Abstract
RNA editing that converts adenosine to inosine replaces the gene-encoded Ile, Asn, and Ile (INI) of serotonin [5-hydroxytryptamine (5-HT)] receptor 2C (5-HT2CR) with Val, Gly, and Val (VGV). Up to 24 different 5-HT2CR isoforms are detected in different brain regions (Burns et al., 1997; Fitzgerald et al., 1999; Wang et al., 2000). To elucidate the physiological significance of 5-HT2CR mRNA editing, we derived mutant mouse lines harboring a knock-in INI or VGV allele, resulting in sole expression of one of two extremely different editing isoforms 5-HT2CR-INI (editing blocked) or -VGV (fully edited). Although INI mice grew normally, VGV mice had a severely reduced fat mass, despite compensatory hyperphagia, as a result of constitutive activation of the sympathetic nervous system and increased energy expenditure. Furthermore, serotonergic neurotransmission was oversensitized in VGV mice, most likely because of the increased cell surface expression of VGV receptors. Melanocortin 4 receptor (MC4R) regulates energy homeostasis (Balthasar et al., 2005; Heisler et al., 2006; Lam et al., 2008), and Mc4r−/− mice are obese because of hyperphagia and reduced energy expenditure (Huszar et al., 1997). However, the elevated energy expenditure of VGV mice could not be rescued in the Mc4r−/− background, indicating the presence of a distinct signaling pathway mediated via 5-HT2CR-VGV that dominates the MC4R-dependent pathway in control of energy expenditure. Our results highlight the importance of regulated 5-HT2CR mRNA editing, because dysregulation could result in the pathological consequences such as growth retardation seen in VGV mice.
Keywords: RNA editing, ADAR, serotonin receptor 2C, energy homeostasis, melanocortin 4C receptor, obesity
Introduction
One type of RNA editing involves the conversion of adenosine residues into inosine (A→I RNA editing) in double-stranded RNA (dsRNA) through the action of adenosine deaminases acting on RNA (ADAR) (Bass, 2000; Nishikura, 2006). Because the translation machinery reads inosines as if they were guanosines, when it occurs within the coding sequence, RNA editing could result in the synthesis of proteins not directly encoded by the genome. A→I RNA editing is involved in alterations of neurotransmitter receptor and channel functions (Reenan, 2001; Greger et al., 2002; Seeburg and Hartner, 2003; Ohlson et al., 2007; Rula et al., 2008) as well as modulation of microRNA expression levels and target selection (Yang et al., 2006; Kawahara et al., 2007a,b, 2008).
Serotonin [5-hydroxytryptamine (5-HT)] receptor 2C (5-HT2CR) is detected exclusively in the CNS and plays roles in various physiological and behavioral processes. Increased anxiety (Heisler et al., 2002), hyperlocomotion (Nonogaki et al., 2003), and adult-onset hyperphagia and obesity (Tecott et al., 1995) have been reported with 5-HT2CR-null mice, confirming the importance of 5-HT2CR in the regulation of emotion, locomotion, and appetite and metabolic rate control. A→I RNA editing occurs at five sites (A, B, C, D, and E sites) located in exon 5 of 5-HT2CR pre-mRNAs (see Fig. 1A) (Burns et al., 1997). Combinatorial editing at the five sites would change three amino acids I156, N158, and I160 (INI) located at the intracellular II region consisting of the G-protein coupling domain of the receptor (Burns et al., 1997; Fitzgerald et al., 1999; Wang et al., 2000). An imperfect dsRNA structure consisting of exon 5 and the downstream intron 5 sequence of 5-HT2CR pre-mRNA is essential for editing (see Fig. 1A) (Burns et al., 1997; Wang et al., 2000). Previous studies conducted in vitro in transfected non-neuronal cells indicated that RNA editing may modulate receptor functions such as 5-HT potency, agonist binding affinity, constitutive activities, and types of Gα subunits to be coupled. The fully edited VGV isoform receptor exhibited the most prominent alterations in G-protein coupling functions compared with the completely unedited INI receptor (Burns et al., 1997; Fitzgerald et al., 1999; Niswender et al., 1999; Wang et al., 2000). Furthermore, RNA editing changes the cell surface expression of the 5-HT2CR by affecting the intracellular trafficking efficacy (Marion et al., 2004).
Figure 1.
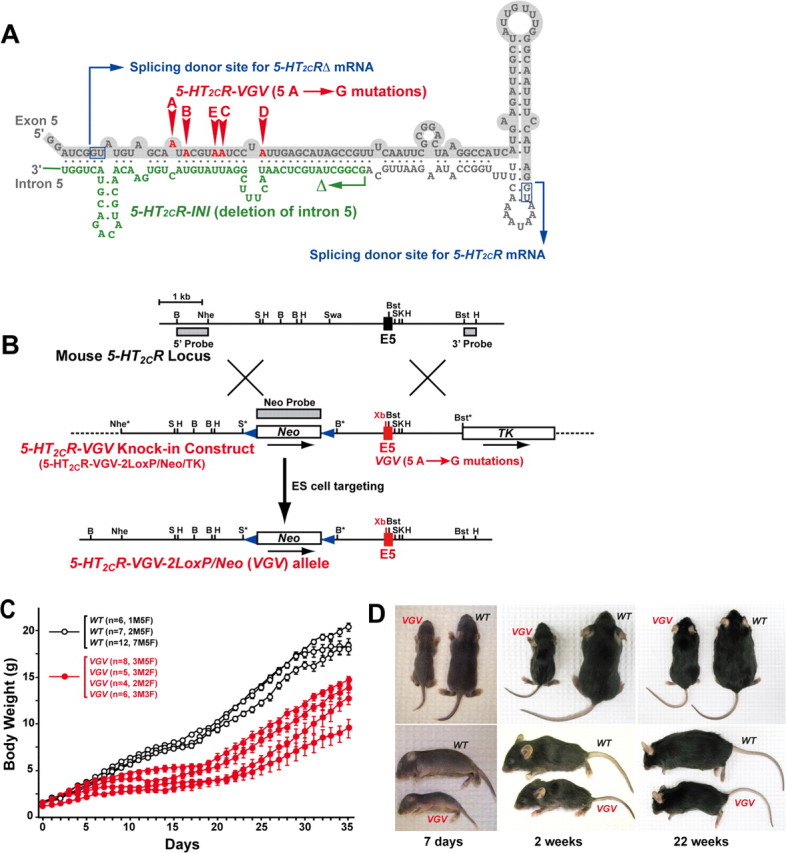
Generation of the VGV mutant mice with reduced body weight. A, In the 5-HT2CR-VGV knock-in vector, adenosine residues were substituted for guanosine at sites A–E, whereas a part of the intron 5 sequence (highlighted in green) was deleted in the 5-HT2CR-INI vector. A dsRNA structure involving exon 5 (lightly shaded) and intron 5 of 5-HT2CR pre-mRNA is essential for site-selective editing. Two GU splicing donor sites, which lead to alternative splicing of the C terminus truncated, nonfunctional 5-HT2CRΔ mRNA or full-length and functional 5-HT2CR mRNA (Wang et al., 2000), are indicated in blue boxes. B, The 5-HT2CR allele, encompassing intron 4, exon 5, and intron 5, the knock-in vector, and steps leading to creation of the VGV allele are shown. Three probes used for identification of allele-specific recombination and two LoxP sequences (blue triangles) are indicated. C, Growth rates of separate litters of wild-type and VGV mice consisting of hemizygous male and homozygous female mice were followed. Error bars indicate SEM. D, Appearance of VGV mice (C57BL/6J) at different postnatal stages. Milk was detected in stomach of VGV mice at 7 d.
To evaluate the significance of 5-HT2CR mRNA editing in vivo, the development of mutant mouse lines harboring a knock-in allele that results in sole expression of a single 5-HT2CR isoform is needed. Here, we derived two mutant mouse lines that resulted in sole expression of two extremely different editing isoforms, one editing blocked (INI) and the other fully edited (VGV). Phenotypic analysis of these mouse lines, INI and VGV, revealed the importance of the tight control of the 5-HT2CR mRNA editing, because overediting of 5-HT2CR mRNAs, represented in VGV mice, results in oversensitization of serotonergic neurotransmission and inappropriately elevated energy expenditure, and consequent growth retardation and reduced fat mass accumulation.
Materials and Methods
Mice.
Mice were maintained on a 12 h light/dark cycle at a temperature of 22 ± 1°C with a humidity of 60–70%. All procedures involving mice were approved by the Institutional Care and Use Committee of the Wistar Institute and performed in accordance with the National Institutes of Health guidelines.
Generation of VGV and INI mice.
