Abstract
Recent work has suggested that the chromosomally encoded TetA(L) transporter of Bacillus subtilis, for which no physiological function had been shown earlier, not only confers resistance to low concentrations of tetracycline but is also a multifunctional antiporter protein that has dominant roles in both Na+- and K+-dependent pH homeostasis and in Na+ resistance during growth at alkaline pH. To rigorously test this hypothesis, TetA(L) has been purified with a hexahistidine tag at its C terminus and reconstituted into proteoliposomes. The TetA(L)–hexahistidine proteoliposomes exhibit high activities of tetracycline–cobalt/H+, Na+/H+, and K+/H+ antiport in an assay in which an outwardly directed proton gradient is artificially imposed and solute uptake is monitored. Tetracycline uptake depends on the presence of cobalt and vice versa, with the cosubstrates being transported in a 1:1 ratio. Evidence for the electrogenicity of both tetracycline–cobalt/H+ and Na+/H+ antiports is presented. K+ and Li+ inhibit Na+ uptake, but there is little cross-inhibition between Na+ and tetracycline–cobalt uptake activities. The results strongly support the conclusion that TetA(L) is a multifunctional antiporter. They expand the roster of such porters to encompass one with a complex organic substrate and monovalent cation substrates that may have distinct binding domains, and provide the first functional reconstitution of a member of the 14-transmembrane segment transporter family.
Keywords: pH homeostasis, antibiotic-resistance
The chromosomally encoded TetA(L) protein of Bacillus subtilis may be among the progenitors of a major category of tetracycline (Tc) efflux proteins that accounts for plasmid-mediated resistance to the antibiotic (1). This protein exhibits 58–81% sequence identity with products of the L and K classes of tet genes that are widespread in plasmids of Gram-positive bacteria, including Bacillus species and Staphylococcus aureus (2–4). The class L and K Tet proteins are predicted to be large hydrophobic proteins with 14 membrane-spanning segments (2, 3), as recently documented for a structurally related transporter (5). The N-terminal part of Tet(L) and Tet(K) proteins that encompasses the first six putative membrane-spanning segments also shows strong sequence similarity to another group of Tet proteins, those produced by tet genes found in plasmids and transposons of Gram-negative bacteria (2, 3, 6). Of the several modes that allow bacteria to resist growth inhibition by Tc, active exclusion of the antibiotic by Tet proteins is the major one and accounts for the significant loss of utility for this important class of antibiotics during the past decade (2, 4, 7). If the details of the efflux mechanism are elucidated, however, strategies for renewed use of tetracyclines, development of more useful analogs, or novel inhibitors of the same pumps may emerge. An encouraging example of such strategies has already been advanced (8). However, work in this area has been impeded by the unavailability of a preparation of a purified Tet protein that catalyzes transport at high specific activity in the absence of other proteins. Here, we report such a preparation of TetA(L).
Successful reconstitution of this particular Tet protein is of added interest because of our recent evidence that the B. subtilis tetA(L) gene has important physiological functions that are independent of Tc, while it also serves as a natural defense against low levels of the antibiotic (9, 10). Specifically, TetA(L) has been proposed to be both a Tc–metal/H+ antiporter and a monovalent cation/H+ antiporter, i.e., it can use either Na+ or K+ as substrates that exchange with external protons in addition to using the antibiotic–metal complex. Deletion of tetA(L) from the B. subtilis chromosome results in a Na+- and alkali-sensitive phenotype that is accompanied by diminished capacity for energy-dependent Na+ efflux and almost complete loss of the capacity for Na+- and K+-dependent pH homeostasis at alkaline pH. The deletion strain is also more sensitive than wild type to low Tc concentrations (10). Enhanced activity of membrane-associated Tc–cobalt/H+ antiport and Na+/H+ antiport was observed in membrane vesicles of Escherichia coli that was transformed with a recombinant plasmid expressing tetA(L) (1, 11). Moreover, TetA(L) is likely to have an additional, endogenous substrate whose efflux is necessary for optimal growth even at pH 7.0 in the absence of added Na+. The tetA(L) deletion strain of B. subtilis exhibits a significant growth deficit at pH 7.0 even without added Na+ and probably would not be viable were it not for a second site mutation that apparently up-regulates a partially compensatory transporter (10). All the growth and pH homeostasis phenotypes are complemented by restoration of either tetA(L) or plasmid-derived tet(K) (10).
