old.8. In this study, we compared patients <40 years old to patients 40-59 and 60-79 years old and further evaluated age as a continuous variable. Our results do not support that cancer-specific survival is either better or worse for younger RCC patients. Additionally, we did not observe an association between age and cancer-specific survival when age was evaluated as a continuous variable.
Interestingly, our data demonstrate that younger patients were more likely to be treated with partial nephrectomy compared with older counterparts. This is true despite the fact that all three age groups had similar size tumors (median 4.3-4.5 cm). In fact, nearly 50% of younger patients were treated with a nephron-sparing approach over the 16-year time-period compared with 35% and 30% of patients 40-59 and 60-79 years old, respectively. Additionally, when evaluating the subset of patients found to have benign histology, younger patients were also more likely to be treated with partial nephrectomy. Previous observations suggest that chronic kidney disease is increased for patients treated with radical compared with partial nephrectomy9 and recent results from our institution confirm that new onset chronic kidney disease is significantly higher for pT1a patients managed with radical compared with partial nephrectomy.10 Moreover, recent data from the Mayo Clinic suggest that overall survival is compromised for younger patients treated with radical compared with partial nephrectomy.11 This impact on survival may relate to surgically induced renal dysfunction as Go and colleagues12 have reported an independent and graded association between reduced glomerular filtration rate and risk of cardiovascular events, hospitalization, and death. Since older patients are more likely to have baseline renal dysfunction, we have now altered our surgical approach to ensure we are not placing them at undue risk of chronic kidney disease. Nevertheless, our rate of partial nephrectomy in the current series for patients >60 years old (30%) is higher than that reported by other institutions1, 3 and is significantly higher than what is observed in the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program (7%).13 Additionally, our rate of partial nephrectomy in patients <40 years old is also higher than that reported by other institutions.1, 3, 14, 15
In other malignancies such as breast cancer, observations suggest that patients <40 years old fare worse with respect to cancer-specific survival compared with older patients.16, 17 However, this does not appear to be the case in RCC. Our results suggest no significant difference in cancer-specific survival between young and old patients which is consistent with previous observations.1, 4, 14 Other groups have reported a significantly improved cancer-specific survival for young RCC patients.2, 3, 6 Additionally, to our knowledge, no group has observed a significantly worse cancer-specific survival in young adult RCC patients compared with older counterparts.2 Thus, these collective observations strongly suggest that RCC in younger adult patients does not behave biologically in a more aggressive fashion.
This study is not without limitations and our data is subject to many of the inherent biases of a retrospective review. The database we used includes patients who were treated surgically at our institution; thus, our results may not be reflective of all patients with renal cell carcinoma. For example, a patient with widely metastatic disease at presentation or significant medical comorbidites may not have been offered nephrectomy and thus, would have been excluded from our analyses. Additionally, referral bias to our tertiary care facility may partially explain some of our observed differences according to age. Furthermore, we did not observe a difference in cancer-specific survival between the arbitrary pre-determined age groups; however, with only 8 events in the <40 years old age group, we had limited power to detect a statistically significant difference. In order to account for this limited power, we evaluated age as a continuous variable and our results support, but do not confirm, that age is not an independent prognostic factor. Additionally, our results may not apply to pediatric (<18 years old) RCC where presentation at advanced stage with compromised outcomes have been observed.18
CONCLUSION
Younger RCC patients are more likely to have symptomatic tumors with chromophobe histology although prognosis appears similar across age groups. Older patients are more likely to be treated with radical nephrectomy and this requires careful scrutiny for current clinical practice.
ACKNOWLEGDEMENTS
This project was supported in part by generous gifts from the Stephen Hanson Family Fellowship. This project was also funded by NIH T32-CA82088.