Inosine is interpreted as guanosine by the translational machinery. In the 5-HT2CR-VGV knock-in vector, five A→G mutations at sites A–E were introduced, whereas the editing complementary sequence in intron 5 (160 bp) was deleted in the 5-HT2CR-INI targeting vector (see Fig. 1A). A silent XbaI site created upstream of the editing sites in exon 5 facilitated screening of the mutated allele in ES cells and knock-in mice (see Fig. 1B). ES cell clones selected for G418 resistance and ganciclovir sensitivity were screened by Southern hybridization analysis using three probes specific for the 5′ and 3′ regions of the 5-HT2CR gene and the Neo gene (see Fig. 1B) or by PCR using a set of primers: the intron 4 primer, 5′-ACTGATACTACCATCACTGG-3′, and the intron 5 primer, 5′-AGCATATATAGGAAATTGCAGTAACCCT-3′, followed by XbaI digestion. By using one of the three positive ES clones identified, germ-line transmitting male founder chimeric mice were generated, which led to establishment of VGV and INI alleles in mice. The 5-HT2CR gene is X-linked, and thus hemizygous male and heterozygous and homozygous female mutant mice in both C57BL/6J and BALB/c genetic backgrounds (more than six times backcrossed) were used for phenotypic analysis.
Drugs. Disodium (RR)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]propyl]-1,3-benzodioxazole-2,2-dicarboxylate (CL316243), 1-(m-chlorophenyl)-piperazine (mCPP), 6-chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]-indoline (SB242084), ketanserin, N-(1-methyl-1H-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea (SB204741), haloperidol, R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride [R(+)-SCH23390], S(−)-eticlopride, spiperone, mianserin, and (±)-8-hydroxy-2-(di-n-propylamino)tetralin hydrobromide (8-OH-DPAT) were obtained from Sigma-Aldrich. 6-Chloro-2-(1-piperazinyl)pyrazine hydrochloride (MK212) and (aS)-6-chloro-5-fluoro-a-methyl-1H-indole-1-ethanamine fumarate (Ro60-0175) were obtained from Tocris Bioscience. SB242084 was dissolved in 8% 2-hydroxypropl-β-cyclodextrin (Sigma-Aldrich). Ketanserin, SB204741, and haloperidol were dissolved in DMSO. Spiperone was dissolved in ethanol, and others were dissolved in saline.
Blood chemistries.
Whole blood was collected from the heart and kept at room temperature for 1 h. After centrifugation at 8000 rpm for 10 min, the serum was transferred to new tubes and kept at −80°C until use. Serum cholesterol and triglyceride levels were measured by Ani-Lytics. Serum hormone levels were measured by the University of Pennsylvania Diabetes Center Core Service Facilities. Serum insulin-like growth factor-1 (IGF-1), growth hormone (GH), insulin, leptin, corticosterone, and thyroxine (T4) were measured using ELISA kits [IGF-1 and GH (Diagnostic Systems Laboratories); insulin (ALPCO); leptin and corticosterone (Linco Research); T4 (MP Biomedicals)]. Serum active ghrelin was measured using radioimmunoassay kit (Linco Research) by adding 1N HCl at a final concentration of 0.1N immediately after plasma separation according to the manufacturer's instruction. Blood glucose levels in tail vein bleeds were measured using OneTouch Ultra (LifeScan).
Metabolic rate and activity measurements.
Oxygen consumption rate was measured by indirect calorimetry using a four-chamber Oxymax system (Columbus Instruments). Motor activity was simultaneously determined by infrared beam interruption analysis. An individual mouse was placed into the chamber 30 min before the measurement. The oxygen consumption, respiratory exchange ratio (RER), and motor activity were measured every 30 min for 5 h, and data from the last 4 h were combined to calculate the mean value. The effect of the β3-AR-specific agonist CL316243 on metabolic rate was measured as previously described (Xie et al., 2006). The effect of the 5-HT2CR-selective agonist MK212 (0.2 mg/kg), the 5-HT2CR-selective antagonist SB242084 (0.01, 0.5, and 4.0 mg/kg), the 5-HT2AR-selective antagonist ketanserin (0.6 mg/kg), and the 5-HT2BR-selective antagonist SB204741 (3.0 mg/kg) on metabolic rate and motor activity was also measured for 30 min before and for 5 h after intraperitoneal injection. In an attempt to identify the pathway activated during hyperlocomotion induced by SB242084, the D2, D3, and D4 dopamine receptor antagonist haloperidol (0.75 mg/kg), the D1 dopamine receptor antagonist R(+)-SCH23390 (0.2 mg/kg), and the D2 dopamine receptor antagonist S(−)-eticlopride (0.3 mg/kg) was injected intraperitoneally 30 min before injection of SB242084 (0.5 mg/kg) followed by determination of the motor activity for 5 h.
Genotyping of Mc4r−/− mice.
Genotyping of LoxTB:Mc4r (Mc4r−/−) mice was previously described (Balthasar et al., 2005). Briefly, pups were genotyped by PCR using three primers (MC4RFW1, 5′-GCACTACAGCGAGTCTCAGG-3′; MC4RFW2, 5′-GTGCAAGTGCAGGTGCCAG-3′; MC4RDW1, 5′-CTCCAACAGGCTTATGACACC-3′). MC4RFW2/MC4RDW1 primer pairs detect the loxP modified allele, whereas MC4RFW1/MC4RDW1 primer pairs detect an endogenous genomic Mc4r fragment.
Food intake studies.
Mice were individually caged and had ad libitum access to water and a standard rodent diet containing 20% (w/w) protein and 30% (w/w) fat. Body weight was determined at the beginning of the dark cycle (6:00 P.M.). After 24 h, remaining food and body weight were determined again everyday between 3 and 8 weeks of age and also between 15 and 16 weeks of age or between 5 and 8 weeks of age.
Food intake studies with 5-HT2CR agonist and antagonist.
The effect of 5-HT2CR agonist and antagonist on food intake was measured as previously described (Stark et al., 2006) with minor modification. Adult wild-type and VGV male mice were individually caged and fasted overnight. At light off (6:00 P.M.), mice received a single dose of the 5-HT2CR-preferential agonist mCPP (0.2, 5.0 mg/kg), the 5-HT2CR-selective agonist MK212 (0.2 mg/kg), the 5-HT2CR-selective antagonist SB242084 (0.01 mg/kg), or control saline intraperitoneally (n = 7 for each group). Food intake was determined 2 and 24 h after presentation of food.
Temperature measurements and time course of 8-OH-DPAT-induced hypothermia.
In all the experiments, body temperature of mice was measured with a digital thermometer (TH5 Thermalert Monitoring Thermometer; Physitemp Instruments) with a probe for mice inserted 1.0 cm into the rectum. All temperatures were measured at ambient temperature (21 ± 1°C). Basal temperature was measured 30 min and immediately before the subcutaneous injection of 8-OH-DPAT (1 mg/kg). Body temperature was recorded every 15 min for 1 h after injection.
Preparation of RNA.
Total RNA was extracted from dissected tissues by homogenizing in TRIzol reagent (Invitrogen).
Quantitative reverse transcription-PCR.
To prepare an internal standard, gene-specific PCR products of >1 kb in length were amplified. After gel purification, serial dilutions of each PCR product ranging from 103 to 108 copies per 1 μl of the DNA solution, which contained 50 ng of salmon sperm DNA were prepared. First-strand cDNA was synthesized using 1 μg of total RNA and oligo-dT primer, followed by real-time PCR using a 7000 Sequence Detection System (Applied Biosystems). The following primer sets were used (amplified product lengths are also indicated): primer set for 5-HT2CR mRNA, 156 bp, 5′-ATAGCCGGTTCAATTCGCG-3′ and 5′-AAGTTCGGGTCATTGAGCACG-3′; β3-adrenergic receptors (β3-AR) mRNA, 124 bp, 5′-CTCCAGAAAGACAAGCAACGG-3′ and 5′-CCTCGTGGTCTAGTCATCGGTT-3′; uncoupling protein 1 (UCP1) mRNA, 211 bp, 5′-TACCAAGCTGTGCGATGT-3′ and 5′-AAGCCCAATGATGTTCAGT-3′; β-actin mRNA, 143 bp, 5′-GTATCCATGAAATAAGTGGTTACAGG-3′ and 5′-GCAGTACATAATTTACACAGAAGCAAT-3′. The relative amount of mRNA was calculated with β-actin mRNA as an internal control.
Analysis of adipose tissues.
Dorsoscapular white adipose tissue (DWAT), epididymal white adipose tissue (EWAT), brown adipose tissue (BAT), total brain, and various tissues were dissected and stored at −80°C until analysis after weight measurement.
Determination of 5-HT2CR agonist toxic effects.
The effect of the 5-HT2CR-selective agonist MK212 and Ro60-0175, and the 5-HT2CR-preferential agonist mCPP were measured as previously described (Lucki et al., 1989). Briefly, both drugs were dissolved in 0.9% saline and injected intraperitoneally at the dose of 0.2, 0.3, 0.5, 1.0, 10, 20, and 50 mg/kg. Each drug was injected for adult wild-type, VGV, and INI male mice (n = 4) to measure the sufficient dose to induce seizure to death for all the mice within 30 min.
5-HT2CR autoradiography.