If TetA(L) is indeed a multifunctional transporter with a variety of important physiological roles, there are implications with respect to the origins of such antibiotic-resistance determinants as well as with respect to transporter structure and mechanisms. However, rigorous proof for the multi-substrate nature of TetA(L) requires demonstration that the purified protein alone reconstitutes all the activities attributed to it. This is especially important because of the lack of cross-inhibition found by Na+ of Tc–Co2+ transport, and by Tc–Co2+ of Na+ transport mediated by TetA(L) in E. coli vesicles (11). This could reflect rather distinct domains for the alternate substrates. However, it might instead reflect the fact that TetA(L) mediates only one of the antiport activities directly and enhances the other by indirect stimulation of a distinct porter. The experiments reported here provide the requisite demonstration that, in fact, TetA(L) catalyzes both Tc–Co2+/H+ antiport and Na+(K+)/H+ antiport.
MATERIALS AND METHODS
Plasmids, Bacterial Strains, and Growth Conditions.
Wild-type B. subtilis BD99 and mutant strain JC112 [ΔtetA(L)] (10) were grown in malate-containing Spizizen salts medium as described (9). For construction of a tetA(L) derivative that would encode a product with an added hexahistidine (H6) tag at the C-terminal end, the wild-type tetA(L) gene was amplified by PCR from chromosomal DNA. The two primers used were TF (5′-GGAGGGGGATCCATGAATACGTCTTATTCACAG) and TR (5′-TTTCACGGATCCAGCCATGTCTCCGCGAACG). Both were customized with a BamHI-cut site. The PCR product was digested with BamHI, gel-purified, and then inserted into the BamHI site of plasmid pQE12 (Qiagen). The new construct, pJQ2, with the tetA(L) gene under control of the T5 phage promoter of the plasmid was selected. The expression of the modified tetA(L) gene was under the control of two lac operator sequences. E. coli DH5αMCR (GIBCO/BRL) was used as the host strain to overexpress the tagged tetA(L) gene. Before undertaking overexpression and purification, growth experiments were conducted that confirmed that in spite of the plasmid’s apparent toxicity, basal expression of the modified tetA(L) in pJQ2 conferred enhanced Tc resistance upon E. coli DH5αMCR. To minimize the toxicity of the gene product to the host, plasmid pREP4 (Qiagen), which contains a lacIq gene, was first transformed into the host bacterium. A transformant into which pJQ2 was then transformed was isolated. The new double transformant was grown in Luria–Bertani medium at 30°C, with 100 μg/ml ampicillin, 25 μg/ml kanamycin, and 2% glucose.
Overexpression and Purification of TetA(L)–H6.
The E. coli DH5αMCR transformant with pJQ2 and pREP4 was used for overexpression. The approach used for both overexpression and purification was that recommended by Qiagen. An overnight culture was inoculated by a 1:200 dilution in Luria–Bertani glucose medium. After growth, at 30°C, to an A600 of about 0.7, the cells were pelleted by centrifugation and resuspended into the same volume of fresh Luria–Bertani medium containing 2 mM isopropyl-1-β-d-thiogalactopyranoside. The cells were grown for an additional 2 hr at 30°C and then harvested by centrifugation. The pellets were kept at −20°C. Everted membrane vesicles were prepared by passing cells through a French press as described (12). The membrane pellets were resuspended in a buffer containing 20% glycerol, 20 mM Tris·HCl (pH 8.0), and 600 mM NaCl, and stored at −70°C. For extraction, a total of 100 mg of membrane protein was resuspended in 10 ml of the same buffer with addition of asolectin (soybean phosphatidylcholine, type II-S, Sigma) and laurylmaltoside to final concentrations of 0.25 mg/ml and 1%, respectively. The asolectin was partially purified by acetone/ether washes as described (13). The mixture was incubated at 4°C with occasional shaking for 1 hr, followed by ultracentrifugation at 150,000 × g for 1 hr. The supernatant was mixed with 2 ml of TALON metal affinity resin (CLONTECH), which had been equilibrated with buffer A (20% glycerol/20 mM Tris, pH 8.0/200 mM NaCl/0.25 mg/ml asolectin/0.04% laurylmaltoside), by gentle mixing at 4°C for 1 hr. The bound resin was then transferred to a column washed with 40 ml of buffer A, followed by 20 ml of buffer A containing 2 mM imidazole. Remaining contaminating proteins and some TetA(L)–H6 were eluted with 5 ml of 50 mM imidazole in buffer A. Highly purified TetA(L)-H6 could then be eluted from the column by 100 mM imidazole in buffer A. Purification was monitored by SDS/PAGE (10% polyacrylamide gel). The TetA(L)–H6 protein stained poorly with Coomassie, and was routinely visualized by silver staining (14). Correlating with the poor Coomassie staining was the finding that a micro Bradford Coomassie G binding assay (15) yielded very low values of protein when applied to TetA(L)–H6. A better protein estimate that more closely correlated with the intensity of silver staining observed on gels was obtained by a micro bicinchoninic acid assay that was carried out according to the manufacturer’s instructions (Pierce), with BSA as the standard. The purified protein was dialyzed against a buffer containing 10% glycerol, 20 mM Tris (pH 8.0), and 0.01% laurylmaltoside and stored in aliquots at −70°C.
