Abstract
HIV-infected children (n = 243), ≥5 to <18 years old, receiving stable antiretroviral therapy, were stratified by immunologic status and randomly assigned to receive intranasal live attenuated influenza vaccine (LAIV) or intramuscular trivalent inactivated influenza vaccine (TIV). The safety profile after LAIV or TIV closely resembled the previously reported tolerability to these vaccines in children without HIV infection. Post-vaccination hemagglutination inhibition (HAI) antibody responses and shedding of LAIV virus were also similar, regardless of immunological stratum, to antibody responses and shedding previously reported for children without HIV infection. LAIV should be further evaluated for a role in immunizing HIV-infected children.
Keywords: HIV-infected children, Live attenuated influenza vaccine, Vaccine virus shedding
INTRODUCTION
Influenza virus infections occur regularly in children in annual winter-spring epidemics. The resulting illness usually causes mild or moderately severe upper respiratory and systemic symptoms, but more significant tracheobronchial involvement is common. Rarely central nervous system and other complications accompany influenza infection (1,2), but the most common complications are bacterial superinfection, such as otitis media, sinusitis, and pneumonia (3–7). Influenza has an even greater potential to cause bacterial complications in some HIV-infected children, and is likely to complicate their routine care during epidemic periods (8). Influenza was associated with an excess of hospitalization, emergency room visits, and prolonged symptoms in a subset of adults with HIV (9–12).
The current standard of care for HIV-infected adults and children recommends immunization with an inactivated trivalent influenza vaccine (TIV) (4, 13–14). The immunogenicity of TIV in HIV-infected adults and children with advanced disease is limited, whereas it is more likely to induce a strong response in those in earlier phases of HIV infection (15–18). Although the efficacy of TIV in HIV-infected children has not been established, there is some evidence of its efficacy in HIV-infected adults (9, 19).
The live, attenuated cold-adapted influenza vaccine (LAIV) has potential advantages for HIV-infected children. Intranasal administration of LAIV simplifies immunization and avoids the pain of intramuscular injection, thereby making LAIV more acceptable to most patients and avoiding missed opportunities because of deferred administration. Moreover, because of intranasal administration, LAIV has the potential to stimulate greater local anti-influenza immune responses than TIV (20–22). LAIV demonstrated greater reductions in influenza than TIV in HIV-uninfected children, against strains that were well-matched with strains in the vaccine, as well as against antigenically drifted strains not included in the vaccine administered (23–28), and several studies have shown that the LAIV protective effect can extend beyond the year of administration (27, 28).
LAIV was demonstrated to be, well tolerated, and immunogenic in 57 asymptomatic or mildy symptomatic HIV-infected adults (29), and was also safely administered to 24 HIV-infected children (30). In these children LAIV induced a influenza-specific hemagglutination inhibition antibody (HAI) response similar to that observed in HIV-uninfected children; a significant serological response occurred in 65–95% of HIV-infected children, depending on their pre-existing immunity against influenza and the serotype-specific antibody measured. Prolonged shedding of vaccine virus did not occur.
The current report compares the safety and serotype-specific anti-influenza antibody responses of 234 HIV-infected children one and six months after they received either LAIV or TIV, and documents limited nasal shedding of live vaccine virus after its administration.
METHODS
Population
Vaccine recipients were HIV-infected children and adolescents (≥ 5 to < 18 years of age) on a stable highly active antiretroviral therapy (HAART) regimen for ≥ 16 weeks, with no change in their HAART regimen anticipated. HAART was defined as ≥ 3 antiretrovirals from at least two different therapeutic classes or the combination of ZDV/3TC/ABC (Trizivir®). HIV-1 plasma RNA had to be < 60,000 copies/mL within 60 days prior to screening, and the subject must have received TIV in at least one of the prior two years. Potential subjects were excluded if: they had received immunosuppressive or immunomodulatory therapy within 60 days prior to immunization; any aspirin or aspirin-containing therapy at the time of vaccination or planned for 42 days thereafter; any other inactivated vaccine within 14 days prior to vaccination or live vaccine within 30 days prior to vaccination; any vaccine planned for the 30 days following vaccination; or received any blood product within 3 months prior to vaccination or planned during the study. Additional influenza vaccines and prophylactic anti-influenza drugs were precluded. Children with chronic pulmonary disease, obstructive or restrictive, that was moderate or greater in severity, as judged by clinical assessment by the site medical staff and available pulmonary function tests, were excluded. Medically-diagnosed wheezing, bronchodilator use, or steroid use (systemic or inhaled), within the 42 days prior to study entry, including children with recent persistent asthma, were excluded.