Receptor binding assay was done as previously described (López-Giménez et al., 2002) with modification. Whole brains from adult wild-type, VGV, and INI male mice (n = 3) were rapidly frozen after removal, and then kept at −80°C until use. Frozen sections of 20 μm thickness were prepared with the cryostat and mounted on slide glasses, and then quickly dried at room temperature. The slides were kept at −80°C until use. Tissues were preincubated in buffer C (50 mm Tris-HCl, pH 7.6, 4 mm CaCl2, 0.1% ascorbic acid) at room temperature for 30 min, and then incubated with a mixture of 0.14 nm (±)-1-(2,5-dimethoxy-4-[125I]iodophenyl)-2-amino-propane [(±)-[125I]DOI] (5-HT2A/2CR agonist; PerkinElmer Life and Analytical Sciences) and 10−7 m cold spiperon (5-HT2AR-specific antagonist) in buffer C at room temperature for 60 min. After washing twice in ice-cold buffer C, tissues were dipped in distilled ice-cold water and dried rapidly under a cold air stream, and then exposed to films for 10 d. We confirmed that a combination of (±)-[125I]DOI with 10−5 m cold mianserin (5-HT2A/2CR antagonist) generated complete blank, whereas the labeling was not significantly changed when spiperon was used as a cold competitor at the concentration ranging from 10−5 to 10−7 m. 5-HT2CR binding sites within select brain regions such as choroid plexus, arcuate nucleus of the hypothalamus (Arc), and paraventricular nucleus of the hypothalamus (PVH) were determined by measuring the optical density of autoradiographs. Nonspecific signal (background density) was subtracted from the total signal, resulting in the specific signal for each tissue section as previously described (Bale et al., 2001).
Statistical analyses.
All the statistical analyses except for body temperature measurements were performed using the Mann–Whitney U test. Two-way ANOVA with one repeated measure (time) followed by Fisher's post hoc tests were used for analysis of body temperature measurement data. All values are displayed as the mean ± SEM. Differences were considered to be statistically significant when p < 0.05.
Results
Perturbation of growth in VGV mice
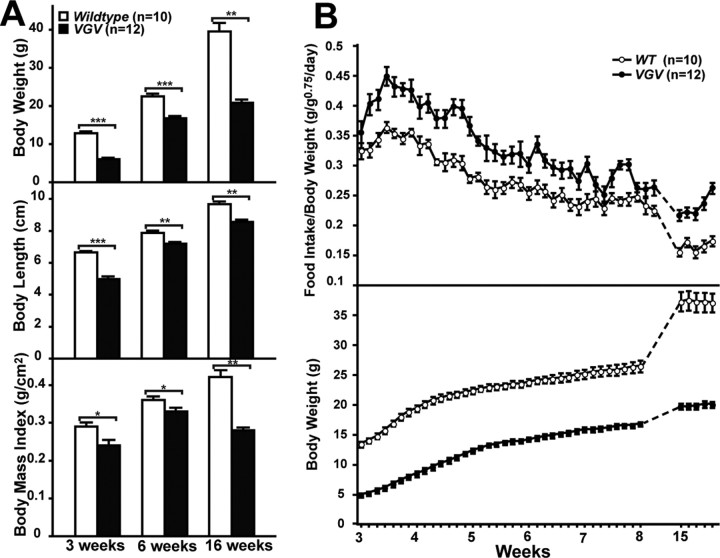
Two mutant mouse lines harboring a knock-in VGV or INI allele were generated (Fig. 1A,B). The hemizygous male and homozygous female mice of these X-linked mutated alleles solely express the fully edited VGV isoform or the completely unedited INI isoform receptor (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). It has been reported that the editing pattern controls the alternative splice site selection and the expression of full-length and functional receptors (Fig. 1A) (Wang et al., 2000; Flomen et al., 2004). However, splicing patterns, and tissue and brain-region specific distribution of 5-HT2CR mRNAs and proteins and their expression levels were identical between mutant and wild-type mice (supplemental Figs. S1A, S2, S3, available at www.jneurosci.org as supplemental material). INI mice grew normally (supplemental Fig. S4, available at www.jneurosci.org as supplemental material), whereas VGV mice, although born with normal body weight, demonstrated a dramatic decrease in growth rate especially during the first 3 weeks of postnatal development (>50% reduction in body weight gain) (Figs. 1C,D, 2A,B). It has been reported that the pattern of 5-HT2CR mRNA editing is significantly different among inbred strains of mice. In C57BL/6 mice, the frequency of 5-HT2CR mRNA editing is substantially higher than that in BALB/c mice. In BALB/c mice with lower serotonin levels caused by a defective serotonin synthesis pathway, ∼80% of 5-HT2CR mRNA remains unedited, resulting in the dominant expression of the INI receptor (Englander et al., 2005). Thus, we anticipated more severe phenotypic changes in BALB/c VGV mice, but no strain difference was observed between INI or VGV mice made in the C57BL/6J and BALB/c backgrounds (data not shown). Accordingly, most experiments hereafter were conducted with mice in the C57BL/6J background. The growth retardation was not caused by poor maternal care or nursing capability of mutant mother mice, because the phenotype of mutant pups raised by wild-type foster mothers did not revert (data not shown). Although the survival rate of hemizygous male VGV mice nursed together with wild-type and heterozygous female littermates was <20% during the first 2 weeks after birth, the rate recovered to 80% when littermate competition was removed (supplemental Fig. S5A, available at www.jneurosci.org as supplemental material). The reduced growth rate was detected also in heterozygous VGV female mice (supplemental Fig. S5B, available at www.jneurosci.org as supplemental material) as well as VGV:INI hybrid female mice generated from crossing of VGV and INI mice (data not shown), indicating the dominant effects of VGV isoform receptor expression. After weaning, the growth rate of mutant mice increased and became parallel to that of their wild-type littermates between 3 and 5 weeks (Fig. 1C). However, the body weight increase of VGV mice thence flattened, leading to a weight disparity of 7–10 g (3–8 weeks) and 20 g (later adult life) for VGV mice (Fig. 2A,B; supplemental Fig. S5B, available at www.jneurosci.org as supplemental material). The body length difference between wild-type and VGV mice became less significant after weaning, resulting in an additional reduction in body mass index of the mutant mice at 16 weeks (Fig. 2A). Sexual maturation of hemizygous male and homozygous female VGV and INI mice was normal, and they produced normal-size litters (Fig. 1C). Because growth retardation of VGV mice was detected regardless of sex, most experiments were conducted with male mice unless noted otherwise.
Figure 2.
Analysis of growth rate and food intake of VGV mice. A, Body weight, nasoanal body length, and body mass index of wild-type and VGV male mice presented as the mean ± SEM. Significant differences are indicated by asterisks (Mann–Whitney U test; *p < 0.05, **p < 0.01, ***p < 0.001). B, Daily food intake and body weight change.
Growth retardation of VGV mice is not caused by growth hormone deficiency
Reduced circulating IGF-1 levels were detected in VGV mice (supplemental Fig. S6A, available at www.jneurosci.org as supplemental material). Because hepatic IGF-1 synthesis is GH dependent, and serotonin is one of the neuromodulators that stimulates GH secretion by the pituitary, GH deficiency was suspected in VGV mice. However, on measurement, circulating GH levels were high, not low (supplemental Fig. S6B,C, available at www.jneurosci.org as supplemental material). In addition, daily injections of mouse recombinant GH from the postnatal day 4 to day 21 failed to rescue the growth retardation phenotype of VGV mice (supplemental Fig. S6D, available at www.jneurosci.org as supplemental material). Together, these findings indicate that VGV mice have no deficiency in GH synthesis or secretion by the pituitary through neonatal and/or adult life. Normal pituitary function was further supported by normal sexual maturation as well as normal thyroxine (T4) and corticosterone levels in VGV mice (Fig. 3A,B).
Figure 3.
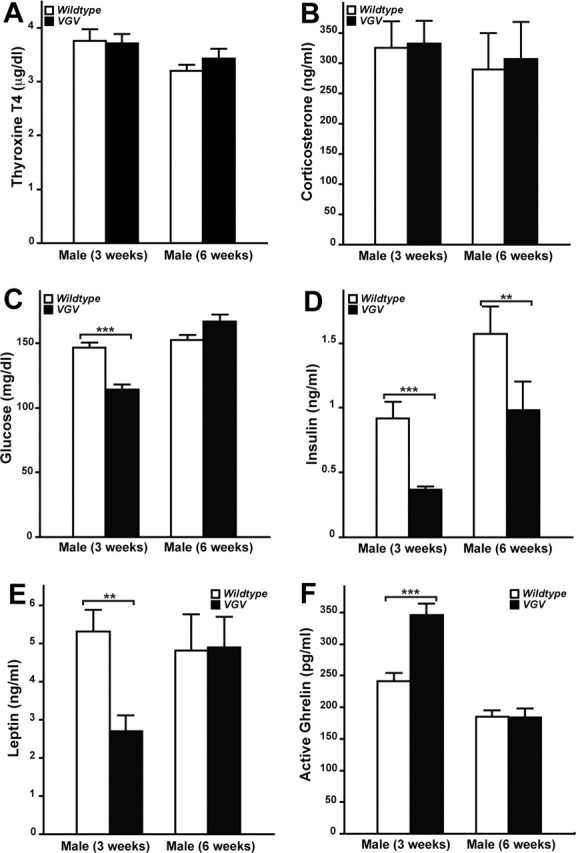
Levels of plasma parameters of energy homeostasis and hormones affecting appetite in VGV mice. T4 (A) and corticosterone (B) levels of wild-type and VGV male mice at two different postnatal stages (3 and 6 weeks) were displayed as the mean ± SEM (n = 11–22). No significant change of serum T4 and corticosterone levels was observed in VGV mice at either stage (Mann–Whitney U test). Serum glucose (C) and insulin levels (D) (n = 24–43) as well as serum leptin (E) and active ghrelin (F) (n = 11–16) were also examined. Insulin levels of VGV mice were significantly decreased at 3 and 6 weeks (**p < 0.01; ***p < 0.001). Serum glucose and leptin levels of VGV mice were significantly decreased at 3 weeks (**p < 0.01; ***p < 0.001), but there was no difference at 6 weeks (p > 0.05). In contrast, active ghrelin levels of VGV mice were significantly increased at 3 weeks (***p < 0.001), but not at 6 weeks (p > 0.05).