Western Immunoblot Analysis and N-Terminal Amino Acid Sequence Analysis.
Rabbit polyclonal antibodies were raised against a synthetic peptide corresponding to the first 14 amino acid residues of TetA(L). Samples of membranes or of purified protein were electrophoresed on an SDS/10% polyacrylamide gel (16) and transferred to nitrocellulose membranes (Bio-Rad). Before electrophoresis, samples were denatured at room temperature in 50 mM Tris (pH 6.8), 4% SDS, 50 mM DTT, and 0.01% bromophenol blue. Western blotting was carried out according to the manufacturer’s instructions except that BSA was substituted for gelatin. The second antibody was conjugated to alkaline phosphatase. To determine the N-terminal sequence of purified TetA(L), the protein was electrophoresed as above and transferred to a poly(vinylidene difluoride) membrane (Bio-Rad). The first six N-terminal amino acids of the purified protein were determined to be SQSTLR, which correspond to amino acid residues 10–15 of the deduced N-terminal sequence of the tetA(L) gene product. Missing were four amino acids from the vector and five from TetA(L) that would have been anticipated to be at the N terminus of the modified tetA(L) product of the construct used for expression and purification; this may be a normal processing or a result of the modification and/or heterologous expression system.
Reconstitution of TetA(L)–H6 into Proteoliposomes.
The protocol for reconstitution followed those developed by others (17, 18). A 40 mg/ml suspension of the partially purified asolectin was bath sonicated to near clarity in a buffer containing 100 mM Mops (pH 7.0), 1 mM DTT, and 1.2% octyl glucoside. Purified TetA(L)–H6 protein (100 μl containing 10–20 μg), to which octyl glucoside was added to a concentration of 1.2%, was mixed with the same volume of asolectin suspension. After brief sonication, the mixture was diluted 50-fold into loading buffer and mixed by stirring for 20 min at room temperature. Proteoliposomes were collected by centrifugation at 150,000 × g for 1 hr. The tubes were drained carefully, and the pellets were resuspended in 100 μl of the loading buffer.
For uptake assays, the loading buffer consisted of 1 mM DTT, 150 mM NH4Cl, and 15 mM Tris (pH 7.0). A transmembrane gradient of pH (ΔpH), acid in, was generated across the proteoliposomes upon dilution of 4 μl of the initial proteoliposome suspension into 500 μl of 150 mM choline chloride and 15 mM Tris at various pH values. Dilution into buffer containing 150 mM NH4Cl in place of choline chloride served as a control in which no ΔpH was generated except for an additional component of the ΔpH that was formed when the dilution medium was at a higher pH than the intraproteoliposomal pH of 7.0. In these instances, an additional control was conducted in which the dilution was carried out into ammonium-containing buffer at pH 7.0. For assays of ΔpH-driven sodium uptake, 1 mM 22NaCl (4 μCi/μmol; 1 Ci = 37 GBq) was added to the dilution buffer. Assays of 86Rb+ were conducted identically except that 1 mM KCl was used in the dilution buffer instead of NaCl and 86Rb+ was added at 15 μCi/ml. For assays of ΔpH-driven uptake of Tc and Co2+, 50 μM [3H]Tc (20 μCi/μmol) was used together with 100 μM nonradioactive Co2+, and 100 μM 60CoCl2 (40 μCi/μmol) was used together with 50 μM nonradioactive Tc; controls using each of the radiolabeled substrates without the cosubstrate were also conducted.
For assays of sodium efflux in response to a diffusion potential (positive out), or a ΔpH (acid out), the proteoliposomes were prepared in buffer consisting of 100 mM potassium acetate, 10 mM choline phosphate (pH 7.0), and 200 μM sodium phosphate; for assays of cobalt efflux, 100 μM CoCl2 and 50 μM Tc were substituted for the sodium phosphate. For these assays, the proteoliposomes were loaded with either 22NaCl or 60CoCl2 (at 50 μCi/ml) plus 50 μM nonradioactive Tc for 1 min. During this incubation, valinomycin was present at a final concentration of 2.5 μM in some samples. The assay was initiated by diluting 4 μl of proteoliposomes into 500 μl of 10 mM choline phosphate (pH 7.0), 5 μM valinomycin, and either 100 mM potassium acetate (control in which no gradients were established), potassium gluconate (ΔpH), or choline acetate (diffusion potential).