REFERENCES
- 1.Gillett MD, Cheville JC, Karnes RJ, Lohse CM, Kwon ED, Leibovich BC, et al. Comparison of presentation and outcome for patients 18 to 40 and 60 to 70 years old with solid renal masses. J Urol. 2005;173:1893. doi: 10.1097/01.ju.0000158157.57981.80. [DOI] [PubMed] [Google Scholar]
- 2.Taccoen X, Valeri A, Descotes JL, Morin V, Stindel E, Doucet L, et al. Renal cell carcinoma in adults 40 years old or less: young age is an independent prognostic factor for cancer-specific survival. Eur Urol. 2007;51:980. doi: 10.1016/j.eururo.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Ortiz RF, Rosser CJ, Madsen LT, Swanson DA, Wood CG. Young age is an independent prognostic factor for survival of sporadic renal cell carcinoma. J Urol. 2004;171:2160. doi: 10.1097/01.ju.0000125487.96469.2e. [DOI] [PubMed] [Google Scholar]
- 4.Goetzl MA, Desai M, Mansukhani M, Goluboff ET, Katz AE, Sawczuk IS, et al. Natural history and clinical outcome of sporadic renal cortical tumors diagnosed in the young adult. Urology. 2004;63:41. doi: 10.1016/j.urology.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez A, Patard JJ, Lobel B. Renal cell carcinoma in young adults: incidence, disease outcome and review of the literature. Arch Esp Urol. 2002;55:969. [PubMed] [Google Scholar]
- 6.Schiff M, Jr., Herter G, Lytton B. Renal adenocarcinoma in young adults. Urology. 1985;25:357. doi: 10.1016/0090-4295(85)90486-8. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez Gomez JM, Rabade Rey CJ, Perez Garcia FJ, Sahagun Anguello JL, Martinez Gomez FJ, Alonso Sainz F. Renal adenocarcinoma in young patients. Actas Urol Esp. 1997;21:22. [PubMed] [Google Scholar]
- 8.Rainwater LM, Zincke H, Farrow GM, Gonchoroff NJ. Renal cell carcinoma in young and old patients. Comparison of prognostic pathologic variables (cell type, tumor grade and stage, and DNA ploidy pattern) and their impact on disease outcome. Urology. 1991;38:1. doi: 10.1016/0090-4295(91)80002-o. [DOI] [PubMed] [Google Scholar]
- 9.Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75:1236. doi: 10.4065/75.12.1236. [DOI] [PubMed] [Google Scholar]
- 10.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468. doi: 10.1016/j.juro.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 13.Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2007;112:511. doi: 10.1002/cncr.23218. [DOI] [PubMed] [Google Scholar]
- 14.Eggener SE, Rubenstein JN, Smith ND, Nadler RB, Kontak J, Flanigan RC, et al. Renal tumors in young adults. J Urol. 2004;171:106. doi: 10.1097/01.ju.0000099028.95679.52. [DOI] [PubMed] [Google Scholar]
- 15.Abou El Fettouh HI, Cherullo EE, El-Jack M, Al Maslamani Y, Novick AC. Sporadic renal cell carcinoma in young adults: presentation, treatment, and outcome. Urology. 2002;60:806. doi: 10.1016/s0090-4295(02)01884-8. [DOI] [PubMed] [Google Scholar]
- 16.Chung M, Chang HR, Bland KI, Wanebo HJ. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer. 1996;77:97. doi: 10.1002/(SICI)1097-0142(19960101)77:1<97::AID-CNCR16>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.de la Rochefordiere A, Asselain B, Campana F, Scholl SM, Fenton J, Vilcoq JR, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039. doi: 10.1016/0140-6736(93)92407-k. [DOI] [PubMed] [Google Scholar]
- 18.Aronson DC, Medary I, Finlay JL, Herr HW, Exelby PR, La Quaglia MP. Renal cell carcinoma in childhood and adolescence: a retrospective survey for prognostic factors in 22 cases. J Pediatr Surg. 1996;31:183. doi: 10.1016/s0022-3468(96)90344-9. [DOI] [PubMed] [Google Scholar]