Hyperphagic and lean VGV mice
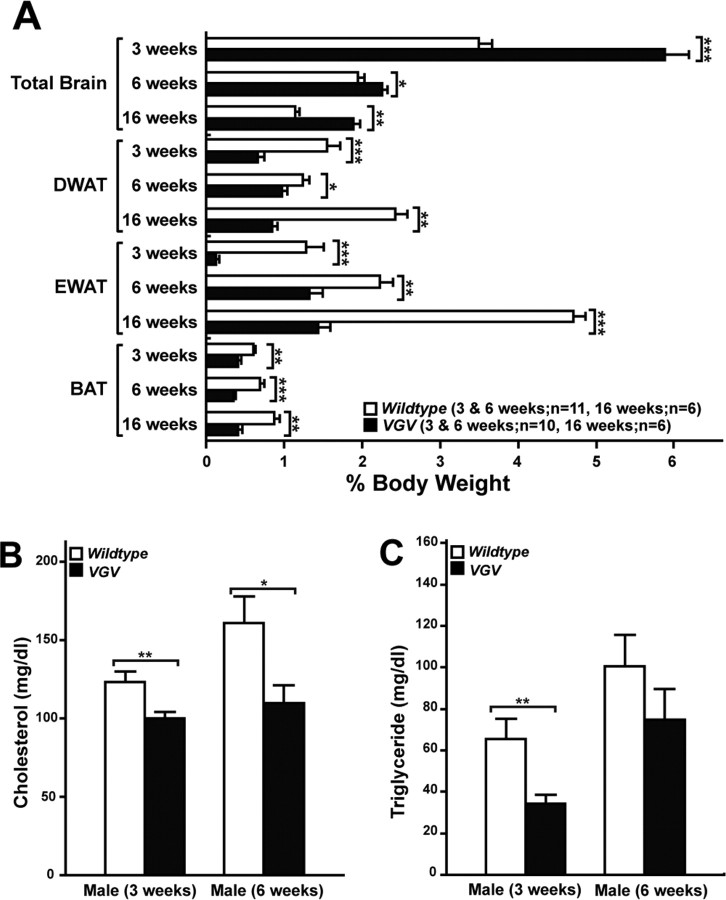
Reduced blood glucose levels at 3 weeks and consistently lower insulin levels (Fig. 3C,D) but normal glucose tolerance (data not shown) were also detected in VGV mice. In addition, reduced leptin and increased ghrelin levels, both stimulating appetite, were noted in VGV hemizygous males at 3 weeks, but they reached normal levels by 6 weeks (Fig. 3E,F). Most noticeably, VGV mice had dramatically reduced fat accumulation in adipose tissues (white and brown) (Fig. 4A). Furthermore, blood cholesterol and triglyceride levels were substantially reduced (Fig. 4B,C). These results collectively indicate negative balance of energy homeostasis in VGV mice, possibly because of hypophagia. However, VGV mice were hyperphagic, exceeding wild-type mice in their daily food intake when normalized by their body weight (Fig. 2B, top panel).
Figure 4.
Fat mass and metabolism of VGV mice. A, Total brain, DWAT, EWAT, and interscapular BAT were dissected and weighed. The brain develops almost normally, resulting in its increased relative ratio to the remaining body weight of VGV mice. B, C, Serum total cholesterol (B) and triglyceride (C) levels were also examined for wild-type (n = 11) and VGV mice (3 weeks, n = 11; 6 weeks, n = 13). Significant differences were detected for cholesterol levels between wild-type and VGV mice at 3 and 6 weeks and for triglyceride levels at 3 weeks (*p < 0.05, **p < 0.01; ***p < 0.001). Error bars indicate SEM.
Increased energy expenditure in VGV mice
Hyperphagic yet lean phenotypes indicated the possibility that physical activity and energy expenditure might be increased in VGV mice. Oxygen consumption measurements conducted with 6-week-old VGV mice indeed revealed an ∼40% increase of the metabolic rate in VGV mice compared with wild-type mice (Fig. 5A), although physical activity was reduced twofold in VGV mice (Fig. 6A). Hyperphagia, observed after weaning of VGV mice, may be a compensatory reaction to the consistent negative balance of energy homeostasis caused by dramatically increased energy expenditure. Nutrition supplied solely in the form of milk during the preweaning period may not be sufficient to balance the overly increased energy expenditure of growing VGV pups. Milk intake is primarily limited by maternal milk provision, because the satiety mechanism does not develop until midway through the postnatal phase (Bouret et al., 2004; Plagge et al., 2004). Thus, substantially reduced IGF-1, glucose, and insulin levels are likely to reflect states of undernutrition in VGV mice. Furthermore, reduction in leptin and increase in ghrelin levels, detected particularly immediately after weaning, indicates that the hormonal feedback mechanisms stimulate appetite to compensate for the persistent overexpenditure of energy in the mutant mice.
Figure 5.
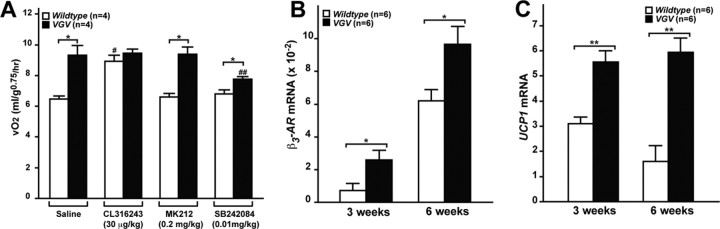
Constitutively activated sympathetic nervous system and increased energy expenditure in VGV mice. A, Metabolic rates of wild-type and VGV male mice (6 weeks of age) measured with or without intraperitoneal injection of the β3-AR agonist (CL316243), 5-HT2CR-selective agonist (MK212), or antagonist (SB242084) are presented as the mean ± SEM. No difference in RER was detected (data not shown). B, The β3-AR mRNA levels in BAT. The relative β3-AR mRNA amount is presented as the mean ± SEM. C, The UCP1 mRNA levels in BAT. The relative UCP1 mRNA amount is presented as the mean ± SEM. A–C, Significant differences are indicated by asterisks (*p < 0.05; **p < 0.01). A, #Significantly different from three other values of the wild-type experiments (p < 0.05). ##Significantly different from three other values of the VGV experiments (p < 0.05).
Figure 6.
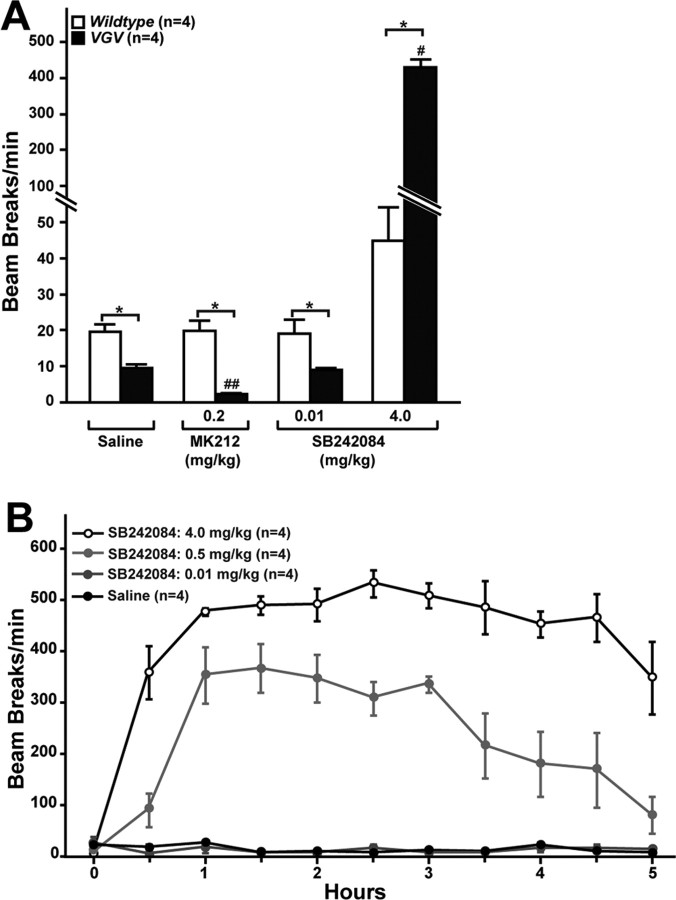
Hyperlocomotion induced in hypoactive VGV mice by the 5-HT2CR antagonist. A, Total activity levels detected in mice administered with the 5-HT2CR-selective agonist or antagonist as infrared beam disturbance of xy-axis movement are presented as the mean ± SEM. Significant differences are indicated by asterisks (*p < 0.05). #Significantly different from three other values of the same VGV experiments (p < 0.05). ##Significantly different from three other values of the same VGV experiments (p < 0.05). B, The dose-dependent hyperlocomotion induced in VGV mice by the 5-HT2CR-selective antagonist.