In both uptake and efflux assays, reactions were stopped by filtering samples onto 0.22 μm pore size GS filters (Millipore) which were then washed with 4 ml of cold reaction buffer. Radioactivity of the filters was measured in a scintillation counter. Numbers presented are the average of at least two independent experiments and in each experiment the values were determined in duplicate. For calculations of the rates of transport, the amount of protein in the proteoliposome preparations was determined by visualizing different volumes of proteoliposomes on silver-stained gels that also contained a standard curve of purified TetA(L). The intensity of the bands was quantitated using imagequant software. The protein content of the everted vesicles used as the starting material was determined by the method of Lowry and coworkers (19) using BSA as a standard.
RESULTS
Purification of TetA(L)–H6.
Western analyses of everted membrane vesicles from uninduced, glucose-repressed and induced transformants of E. coli expressing the modified tetA(L) gene indicated nearly undetectable levels of the gene product before induction. Thus while the isopropyl-1-β-d-thiogalactopyranoside treatment clearly caused a very large increase in TetA(L)–H6 content of the membranes, this increase could not be accurately quantitated (data not shown). The purified TetA(L)–H6 preparations showed a single band, with no major contaminants, on silver-stained SDS/polyacrylamide gels and upon Western blot analysis (Fig. 1). The molecular mass for the purified protein was calculated to be 37.5 kDa, based on mobility in the gels, as compared with that of 49.9 kDa calculated from the deduced sequence of the modified TetA(L).
Figure 1.
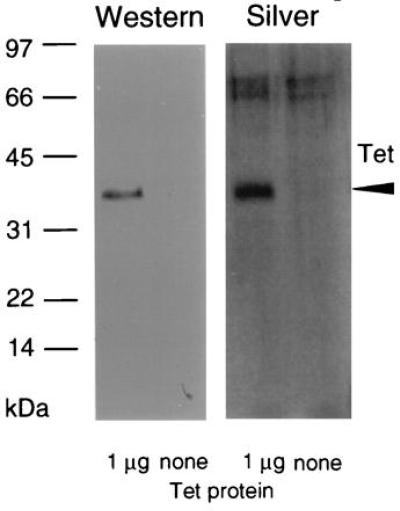
Electrophoretic characterization of TetA(L)–H6 purified from E. coli expressing a modified B. subtilis tetA(L) gene on a multicopy plasmid. Purified TetA(L)–H6 (1 μg) next to empty lanes containing no protein. After electrophoresis, the sample was silver-stained (Right) or transferred and analyzed for cross-reaction with antibody raised against a synthetic peptide corresponding to the N-terminal 14 amino acids of TetA(L) (Left); although not shown, preimmune rabbit serum showed no cross-reaction. The arrowhead indicates the position of TetA(L)–H6. A higher set of bands found in both lanes of the silver-stained gel is most likely a staining artifact.
Activities of TetA(L)–H6 in Proteoliposomes.
Preliminary assays of Na+ uptake and of Tc uptake in the presence of cobalt were conducted in proteoliposomes that were energized by generation of an outwardly directed gradient of protons using the ammonium-loading strategy described in Materials and Methods. In the initial experiments, energization by imposition of this gradient was compared at various pH values. Although the data are not shown, Tc uptake by the proteoliposomes was optimal when the dilution buffer was at pH 7.5, whereas Na+ uptake was optimal when the dilution buffer was at pH 8.5. As shown in Fig. 2, establishment of an outwardly directed gradient of protons by dilution of the proteoliposomes into ammonium-free dilution buffers at those pH values resulted in rapid uptake of both Tc and Na+. Because part of the driving force was in the form of a pH shift, resulting from the use of a dilution buffer that was more alkaline than the loading buffer, some uptake was observed even when the dilution buffer contained ammonium; although not shown, no uptake of either substrate occurred over time when the dilution buffer contained ammonium and was at pH 7.0. For Tc uptake, the highest specific activity of the proteoliposomes was 222 μmol/min/mg of protein, and for Na+ uptake, it was 114 μmol/min/mg of protein. The turnover number of the most active TetA(L) preparation was about 11,100 per min for Tc and 5,700 per min for Na+ in the solute uptake direction; it is likely that transmembrane potential-driven efflux would yield even higher values, as observed for the E. coli nhaA antiporter product (20), but those initial rates were too fast to determine accurately (see below). Importantly, there was again little cross-inhibition between the alternate substrates, especially in the early time points. Tc uptake in the presence of cobalt was not dramatically inhibited by Na+, and Na+ uptake was not initially inhibited much by Tc–cobalt. As shown in Table 1, the energy-dependent Na+ uptake by the proteoliposomes was more markedly inhibited by the presence of either Li+ or K+, and the proteoliposomes took up Rb+ at approximately the same rate as that of Na+. The finding of TetA(L)-mediated, proton-driven antiport of both Na+ and Rb+, the latter being taken as a reporter of some K+ flux pathways, was consistent with the earlier indications of TetA(L)’s important involvement in both Na+- and K+-dependent pH homeostasis upon an alkaline shift (10).