Regimen
All subjects received influenza immunization as soon as possible in September 2004 and as late as November 19, 2004. Subjects were randomly allocated to receive either LAIV (Arm A) or TIV (Arm B). In each study Arm the vaccinees were stratified by the following CD4% criteria: Immunological Group 1 = CD4% <15 at nadir and ≥ 15 at screening; Immunological Group 2 = CD4% ≥ 15 but < 25 at nadir and ≥ 15 at screening; and Immunological Group 3 = CD4% ≥ 25 at nadir and ≥ 25 at screening. Nadir was defined as the lowest CD4% ever recorded during the subject’s lifetime. The subject remained in his/her assigned Group throughout the study for purposes of analysis.
Vaccines
Arm A (LAIV) received the frozen formulation of Influenza Virus Vaccine Live, Intranasal, (FluMist®; MedImmune) 0.5 mL (0.25 mL per nostril); Arm B (TIV) received Influenza Viral Vaccine, Intramuscular, (Fluzone®; Aventis Pasteur, Inc.) 0.5 mL in the deltoid muscle region. Both vaccines were stored and administered according to the package insert. The strains represented in the vaccines were those recommended by the U.S. Public Health Service (USPHS) for the 2004/2005 season: A/New Caledonia/20/99 (H1N1); A/Wyoming/3/2003 (H3N2) [an A/Fujian/411/2002-like virus]; and B/Jilin/20/2003 (LAIV) or B/Jiangsu/10/2003 (TIV); both are B/Shanghai/361/2002-like viruses.
Clinical evaluation
Subjects or their caretakers had information collected about adverse events on days 3, 7, 14, and 21 post-vaccination by telephone if they were in Arm B, and by telephone (days 7 and 21) and during scheduled study visits (days 3 and 14) if they were in Arm A. Subjects in both Arms were seen in clinic on day 28 post-vaccination. A diary card was kept for 42 days after vaccination, and information concerning serious adverse events was obtained during a day 42 post-vaccination telephone call and a 6-month clinic visit. The etiology of any lower respiratory illnesses was assessed with viral cultures and/or rapid diagnostic tests.
Specimen Collection
Blood was obtained on all subjects prior to vaccination, at 28 days, and 6 months after vaccination in order to measure plasma HIV RNA, lymphocyte phenotypes, and serum levels of HAI antibody against influenza serotypes in the vaccine. Subjects receiving LAIV had their nares swabbed on day 3, 14, and 28 in order to detect vaccine strain influenza. Any culture that was positive at day 14 or later was followed with a repeat negative culture within the subsequent 14 days.
Influenza-specific HAI assay
Serum samples were treated with receptor-destroying enzyme from Vibrio cholerae (Denka-Seiken). These were diluted 1:10 in saline and subsequent serial 2-fold dilutions of the sera were used in a standard HAI assay using 4 hemagglutinating units of the viruses or antigen and 0.75% guinea pig red blood cells (31). Serum samples with titers ≥10 and ≥40 were considered indicative of immunity and protection, respectively. The antigens used were: A/New Caledonia/20/99 (H1N1) and A/Wyoming/03/2003 (H3N2) cold-adapted viruses, and B/Yamanashi/166/98 (Shanghai-like) antigen generously provided by Dr. Alexander Klimov Centers for Disease Control and Prevention).