The sympathetic nervous system is constitutively activated in VGV mice
The sympathetic nervous system (SNS) plays a critical role in the control of energy expenditure. The innervation of sympathetic preganglionic neurons to adipose tissues stimulates lipolysis and thermogenesis via β3-AR (Hücking et al., 2003; Lowell and Bachman, 2003; Xie et al., 2006). There is no reliable antagonist that suppresses β3-AR activities in vivo. Therefore, we used a β3-AR-specific agonist CL316243 (30 μg/kg, i.p.), which increased the metabolic rate of wild-type mice to a level comparable in VGV mice, whereas it had no effect on VGV mice (Fig. 5A), indicating that in the mutant mice β3-ARs are constitutively already at their maximal activities. Activation of SNS is known to increase β3-AR expression followed by upregulation of UCP1, a mitochondrial protein specifically expressed in brown adipose tissue (Lindqvist et al., 2007). β3-AR mRNA levels were elevated in brown adipose tissue of VGV mice by 3.5- and 1.6-fold at 3 and 6 weeks, respectively, compared with wild-type mice (Fig. 5B). In addition, UCP1 mRNA levels were increased in brown adipose tissue of VGV mice by twofold and fourfold, respectively, compared with wild-type mice (Fig. 5C). Together, these results indicate that the elevated energy expenditure and reduced fat accumulation of VGV mice are likely to have resulted from constitutively activated SNS activity.
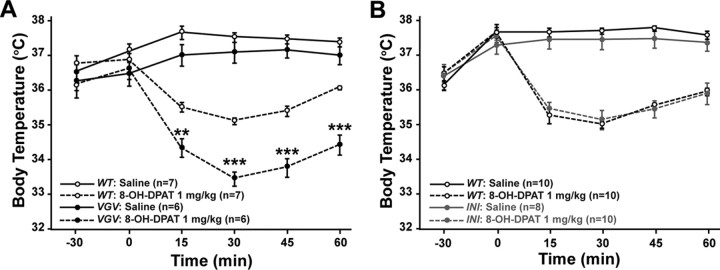
Normal body temperature of VGV mice regulated by a 5-HT1AR-mediated feedback mechanism
We reasoned that the elevated sympathetic tone and BAT thermogenesis might result in increase of the body temperature in VGV mice. However, there was no significant difference in the basal body temperature between VGV and counterpart wild-type mice (Fig. 7A). The normal body temperature of VGV mice may indicate the presence of a counteracting mechanism operating in VGV mice. It has been reported that normal levels of serotonin neuron firing that regulates heart rate, respiration, and body temperature are maintained by a negative-feedback inhibition mechanism via 5-HT1A receptors (5-HT1AR) in the medullary raphe (Richer et al., 2002; Nakamura and Morrison, 2007). Therefore, we investigated whether the 5-HT1AR-mediated mechanism contributes to normal body temperature of VGV mice by using a 5-HT1AR-specific agonist 8-OH-DPAT (Fig. 7A). Overall, effects of 8-OH-DPAT confirmed that 5-HT1AR activation elicited hypothermia. However, more prominent 8-OH-DPAT-induced hypothermia was observed with VGV mice compared with wild-type mice (Fig. 7A). In contrast, INI mice exhibited similar basal body temperature and 8-OH-DPAT-induced hypothermia compared with wild-type mice (Fig. 7B). The results indicate that the body temperature of VGV mice, which would be otherwise elevated, is set back to a normal level, most likely via a 5-HT1AR-mediated compensatory mechanism.
Figure 7.
Enhanced hypothermic response induced in VGV mice by 8-OH-DPAT. Time course effects of 8-OH-DPAT (1 mg/kg, s.c.) on the decrease of body temperature in VGV and wild-type (A) or INI and wild-type mice (B) are shown. Temperature values plotted are means ± SEM of 6–7 mice for VGV and 8–10 mice for INI. Significant difference between VGV and wild-type mice treated with 8-OH-DPAT was noted (**<0.01; ***<0.001).
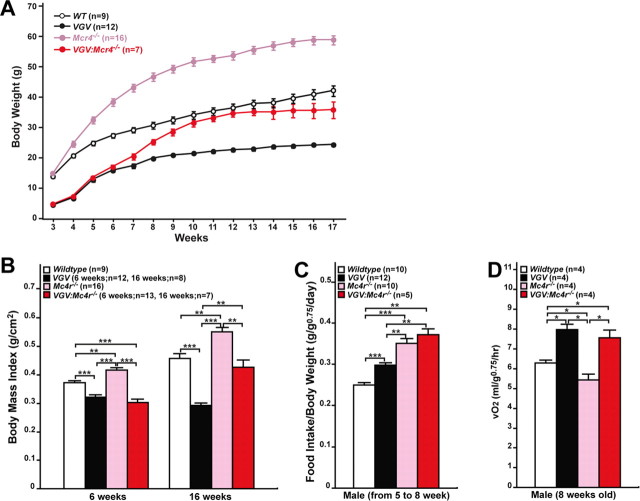
Elevated energy expenditure of VGV mice is regulated independent of the MC4R-mediated pathway
The Arc of the hypothalamus plays an important role in energy homeostasis. The Arc contains two distinct populations of neurons: one, preopiomelanocortin neurons expressing the anorexigenic α-melanocyte-stimulating hormone (α-MSH), and the other expressing the orexigenic agouti-related protein (AgRP). α-MSH and AgRP act as agonist and antagonist, respectively, for MC4R, which has been reported to regulate food intake (Heisler et al., 2006). In addition, MC4R regulates energy expenditure, most likely through SNS (Balthasar et al., 2005). Mc4r−/− mice are obese because of hyperphagia and reduced energy expenditure (Huszar et al., 1997). It has been postulated that 5-HT exerts its effects upstream of the MC4R signaling pathway via 5-HT1BR and 5-HT2CR. 5-HT1BR is positioned to inhibit the pathway leading to release of AgRP, whereas 5-HT2CR is positioned to exert its stimulatory effects on α-MSH activity upstream of MC4R to regulate food intake (Heisler et al., 2002, 2006; Lam et al., 2008). It has recently been reported that 5-HT2CR agonist also stimulates SNS, at least in part, through MC4R activation in the intermediolateral nucleus of the spinal cord (Zhou et al., 2007). To determine the relationship between 5-HT2CR-VGV-mediated and MC4R-mediated signaling pathways, the growth rate of VGV mice in the Mc4r−/− background was determined (Fig. 8A,B). Although the growth rate of VGV:Mc4r−/− mice became almost parallel to that of wild-type mice, especially after 9 weeks, it was still substantially lower than that of Mc4r−/− mice, suggesting that obesity of Mc4r−/− mice was rescued in the VGV background (Fig. 8A,B). Substantially increased food intake detected with VGV:Mc4r−/− mice compared with VGV mice (from 5 to 8 weeks) (Fig. 8C) must be responsible for larger body mass index of VGV:Mc4r−/− mice than that of VGV mice detected at 16 weeks (Fig. 8B). Most importantly, the energy expenditure increase detected with VGV mice remained in VGV:Mc4r−/− mice, indicating that elevated energy expenditure of VGV mice cannot be rescued in the Mc4r−/− background (Fig. 8D).
Figure 8.
No rescue of increased energy expenditure of VGV mice in the Mc4r−/− genetic background. A, Growth rates of wild-type, VGV, Mc4r−/−, and VGV:Mc4r−/− mice. B, Body mass index of VGV: Mc4r−/− mice was significantly smaller than that of Mc4r−/− mice at 16 weeks. C, More enhanced hyperphagia of VGV mice in the Mc4r−/− background. D, Elevated energy expenditure detected also in VGV:Mc4r−/− mice. All experiments were conducted with male mice. B–D, Significant differences are indicated by asterisks (*p < 0.05; **p < 0.01; ***p < 0.001). Error bars indicate SEM.