Figure 2.
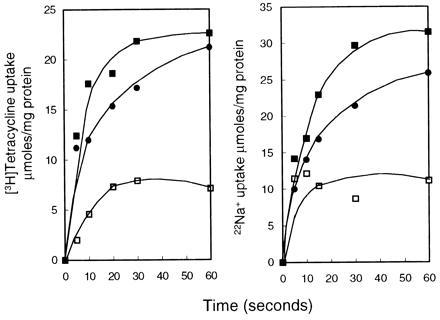
Uptake of Tc and Na+ into TetA(L)–H6 proteoliposomes upon imposition of an outwardly directed proton gradient. The proteoliposomes were loaded at pH 7.0 with 100 mM NH4Cl and then diluted into the following: (Left) ammonium-free buffer at pH 7.5 containing 50 μM [3H]Tc and 100 μM CoCl2 (▪) alone or also containing either 10 mM NaCl (•) or 100 mM NH4Cl (□); (Right) ammonium-free buffer at pH 8.5 containing 1 mM 22Na+ alone (▪) or also containing either 50 μM Tc plus 100 μM CoCl2 (•) or 100 mM NH4Cl (□). Samples were filtered at intervals and analyzed as described in Materials and Methods.
Table 1.
Uptake of 22Na+ and 86Rb+ upon imposition of an inwardly directed pH gradient across TetA(L) proteoliposomes, and cross-inhibition by monovalent cations
| Cation solute | Uptake,* μmol/mg of protein at 1 min | % inhibition by
|
||
|---|---|---|---|---|
| Na+ | Li+ | K+ | ||
| 22Na+ | 20.5 | — | 73 | 83 |
| 86Rb+ | 18.4 | 93 | ND | — |
ND, Not determined.
TetA(L) proteoliposomes were prepared and loaded with NH4Cl as described in Materials and Methods. Uptake was initiated by dilution into ammonium-free buffer containing either 1 mM 22Na+ or 1 mM 86Rb+ (K+) plus no added cations, 10 mM LiCl, 100 mM KCl, or 100 mM NaCl, as indicated. Samples were filtered at intervals. The amount of uptake of the radiolabeled cation in the absence of inhibitors was calculated from the 1-min point.
Consistent with the calculations of Yamaguchi and colleagues (21) for the TetA(B) protein assayed in membrane vesicles, the Tc/cobalt ratio was 1:1 in experiments in which Tc–cobalt was the substrate with either uptake of [3H]Tc or 60Co2+ being monitored (Fig. 3). No uptake of labeled Tc occurred in the absence of cobalt, and no uptake of labeled cobalt occurred in the absence of Tc (Fig. 3); monovalent cations could not substitute for cobalt in support of Tc uptake (data not shown).
Figure 3.
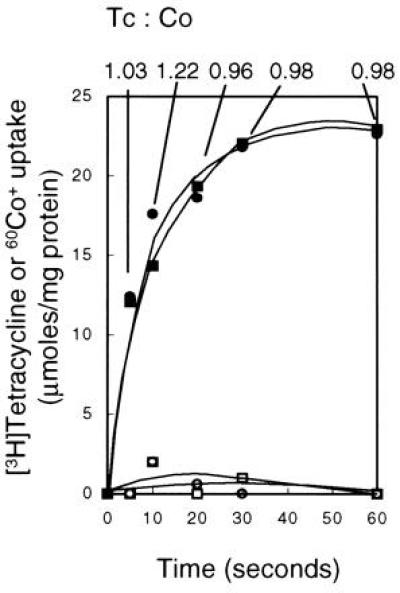
Uptake of Tc and cobalt into TetA(L)–H6 proteoliposomes upon imposition of an outwardly directed proton gradient. The proteoliposomes were loaded with NH4Cl at pH 7.0 as described in Materials and Methods and were diluted into ammonium-free buffer at pH 7.5 containing 50 μM [3H]Tc and 100 μM nonradioactive CoCl2 (•); 50 μM nonradioactive Tc and 100 μM 60CoCl2 (▪); 50 μM [3H]Tc alone (○); or 100 μM 60CoCl2 alone (□). Samples were filtered at intervals and processed as described in the text. The numbers at the top of the figure indicate the Tc/Co ratios calculated from total accumulation at the points shown by the lines.