Cultures of nasal swab specimens for influenza virus
Each nostril was sampled using a Dacron swab. These swab samples were pooled, placed immediately in viral transport media, stored at 2° C to 8° C, and shipped at this temperature to the University of Colorado Hospital Clinical Virology Laboratory.
Viral isolation
Clinical specimens (0.3 ml) were inoculated into each of two RhMK tubes, each from a different vendor (BioWhitaker and Viromed). Tubes in maintenance medium consisting of Eagle’s medium (BioWhitaker) with penicillin, streptomycin and amphotericin B were incubated at 37°C for up to 14 days. Medium was changed at 24 to 48 h after inoculation, after each HAI assay, and as dictated by the appearance of the monolayer. Tubes were examined daily during the first week of culture and thrice weekly thereafter by light microscopy. Hemagglutination assay with guinea pig red blood cells was performed weekly. At the end of the observation period monolayers were trypsinized and the cell suspension spotted onto slides, followed by acetone fixation and staining with specific monoclonal antibodies (Dako). Slides were read with a fluorescence microscope. A positive result was defined as the presence of bright green fluorescence in the cytoplasm of ≥2 cells/slide.
Titration of influenza in positive specimens
Virus from influenza-positive cultures was quantified in an assay that measured infectious, cytocidal virus in confluent Madin-Darby canine kidney (MDCK) cells in 96-well plates. Serial dilutions of thawed influenza-positive nasal swab samples were prepared and added to the plates with MDCK cells, resulting in a final dilution of 1:5 to 1:50,000 (−0.7 to −4.7 log10 TCID50/mL) with 2 replicates of each dilution. Inoculated plates were incubated at 33°C±1°C with 5% CO2 for 6 days. Metabolically active cells were identified using an Alamar Blue® dye colorimetric assay (excitation at 535 nm, emission at 590 nm). Data were converted to virus titer using the modified Karber formula. Replicates of all samples below the limit of detection (<1 log10 TCID50/mL) were reported as 0.5 log10 TCID50/mL.
Genotyping and subtyping of influenza isolates
A PCR assay was used to identify and confirm the presence of wild-type (wt) and cold-adapted (ca) influenza virus in nasal swab samples using two separate PCR amplification reactions (32). One reaction differentiated between wt A/H1, A/H3, and B viruses; the second differentiated ca A from ca B viruses. The PB1 gene segment of wt A/H1, wt A/H3 and ca A viruses or the PA gene segment of wt B and ca B viruses were selected for primer design. Briefly, RNA was extracted, reverse-transcribed and PCR-amplified in separate reactions using commercial kits (Qiagen). PCR amplification of wild-type isolates used a mixture of primer pairs specific for wt A/H1, A/H3 or B genotypes. PCR amplification of ca isolates used a mixture of primers specific for ca A and B genotypes. Identification of each genotype was made by its unique PCR product size as determined by electrophoresis on microfluidics chips (Agilent Bioanalyzer). Appropriate wt and ca positive detection controls and negative controls were included in all assays; the lower limit of detection (LOD) was 2.0 log10 TCID50/mL.
Since ca A/H1 and A/H3 genotypes were recognized by the same primer pair, they could not be differentiated in this assay and a PCR subtyping assay was used to identify and confirm the presence of influenza A/H1, A/H3 or B subtype viruses in clinical trial samples based on selective amplification of the HA gene segment of each influenza subtype. The primers specific for each subtype were designed to generate PCR products of defined size to enable subtype identification based on size. Briefly, RNA was extracted, reverse-transcribed and PCR-amplified as above using commercial kits (Qiagen). PCR amplification included primer pairs for any of the three influenza subtypes (A/H1, A/H3 or B). As above, identification of each subtype was made by its unique PCR product size as determined by electrophoresis on microfluidics chips. The LOD of the assay was 2.0 log10TCID50/mL.