Oversensitization of 5-HT2CR-mediated neurotransmission for locomotion, food intake, and energy expenditure in VGV mice
The administration of the 5-HT2CR-selective agonist MK212 or mCPP has been shown to decrease locomotor activity and, at high dose, produce severe convulsions and death in rats (Lucki et al., 1989). To obtain insights into the hypoactive phenotype of VGV mice, we examined the effects of the 5-HT2CR agonists. Similar pharmacological effects (convulsions and death) of MK212 were produced but surprisingly at the 100 times lower dose (0.5 vs 50 mg/kg, i.p.) in VGV mice compared with wild-type mice (Table 1) as well as INI mice (data not shown). Similar low-dose toxic effects of two other 5-HT2CR agonists, mCPP and Ro60-0175, were confirmed (data not shown). The administration of MK212 at the low dose that did not exhibit the toxic effects (0.2 mg/kg, i.p.) resulted in more pronounced hypoactivity in VGV mice, indicating that suppression of locomotion results directly from the activation of 5-HT2CR (Fig. 6A). Finally, we investigated the effects of SB242084, a 5-HT2CR-specific antagonist. Interestingly, the administration of SB242084, but neither a 5-HT2AR-specific antagonist ketanserin nor a 5-HT2BR-specific antagonist SB204741 (data not shown), induced the dose-dependent hyperlocomotion in VGV mice (Fig. 6A,B), confirming the presence of the signaling pathway mediated via 5-HT2CR-VGV that negatively controls the motor activities. The negative control must be released by the treatment of VGV mice with the antagonist. The hyperlocomotion induced in VGV mice was extreme (e.g., 10 times higher than that induced in wild-type mice by 4.0 mg/kg SB242084) (Fig. 6A). The results indicate that the hypoactivity of VGV mice is unlikely to result from a simple feedback mechanism in response to the increased energy expenditure. The constitutive activation of the serotonergic neurotransmission mediated via the VGV receptor appears to form a strong circuitry that negatively regulates locomotion. Sudden release of this inhibitory mechanism by the 5-HT2CR antagonist seems to induce the reactive hyperlocomotion. During the induced hyperlocomotion, VGV mice moved restlessly often in one direction, which was interrupted by occasional vigorous and sudden movement (i.e., jumping). The extreme hyperlocomotion resembled the kinetosis induced by overstimulation of dopaminergic neurotransmission (Ralph et al., 2001). Phasic and tonic inhibitory effects of the neurotransmission mediated via 5-HT2CR over the mesocorticolimbic dopamine system have been reported previously (Di Matteo et al., 2001). To test the possible involvement of the dopaminergic neurotransmission in the mechanism that controls locomotion of VGV mice, several dopamine receptor antagonists were examined. However, the administration of haloperidol (D2, D3, and D4 dopamine receptor antagonist) could not revert the hyperlocomotion induced by the 5-HT2CR-specific antagonist (data not shown). A D1 antagonist R(+)-SCH23390 known to attenuate the hyperlocomotion of the dopamine transporter knock-out mice (Ralph et al., 2001) and/or a D2 antagonist S(−)-eticlopride did not have significant effects either (data not shown). However, the extent of hyperlocomotion is extreme, and additional studies are required to exclude completely the involvement of the dopamine system in the mechanism affecting the locomotive activity of VGV mice. Interestingly, these pharmacological experiments using 5-HT2CR agonists and antagonists revealed that the dose required for induction of certain pharmacological effects (i.e., convulsion or hyperlocomotion) is much lower in VGV mice compared with wild-type mice, indicating that the serotonergic neurotransmission is overly sensitized in VGV mice.
Table 1.
Toxic effects of 5-HT2CR-selective agonists on VGV mice
| Genotype | MK212 (mg/kg) |
||||||
|---|---|---|---|---|---|---|---|
| 0.2 | 0.3 | 0.5 | 1.0 | 10 | 20 | 50 | |
| Wild type | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0) | 4 (4) |
| VGV | 0 (0) | 1 (0) | 4 (4) | 4 (4) | 4 (4) | 4 (4) | 4 (4) |
Four each of wild-type and VGV hemizygous males were examined. Indicated are the number of mice that had seizures and, in parentheses, the number of mice that underwent severe convulsions and death.
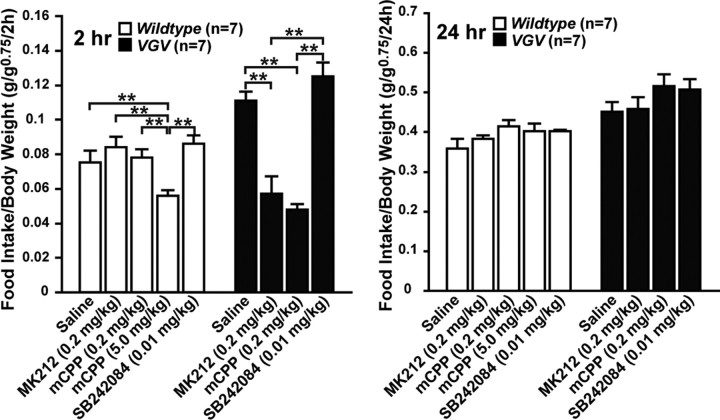
Consequently, we then conducted similar pharmacological studies on food intake and energy expenditure by using the low dose of the 5-HT2CR agonist and antagonist that would not cause significant changes in locomotion. Food intake of VGV mice was reduced substantially at 2 h after administration of the 5-HT2CR-selective agonist MK212 (0.2 mg/kg, i.p.) or mCPP (0.2 mg/kg, i.p.), although their suppressive effects were transient and lost at 24 h most likely because of their short half-lives (Fig. 9). The low-dose administration of the 5-HT2CR-selective antagonist SB242084 (0.01 mg/kg, i.p.) did not have any effects on hyperphagia of VGV mice (Fig. 9). The results indicate that the anorectic effects of 5-HT2CR-mediated pathway (Heisler et al., 2002, 2006) remains intact in VGV mice but is masked by a separate mechanism that has more dominant orexigenic effects, leading to hyperphagia. It is noteworthy that the dose of the agonist that induced transient anorectic effects in VGV mice was very low and did not have substantial effects on food intake of wild-type mice. The much higher dose of the agonist mCPP (5.0 mg/kg, i.p.) was required to observe the anorectic effects in wild-type mice, revealing once again the oversensitized serotonergic neurotransmission in VGV mice. Together, these studies indicate that the hyperphagia of VGV mice likely results from the constitutive suppression of the anorectic signaling pathway mediated via 5-HT2CR (Heisler et al., 2002, 2006).
Figure 9.
Effects of 5-HT2cR-selective agonist and antagonist on appetite. Cumulative food intake at 2 and 24 h after administration of 5-HT2CR-selective agonist (mCPP and MK212) or antagonist (SB242084) presented as the mean ± SEM. Significant differences are indicated by asterisks (*p < 0.05; **p < 0.01; ***p < 0.001).
Finally we found that administration of the low-dose 5-HT2CR-selective agonist MK212 (0.2 mg/kg, i.p.) had no additional effects on energy expenditure, indicating that the serotonergic neurotransmission projecting to SNS has been constitutively activated already to the maximal level (Fig. 5A). However, the low-dose administration of the 5-HT2CR-selective antagonist SB242084 (0.01 mg/kg, i.p.) reduced the energy expenditure significantly in VGV mice, yet higher than that of the level of wild type, indicating that the increased energy expenditure results from the direct activation of 5-HT2CR (Fig. 5A).
Increased agonist binding of the 5-HT2CRs in VGV mice
To investigate the mechanism that results in oversensitization of serotonergic neurotransmission, quantitative receptor autoradiography was conducted. Either (±)-[125I]DOI (5-HT2AR and 5-HT2CR-specific agonist) or [3H]mesulergrine (5-HT2AR and 5-HT2CR-specific antagonist as well as inverse agonist) in the presence of the cold competitor spiperone, a specific antagonist to 5-HT2AR, has been successfully used as the 5-HT2CR tracer, and visualization of high density in choroid plexus but otherwise in general low density of the 5-HT2CR-specific binding sites in wild-type rodent brains has been reported (López-Giménez et al., 2002). Therefore, we used (±)-[125I]DOI in the presence of the cold competitor spiperone for our experiments (Fig. 10A–I). The four times lower binding affinity of (±)-DOI to the VGV receptor compared with the INI receptor has been reported with the in vitro cell transfection system (Fitzgerald et al., 1999). To our surprise, however, the receptor autoradiography revealed that the 5-HT2CR-specific binding of the tracer was dramatically increased in the entire brain of VGV mice compared with wild-type and INI mice. The difference could be clearly seen especially in certain brain regions. For instance, the binding sites in the fourth ventricle choroid plexus (ChP), Arc, and PVH of VGV mice were twofold, fourfold, and fourfold higher than those of wild-type or INI mice, respectively (Fig. 10J). The apparent increase of (±)-[125I]DOI binding is not attributable to the relative increase of its binding to 5-HT2AR because of, for instance, increased binding of the VGV receptor to spiperone, because both VGV and INI receptors barely bind to this 5-HT2AR-specific antagonist (Fitzgerald et al., 1999). No significant difference was detected in various brain regions between wild-type and INI mice. Together, these results revealed dramatically increased ligand binding sites in VGV mice, most likely caused by increased expression of the functional VGV receptors.
Figure 10.