The earlier experiments in which TetA(L)-dependent Tc and Na+ uptake had been measured in everted E. coli vesicles indicated that both antiports were electrogenic (11), in contrast to findings by others with the Gram-negative TetA(B) (22). Both TetA(L)-mediated antiports in vesicles were uninhibited by abolition of the ΔpH by nigericin. This treatment would have left only the transmembrane electrical component of the respiration-derived gradient, a driving component that would not energize an electroneutral flux (11). As shown in Fig. 4 and consistent with the vesicle experiments, efflux of cobalt, in the presence of Tc or of Na+, from preloaded proteoliposomes could be energized by imposition of either a ΔpH, acid out, or a transmembrane electrical potential, positive out, with the latter being somewhat more efficacious.
Figure 4.
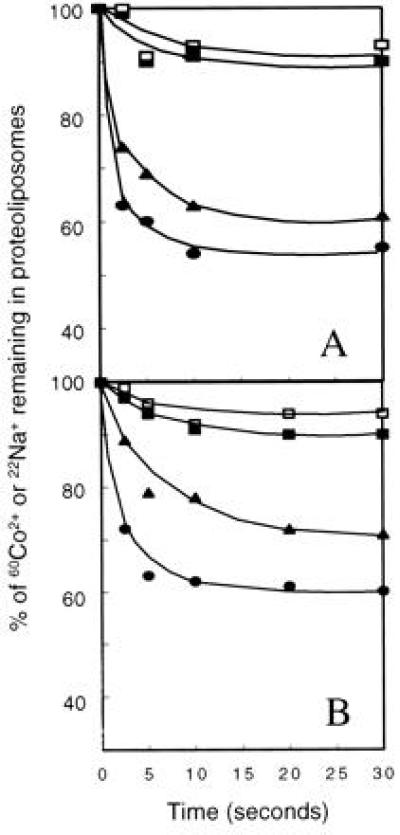
Efflux of 60Co2+ in the presence of Tc or of 22Na+ from preloaded TetA(L)–H6 proteoliposomes upon imposition of either an inwardly directed proton gradient or a diffusion potential, outside positive. Proteoliposomes were loaded with 100 mM potassium acetate and either 100 μM 60Co2+ plus 50 μM nonradioactive Tc (A) or 1 mM 22Na+ (B). During the loading, 2.5 μM valinomycin was also present in all but the control sample with no valinomycin (□). The experiment was initiated by the dilution of the appropriately loaded proteoliposomes into buffers at pH 7.0 and containing 5 μM valinomycin and 100 mM potassium acetate (▪), potassium gluconate (▴), or choline acetate (•). The control with no valinomycin was diluted into buffer lacking valinomycin and containing 100 mM potassium acetate.
DISCUSSION
Successful reconstitution of purified TetA(L) has provided compelling evidence in support of the hypothesis that this protein is a multifunctional antiporter that catalyzes Tc–metal/H+ antiport, Na+/H+ antiport, and K+/H+ antiport. The domains that form the binding sites for the antibiotic–divalent metal complex and for the monovalent cation substrates may be distinct, given the cross-inhibition among monovalent cations but the low level of cross-inhibition seen between monovalent cations and Tc–cobalt transport and vice versa. In fact, the latter cross-inhibitions were very low or zero at early time points. At later time points, modest cross-inhibition may reflect competition for the same driving force rather than for sites or translocation pathways on TetA(L). In the vesicle experiments in which no cross-competition was observed between the two types of substrates, the electrochemical proton gradient was being actively generated by respiration throughout the assay (11). The economy of using the same transporter for diverse physiological functions such as resistance to exogenous antibiotics and for Na+-resistance and Na+(K+)-dependent pH homeostasis in the alkaline range of pH would be preserved without competitive cross-inhibition. Tc is most toxic at pH values near or below neutral, where the inwardly directed proton gradient provides the driving force for noncarrier-mediated accumulation of the neutral, protonated Tc (23). At higher pH values of growth, less Tc would enter even when it was present in the environment; TetA(L) would be used more heavily, on the other hand, for its monovalent cation/H+ antiport activities, which include pH homeostasis under those conditions and the exclusion of Na+, which is more cytotoxic as the growth pH is raised (24, 25). Use of a monovalent cation/H+ antiporter to catalyze net proton accumulation during respiration at high pH would require an electrogenic antiporter that functioned with a H+/Na+ or K+ ratio greater than unity. The data presented here support the electrogenicity of both the Tc–metal/H+ and Na+/H+ antiport activities, similar to observations with the Na+/H+ ratio of the E. coli Nha proteins (18, 26) and in contrast to data for TetA(B) (22). On the other hand, as for TetA(B) (21), the apparent Tc/cobalt ratio for that solute complex was 1:1 for TetA(L).