Statistics
Influenza-specific HAI responses were assessed as "protective" (HAI titer ≥40); 4-fold increases over entry HAI titer; and geometric mean titers (GMTs). Logarithms (base 2) of HAI titers were used to normalize data where appropriate. For the immunogenicity analyses of seroresponse and GMTs, only subjects with pre- and post-vaccination data were included. An HAI titer of 2 was assigned for the analysis if the titer was reported as < 10. The HAI GMTs and GMT ratios, defined as the ratio of GMT in LAIV and GMT in TIV, was summarized by treatment group and 95% CIs were calculated by the bootstrap method. Two-sided exact confidence intervals around the difference between the response rates of LAIV and TIV recipients were constructed using the unconditional exact method proposed by Chan and Zhang (33). Univariate linear regression analyses were performed to identify predictors of baseline and week 4 HAI titers. For categorical variables having >2 categories, Tukey-Kramer simulation-based adjusted P-values were used for pair-wise comparisons when F tests were significant. Variables identified as at least marginally significant (P<0.1) predictors of the week 4 HAI titer for at least one influenza strain were included in the multivariate linear regression. The immunologic stratification group was also included in the multivariate linear regression.
RESULTS
The demographics of the 243 subjects were similar for the LAIV and the TIV recipients in each of the immunologic Groups vaccinated and between the Arms when the three Groups were combined (Table 1). CD4% and CD4 count increased progressively from Group 1 to Group 3 in accordance with the experimental design. HIV viral load was similar between the vaccine Arms and between the immunologic Groups.
Table 1.
Demographics of subjects receiving either LAIV or TIV
| Parameters | Group 1 | Group 2 | Group 3 | Groups Combined | ||||
|---|---|---|---|---|---|---|---|---|
| LAIV | TIV | LAIV | TIV | LAIV | TIV | LAIV | TIV | |
| Number | 45 | 44 | 50 | 48 | 27 | 29 | 122 | 121 |
| Gender | ||||||||
| Male | 23 (51%) | 26 (59%) | 25 (50%) | 28 (58%) | 17 (63%) | 10 (34%) | 65 (53%) | 64 (53%) |
| Female | 22 (49%) | 18 (41%) | 25 (50%) | 20 (42%) | 10 (37%) | 19 (66%) | 57 (47%) | 57 (47%) |
| Ethnicity | ||||||||
| White1 | 5 (11%) | 4 (9%) | 7 (14%) | 4 (8%) | 7 (26%) | 3 (10%) | 20 (16%) | 11 (9%) |
| Black1 | 32 (71%) | 33 (75%) | 29 (58%) | 32 (67%) | 11 (41%) | 17 (59%) | 72 (59%) | 82 (68%) |
| Hispanic | 8 (18%) | 6 (14%) | 13 (26%) | 12 (25%) | 7 (26%) | 8 (28%) | 27 (22%) | 26 (21%) |
| Others | 0 (0%) | 1 (2%) | 1 (2%) | 0 | 2 (7%) | 1 (3%) | 3 (2%) | 2 (2%) |
| Age - years mean (STD) | 13.0 (2.7) | 12.2 (3.0) | 10.8 (3.2) | 12.2 (3.0) | 9.6 (3.1) | 10.9 (2.9) | 11.4 (3.3) | 11.9 (3.0) |
| Entry CD4% mean (STD) | 30.7 (8.9) | 31.9 (7.5) | 32.9 (8.1) | 32.6 (7.8) | 38.0 (5.9) | 39.9 (6.7) | 33.2 (8.4) | 34.1 (8.1) |
| Entry CD4 count mean (STD) | 732 (328) | 825 (373) | 842 (344) | 846 (362) | 1023 (346) | 1089 (349) | 842 (353) | 897 (376) |
| Entry Viral Load (Log10)* (STD) | 2.8 (0.6) | 2.7 (0.8) | 2.7 (0.7) | 2.7 (0.7) | 2.6 (0.7) | 2.7 (0.6) | 2.7 (0.7) | 2.7 (0.7) |
non-Hispanic
Roche Amplicor Monitor HIV RT PCR was used for 179 subjects (if copies/ml<400, then copies/ml was set to 400); Roche UltraSensitive HIV RT PCR was used for 62 subjects (if copies/ml <50, then copies/ml was set to <500); OTC NASBA HIV RNA QT assay was used for 2 subjects (if copies/ml <500, then copies/ml was set to 500).