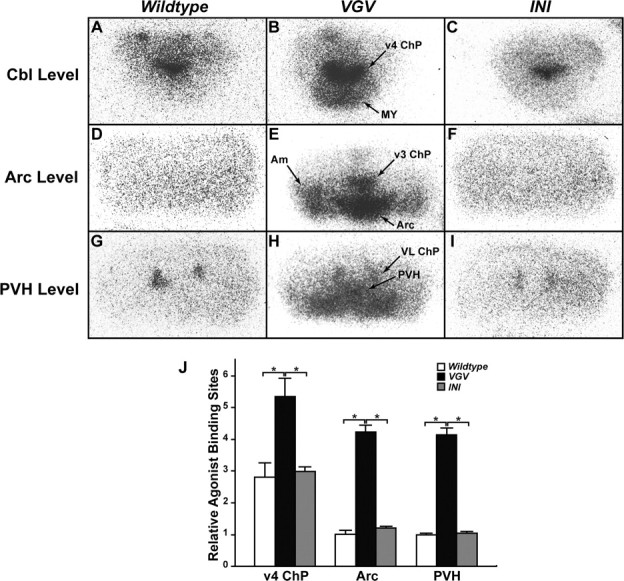
Increased binding of 5-HT2CR-selective radioligands in VGV mouse brains. A–I, The brain structure was visualized by binding of (±)-[125I]DOI agonist to 5-HT2C receptors. Representative autoradiographs are shown. 5-HT2C receptors were visualized with 5-HT2A/2CR agonist (±)-[125I]DOI (0.14 nm) in the presence of 10−7 m cold spiperone, the 5-HT2AR antagonist. PVH, Paraventricular nucleus of the hypothalamus; Arc, arcuate nucleus of the hypothalamus; Cbl, Cerebellum; Am, amygdala; ChP, choroid plexus; v3, third ventricle; v4, fourth ventricle; VL, lateral ventricle; MY, medulla. J, Quantification of 5-HT2CR agonist binding sites were done by measuring the optical density of areas representing the fourth ventricle choroid plexus (v4ChP), Arc, and PVH (n = 3). The relative optical density normalized to the density determined for the PVH area of the wild-type mouse brain is indicated. Significant differences are indicated by asterisks (*p < 0.05). Error bars indicate SEM.
Discussion
Editing isoforms of 5-HT2CR have been characterized previously only in vitro in non-neuronal cells transfected with various editing isoform expression plasmids (Burns et al., 1997; Fitzgerald et al., 1999; Niswender et al., 1999; Wang et al., 2000; Marion et al., 2004). However, the in vivo effects of 5-HT2CR mRNA editing have not been evaluated previously. In the present studies, we created mutant mice that solely express one of two extremely different editing isoform receptors, VGV (fully edited) and INI (editing blocked), to examine the in vivo significance of 5-HT2CR mRNA editing. We provided the first direct evidence through phenotypic analysis of these mutant mice that regulated editing is important, because overediting of 5-HT2CR mRNAs altered dramatically the mechanism that regulates energy homeostasis.
It has been reported that 5-HT2CR-null mice exhibit hyperphagic and late-onset obesity phenotypes (Tecott et al., 1995). In contrast, VGV mice expressing solely the VGV isoform had reduced fat mass and lipid accumulation in adipose tissue, and yet were hyperphagic and hypoactive through their postnatal and adult life. The lean and energy dissipating phenotypes of VGV mice, different from the INI mouse phenotypes with normal growth and metabolic rates or late-onset obesity phenotypes of 5-HT2CR-null mice (Tecott et al., 1995), clearly indicate the presence of a distinct signaling pathway activated via the VGV receptor. This VGV-dependent pathway appears to activate a latent “gain-of-function” type mechanism that upregulates energy metabolism, because no opposite effects (reduced energy expenditure and obesity) were detected in INI mice. The presence of the mechanism is revealed in VGV mice because a feedback mechanism(s), which normally acts to reduce the inappropriately increased energy expenditure by reducing the extent of 5-HT2CR mRNA editing, is not available in this mutant mouse line with editing frequency set to 100% permanently. Serum GH, corticosterone, and thyroxine concentrations were all in the normal range in VGV mice. Furthermore, no abnormality was noted for mating behavior of hemizygous male and homozygous female VGV mice as well as their litter sizes, indicating normal pituitary function.
The increased energy expenditure appears to result from the constitutively activated SNS, because the increased expression of β3-AR and UCP1 in brown fat tissues was detected in VGV mice. Interestingly, basal body temperature of VGV mice expected to be elevated by activated SNS and BAT thermogenesis was normal, perhaps regulated by the 5-HT1AR-mediated feedback mechanism. The normal body temperature may help to prevent additional energy dissipation in VGV mice. MC4R has been proposed to play a major role in the regulation of energy metabolism and appetite by providing an essential node linking various pathways including one mediated via 5-HT2CR, and inactivation of this melanocortin receptor gene leads to increase in food intake and decrease in energy expenditure and consequent obesity (Huszar et al., 1997; Balthasar et al., 2005). However, the reduced energy expenditure of Mc4r−/− mice was reverted in the VGV genetic background, whereas the elevated energy expenditure of VGV mice remained in the Mc4r−/− genetic background. Our results indicate that the pathway activated via the VGV receptor that regulates energy expenditure is independent of the α-MSH–MC4R-mediated pathway. Furthermore, this neurotransmission activated via the VGV receptor has dominant effects over the MC4R-mediated pathway. Our experiments using 5-HT2CR-selective agonists confirmed that the 5-HT2CR–MC4R-mediated signaling pathway that negatively regulates appetite is active in VGV mice, because hyperphagia of the mutant mice can be transiently reverted by agonist administration. However, the administration of the 5-HT2CR-selective antagonist could not enhance hyperphagia of VGV mice. It seems that a feedback mechanism strongly upregulates appetite to compensate the negative balance of energy homeostasis resulted from the overly activated SNS and consequent energy dissipation by reducing and masking the anorectic effects of the 5-HT2CR–α-MSH–MC4R-mediated pathway.
The molecular basis of the VGV isoform action, leading to VGV mouse phenotypes, is currently unknown. Previous in vitro studies in transfected non-neuronal cells suggested that G-protein coupling efficacy, basal level activities, and the agonist affinity of VGV isoform are dramatically reduced compared with the INI isoform receptor. For instance, the 5-HT potency of the VGV receptor is 20–30 times lower than that of the INI receptor, whereas binding affinity of the 5-HT2CR-selective agonist MK212 and mCPP to the VGV isoform is six times lower than that of the INI receptor (Burns et al., 1997; Fitzgerald et al., 1999; Niswender et al., 1999; Wang et al., 2000). Paradoxically, we found that the 5-HT2CR-mediated neurotransmission is overly sensitized in VGV mice as seen by enhanced response to the 5-HT2CR-selective agonists and antagonists. Our radiohistochemical studies indeed revealed that the 5-HT2CR-selective agonist binding sites were significantly higher in the entire brain region including choroid plexus, Arc, and PVH of VGV mice compared with wild-type or INI mice. The results indicate clearly the increased expression of functional receptors capable of binding to the agonist, although the overall expression of the 5-HT2CR receptors detected by analysis of their mRNA or protein levels was not so different in VGV mice compared with wild-type or INI mice (supplemental Figs. S2, S3, available at www.jneurosci.org as supplemental material). It has been reported that the intracellular trafficking efficacy and cell surface expression of the VGV isoform are significantly higher than those of the INI receptor (Marion et al., 2004). In contrast, most INI receptors are internalized and exist mainly as the desensitized form accumulated in endosomes because of formation of a complex with β-arrestins (Marion et al., 2004). The increased trafficking as well as reduced desensitization kinetics may contribute to constitutive high expression of the functional VGV receptor on the cell surface, which in turn may overcome its low agonist binding affinity and reduced G-protein coupling efficacy reported previously for this fully edited receptor (Burns et al., 1997; Fitzgerald et al., 1999; Niswender et al., 1999). Although additional studies are required for understanding the precise mechanism that increases the agonist binding sites, oversensitization of the serotonergic neurotransmission observed in VGV mice most likely resulted from the dramatic increase of functional receptor expression on the cell surface.
Our findings highlight the importance of the regulation of 5-HT2CR mRNA editing. Expression of the fully edited VGV isoform receptor is limited to certain brain regions such as the hypothalamus and frontal cortex, whereas other editing isoforms such as VNV or VSV are more commonly detected (Burns et al., 1997; Fitzgerald et al., 1999; Wang et al., 2000). The 5-HT2CR mRNA editing pattern has been reported to change dynamically in response to stress or medication (Englander et al., 2005). In VGV mice, the spatial and stress or medication-responsive regulation of 5-HT2CR mRNA editing is removed, resulting in exclusive expression of the VGV receptor. Our results clearly demonstrate that dysregulated editing, overediting but not underediting, of 5-HT2CR mRNA can cause pathological consequences at least for control of energy homeostasis as demonstrated by the inappropriately increased energy expenditure and retarded growth phenotypes of VGV mice. This is in contrast to AMPA glutamate receptor B subunit (GluR-B) Q/R site editing (Higuchi et al., 2000). GluR-B mRNAs are edited at the Q/R site almost exclusively by ADAR2, leading to the replacement of the gene-encoded Glu with Arg. In adults, the Q/R site editing efficiency is normally set to almost 100%. In other words, the Q/R site editing is essentially unregulated and produces mostly edited protein. This unregulated and extremely high editing efficiency must be critical, because a slight decrease of the editing efficiency has proven to be pathological, resulting in seizures and premature death in ADAR2−/− mice (Feldmeyer et al., 1999; Higuchi et al., 2000). Thus, too much editing of sites like 5-HT2CR-VGV can be deleterious, as can too little editing of sites like GluR-B Q/R.