This is the first preparation of a purified Tet protein that reconstitutes Tc–metal transport when present as the sole protein in proteoliposomes. It is also the first successful reconstitution among the family of 14-transmembrane segment, drug-resistance antiporters. Two other purified preparations of Tet proteins, both of the Gram-negative TetA(B) version, have been described (27, 28), but the only reported reconstitution of Tc transport activity necessitated the co-reconstitution of an F1F0–ATPase complex to effect successful energization (28). That co-reconstituted TetA(B) preparation had modest activity compared with the activity assayed in membrane vesicles. In contrast, the turnover numbers calculated for TetA(L)–H6 in proteoliposomes fall in the same extraordinarily high range as those observed for the NhaA and NhaB proteins from E. coli (18, 20). The specific activities for Tc–cobalt transport and for Na+ transport were also much higher than that observed in membrane vesicles, by orders of magnitude. It was not possible to precisely assess the relative contributions of overexpression and purification since the vesicles from cells in which tetA(L) overexpression is induced are leaky to ions; however, both contributions were large. Perhaps, the specifics of the reconstitution (e.g., protein/lipid ratio) and lipid purification procedures used in the current study account for the high activity of TetA(L)–H6 in the proteoliposomes. However, it is also possible that for unknown and unanticipated reasons, the packing properties of the larger TetA(L)–H6 protein in the commercial lipids used creates a less leaky preparation than that created with the smaller TetA(B) protein. In natural membranes, even TetA(L) is found to make the membranes leaky, as indicated by the toxicity of this protein when overexpressed just modestly. The enhanced leak of Tc may partially explain why the resistance conferred by a protein with such high in vitro activity represents only a several-fold increase in the minimum inhibitory concentration.
Tc efflux pumps had been thought to originate largely in antibiotic-producing organisms in concert with the development of the synthetic mechanism for the antibiotic (29, 30). The findings of major physiological roles and associated regulatory features for the chromosomally encoded TetA(L) of B. subtilis support the view that this particular family of proteins may have emerged in soil bacteria that are the natural targets rather than producers of Tc (1, 10). A model has been advanced for the advantage that may have been inherent in the use of a monovalent cation/H+ antiporter for the additional function of Tc–metal efflux (1). Refinement of the model may result from the further clarification of the roles of TetA(L) that a functional, purified assay system will facilitate. For example, there is probably an as yet unknown endogenous substrate for TetA(L) that accounts for the phenotype of the tetA(L) deletion strain at pH 7.0 (10). This substrate might be an antibiotic, cell wall layer component or siderophore that B. subtilis produces and must export for it to be useful and nontoxic to the producer. Preliminary data suggest that this substrate uses the divalent cation-complexed pathway, since the tetA(L) deletion strain is more sensitive to growth inhibition by cobalt than the wild type is even in the absence of Tc (A. A. Guffanti, unpublished data). The endogenous substrate may be an additional factor, together with inward leak of Tc, that reduces the apparent efficacy of TetA(L) in vivo. Perhaps this apparent inefficacy relative to in vitro preparations results from competitive inhibition by the endogenous substrate. Presumably, an endogenous substrate that is coeffluxed with Co2+ by TetA(L) could be assayed by its ability to promote energy-dependent cobalt uptake in TetA(L) proteoliposomes. More complete information about the multiple roles of antibiotic efflux proteins is important because plausible models of the origins of antibiotic-resistance genes inform our understanding of the proliferation of such genes and our evaluation of means to limit that proliferation.
Acknowledgments
This work was supported by National Institutes of Health Grant GM52837 and National Science Foundation Grant MCB9600555.
Footnotes
Abbreviations: ΔpH, transmembrane gradient of pH; H6, hexahistidine; Tc, tetracycline.
References
- 1.Cheng J, Baldwin K, Guffanti A A, Krulwich T A. Antimicrob Agents Chemother. 1996;40:852–857. doi: 10.1128/aac.40.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy S B. Antimicrob Agents Chemother. 1992;36:695–703. doi: 10.1128/aac.36.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speer B S, Shoemaker N B, Salyers A A. Clin Microbiol Rev. 1992;5:387–399. doi: 10.1128/cmr.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulsen I T, Skurray R A. Gene. 1993;124:1–11. doi: 10.1016/0378-1119(93)90755-r. [DOI] [PubMed] [Google Scholar]
- 5.Paulsen I T, Brown M H, Littlejohn T G, Mitchell B A, Skurray R A. Proc Natl Acad Sci USA. 1996;93:3630–3633. doi: 10.1073/pnas.93.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marger M D, Saier M H., Jr Trends Biochem Sci. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 7.Nikaido H. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 8.Nelson M L, Park B H, Andrews J S, Georgian V A, Thomas R C, Levy S B. J Med Chem. 1993;36:370–377. doi: 10.1021/jm00055a008. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J, Guffanti A A, Krulwich T A. J Biol Chem. 1994;269:27365–27371. [PubMed] [Google Scholar]
- 10.Cheng J, Guffanti A A, Wang W, Krulwich T A, Bechhofer D H. J Bacteriol. 1996;178:2853–2860. doi: 10.1128/jb.178.10.2853-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guffanti A A, Krulwich T A. J Bacteriol. 1995;177:4557–4561. doi: 10.1128/jb.177.15.4557-4561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambudkar S V, Zlotnick G W, Rosen B P. J Biol Chem. 1984;259:6142–6146. [PubMed] [Google Scholar]
- 13.Viitanen P, Newman M H, Foster D L, Wilson T H, Kaback H R. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- 14.Ansorge W. In: Electrophoresis ’82. Stathakos D, editor. New York: de Gruyter; 1982. pp. 235–242. [Google Scholar]
- 15.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Racker E, Bioland B, O’Neal S, Alfonzo M, Telford J. Arch Biochem Biophys. 1979;198:470–477. doi: 10.1016/0003-9861(79)90521-6. [DOI] [PubMed] [Google Scholar]
- 18.Pinner E, Padan E, Schuldiner S. J Biol Chem. 1994;269:26274–26279. [PubMed] [Google Scholar]
- 19.Lowry O H, Rosebrough N H, Farr A L, Randall R J. J Biol Chem. 1951;193:262–275. [PubMed] [Google Scholar]
- 20.Taglicht D, Padan E, Schuldiner S. J Biol Chem. 1991;266:11289–11294. [PubMed] [Google Scholar]
- 21.Yamaguchi A, Udagawa T, Sawai T. J Biol Chem. 1990;265:4809–4813. [PubMed] [Google Scholar]
- 22.Yamaguchi A, Iwasaki-Ohba Y, Ono N, Kaneko-Ohdera M, Sawai T. FEBS Lett. 1991;282:415–418. doi: 10.1016/0014-5793(91)80527-a. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi A, Ohmori H, Kaneko-Ohdera M, Nomura T, Sawai T. Antimicrob Agents Chemother. 1991;35:53–56. doi: 10.1128/aac.35.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krulwich T A, Cheng J, Guffanti A A. J Exp Biol. 1994;196:457–470. doi: 10.1242/jeb.196.1.457. [DOI] [PubMed] [Google Scholar]
- 25.Padan E, Schuldiner S. Biochim Biophys Acta. 1994;1185:129–151. doi: 10.1016/0005-2728(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 26.Taglicht D, Padan E, Schuldiner S. J Biol Chem. 1993;268:5382–5387. [PubMed] [Google Scholar]
- 27.Aldema M L, McMurry L M, Walmsley A R, Levy S B. Mol Microbiol. 1996;19:187–193. doi: 10.1046/j.1365-2958.1996.359886.x. [DOI] [PubMed] [Google Scholar]
- 28.Someya Y, Moriyama Y, Futai M, Sawai T, Yamaguchi A. FEBS Lett. 1995;374:72–76. doi: 10.1016/0014-5793(95)01079-t. [DOI] [PubMed] [Google Scholar]
- 29.Levy S B. Antimicrob Agents Chemother. 1989;24:1–3. doi: 10.1093/jac/24.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Lewis K. Trends Biochem Sci. 1994;19:119–123. doi: 10.1016/0968-0004(94)90204-6. [DOI] [PubMed] [Google Scholar]