Substantial variations in metabolic rate and obesity detected among individuals of different ages and ethnic backgrounds may be related, at least in part, to differences in editing efficacy of 5-HT2CR mRNAs. It is of special interest to determine whether higher VGV levels are detected in individuals with high metabolic rates. In recent years, 5-HT2CR selective agonists have been used for obesity prevention and treatment (Halford et al., 2005). Our findings suggest that editing of HT2CR mRNA can be a future target of great promise for development of drugs to control body weight. Finally, dysregulation of 5-HT2CR mRNA editing has been implicated in pathological processes of human mental illness such as suicidal depression and schizophrenia (Niswender et al., 2001; Sodhi et al., 2001; Gurevich et al., 2002). Additional behavioral and pharmacological analyses of VGV and INI mice will provide an invaluable animal model system to evaluate the significance of 5-HT2CR mRNA editing not only for energy metabolism but also for processes that regulate emotion and behavior as well as human mental disorders.
Footnotes
This work was supported by grants from the National Institutes of Health (A.G., T.L.B., J.A.B., K.N.), the Juvenile Diabetes Research Foundation (K.N.), and the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health (K.N). We thank B. B. Lowell and J. K. Elmquist for LoxTB:Mc4r mice; R. B. Emeson for sharing unpublished results; J. M. Murray, A. Weber, and H. J. Grill for reading and comments; J. Richa for generation of chimeric mice; H. Collins for measurement of serum hormones; R. Dhir for energy expenditure analysis; and J. T. Lee for preparation of targeting constructs.
References
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bass BL. Double-stranded RNA as a template for gene silencing. Cell. 2000;101:235–238. doi: 10.1016/s0092-8674(02)71133-1. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT(2C) receptors in the control of central dopamine function. Trends Pharmacol Sci. 2001;22:229–232. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- Englander MT, Dulawa SC, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Kask K, Brusa R, Kornau HC, Kolhekar R, Rozov A, Burnashev N, Jensen V, Hvalby O, Sprengel R, Seeburg PH. Neurological dysfunctions in mice expressing different levels of the Q/R site-unedited AMPAR subunit GluR-B. Nat Neurosci. 1999;2:57–64. doi: 10.1038/4561. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Iyer G, Conklin DS, Krause CM, Marshall A, Patterson JP, Tran DP, Jonak GJ, Hartig PR. Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology. 1999;21:82S–90S. doi: 10.1016/S0893-133X(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Flomen R, Knight J, Sham P, Kerwin R, Makoff A. Evidence that RNA editing modulates splice site selection in the 5-HT2C receptor gene. Nucleic Acids Res. 2004;32:2113–2122. doi: 10.1093/nar/gkh536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;34:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Lawton CL, Blundell JE. Serotonin (5-HT) drugs: effects on appetite expression and use for the treatment of obesity. Curr Drug Targets. 2005;6:201–213. doi: 10.2174/1389450053174550. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- Hücking K, Hamilton-Wessler M, Ellmerer M, Bergman RN. Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest. 2003;111:257–264. doi: 10.1172/JCI14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007a;315:1137–1140. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Zinshteyn B, Chendrimada TP, Shiekhattar R, Nishikura K. RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer-TRBP complex. EMBO Rep. 2007b;8:763–769. doi: 10.1038/sj.embor.7401011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Megraw M, Kreider E, Iizasa H, Valente L, Hatzigeorgiou AG, Nishikura K. Frequency and fate of microRNA editing in human brain. Nucleic Acids Res. 2008;36:5270–5280. doi: 10.1093/nar/gkn479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DD, Przydzial MJ, Ridley SH, Yeo GS, Rochford JJ, O'Rahilly S, Heisler LK. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, de la Cour CD, Håkanson R, Erlanson-Albertsson C. Ghrelin affects gastrectomy-induced decrease in UCP1 and beta(3)-AR mRNA expression in mice. Regul Pept. 2007;142:24–28. doi: 10.1016/j.regpep.2007.01.007. [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Tecott LH, Palacios JM, Mengod G, Vilaró MT. Serotonin 5- HT (2C) receptor knockout mice: autoradiographic analysis of multiple serotonin receptors. J Neurosci Res. 2002;67:69–85. doi: 10.1002/jnr.10072. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Bachman ES. Beta-Adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem. 2003;278:29385–29388. doi: 10.1074/jbc.R300011200. [DOI] [PubMed] [Google Scholar]
- Lucki I, Ward HR, Frazer A. Effect of 1-(m-chlorophenyl)piperazine and 1-(m-trifluoromethylphenyl)piperazine on locomotor activity. J Pharmacol Exp Ther. 1989;249:155–164. [PubMed] [Google Scholar]
- Marion S, Weiner DM, Caron MG. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J Biol Chem. 2004;279:2945–2954. doi: 10.1074/jbc.M308742200. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R127–R136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K. Editor meets silencer: crosstalk between editing and RNA interference. Nat Rev Mol Cell Biol. 2006;7:919–931. doi: 10.1038/nrm2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem. 1999;274:9472–9478. doi: 10.1074/jbc.274.14.9472. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Herrick-Davis K, Dilley GE, Meltzer HY, Overholser JC, Stockmeier CA, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-HT2C receptor: alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology. 2001;24:478–491. doi: 10.1016/S0893-133X(00)00223-2. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Abdallah L, Goulding EH, Bonasera SJ, Tecott LH. Hyperactivity and reduced energy cost of physical activity in serotonin 5-HT(2C) receptor mutant mice. Diabetes. 2003;52:315–320. doi: 10.2337/diabetes.52.2.315. [DOI] [PubMed] [Google Scholar]
- Ohlson J, Pedersen JS, Haussler D, Ohman M. Editing modifies the GABA(A) receptor subunit alpha3. RNA. 2007;13:698–703. doi: 10.1261/rna.349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagge A, Gordon E, Dean W, Boiani R, Cinti S, Peters J, Kelsey G. The imprinted signaling protein XL alpha s is required for postnatal adaptation to feeding. Nat Genet. 2004;36:818–826. doi: 10.1038/ng1397. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenan RA. The RNA world meets behavior: A→I pre-mRNA editing in animals. Trends Genet. 2001;17:53–56. doi: 10.1016/s0168-9525(00)02169-7. [DOI] [PubMed] [Google Scholar]
- Richer M, Hen R, Blier P. Modification of serotonin neuron properties in mice lacking 5-HT1A receptors. Eur J Pharmacol. 2002;435:195–203. doi: 10.1016/s0014-2999(01)01607-7. [DOI] [PubMed] [Google Scholar]
- Rula EY, Lagrange AH, Jacobs MM, Hu N, Macdonald RL, Emeson RB. Developmental modulation of GABAA receptor function by RNA editing. J Neurosci. 2008;28:6196–6201. doi: 10.1523/JNEUROSCI.0443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH, Hartner J. Regulation of ion channel/neurotransmitter receptor function by RNA editing. Curr Opin Neurobiol. 2003;13:279–283. doi: 10.1016/s0959-4388(03)00062-x. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, Burnet PW, Makoff AJ, Kerwin RW, Harrison PJ. RNA editing of the 5-HT(2C) receptor is reduced in schizophrenia. Mol Psychiatry. 2001;6:373–379. doi: 10.1038/sj.mp.4000920. [DOI] [PubMed] [Google Scholar]
- Stark JA, Davies KE, Williams SR, Luckman SM. Functional magnetic resonance imaging and c-Fos mapping in rats following an anorectic dose of m-chlorophenylpiperazine. Neuroimage. 2006;31:1228–1237. doi: 10.1016/j.neuroimage.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Wang Q, O'Brien PJ, Chen CX, Cho DS, Murray JM, Nishikura K. Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. J Neurochem. 2000;74:1290–1300. doi: 10.1046/j.1471-4159.2000.741290.x. [DOI] [PubMed] [Google Scholar]
- Xie T, Plagge A, Gavrilova O, Pack S, Jou W, Lai EW, Frontera M, Kelsey G, Weinstein LS. The alternative stimulatory G protein alpha-subunit XLalphas is a critical regulator of energy and glucose metabolism and sympathetic nerve activity in adult mice. J Biol Chem. 2006;281:18989–18999. doi: 10.1074/jbc.M511752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Chendrimada TP, Wang Q, Higuchi M, Seeburg PH, Shiekhattar R, Nishikura K. Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nat Struct Mol Biol. 2006;13:13–21. doi: 10.1038/nsmb1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, Clifton PG, Yueh CY, Evans ML, McCrimmon RJ, Elmquist JK, Butler AA, Heisler LK. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]