Table 2a indicates the adverse events reported within 28 days after administration of each vaccine. The number of subjects with adverse events was similar after either vaccine in all event categories except for the injection site reactions after TIV (23% overall) and more nasopharyngeal symptoms after LAIV compared with TIV (52% vs 31%; p = 0.002). Event frequency did not vary significantly with the immunological Group of the vaccine recipients. Pulmonary signs and symptoms, including the incidence of asthma and wheezing within 28 days after vaccination, were similar in LAIV and TIV recipients.
Table 2.
| Table 2a. Adverse events within 28 days of vaccination: LAIV vs TIV | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group1 | Group2 | Group3 | All Groups | |||||
| Adverse Events1,2 | LAIV | TIV | LAIV | TIV | LAIV | TIV | LAIV | TIV |
| Abdominal Signs & Symptoms | 8 (18%) | 4 (9%) | 7 (14%) | 4 (8%) | 4 (15%) | 2 (7%) | 19 (16%) | 10 (8%) |
| Constitutional Signs & Symptoms | 15 (33%) | 13 (30%) | 13 (26%) | 16 (33%) | 7 (26%) | 4 (14%) | 35 (29%) | 33 (27%) |
| Ear or Eye Signs & Symptoms | 5 (11%) | 2 (5%) | 4 (8%) | 2 (4%) | 0 (0%) | 0 (0%) | 9 (7%) | 4 (3%) |
| Injection Local Reaction | 0 (0%) | 9 (20%) | 0 (0%) | 13 (27%) | 0 (0%) | 6 (21%) | 0 (0%) | 28 (23%) |
| Nasopharyngeal | 28 (62%) | 15 (34%) | 26 (52%) | 15 (31%) | 10 (37%) | 8 (28%) | 64 (52%) | 38 (31%) |
| Other | 3 (7%) | 1 (2%) | 5 (10%) | 1 (2%) | 3 (11%) | 0 (0%) | 11 (9%) | 2 (2%) |
| Pulmonary Signs & Symptoms | 19 (42%) | 13 (30%) | 12 (24%) | 11 (23%) | 8 (30%) | 7 (24%) | 39 (32%) | 31 (26%) |
| Skin Abnormality | 3 (7%) | 0 (0%) | 5 (10%) | 2 (4%) | 2 (7%) | 3 (10%) | 10 (8%) | 5 (4%) |
| Total Subjects | 45 | 44 | 50 | 48 | 27 | 29 | 122 | 121 |
| Table 2b. Toxicity Grades for adverse events within 28 days of vaccination LAIV vs TIV | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group1 | Group2 | Group3 | All Groups | |||||
| Adverse Events1,2 | LAIV | TIV | LAIV | TIV | LAIV | TIV | LAIV | TIV |
| Grade 1 | 22 (49%) | 16 (36%) | 24 (48%) | 14 (29%) | 14 (52%) | 10 (34%) | 60 (49%) | 40 (33%) |
| Grade 2 | 10 (22%) | 8 (18%) | 8 (16%) | 15 (31%) | 5 (19%) | 6 (21%) | 23 (19%) | 29 (24%) |
| Grade 3 3,4 | 2 (4%) | 2 (5%) | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (2%) | 2 (2%) |
| Ungraded Diagnoses5 | 9 [3] | 6 [3] | 8 [2] | 3 [1] | 3 [2] | 3 [1] | 20 [7] | 12 [5] |
| Total Subjects | 45 | 44 | 50 | 48 | 27 | 29 | 122 | 121 |
All adverse events were counted per subject.
Examples of adverse event categories are: abdominal = diarrhea, emesis, pain; constitutional = fever, chills, headache, malaise, fatigue, anorexia; ear or eye = conjunctivitis, ear pain, otitis media; injection site = tenderness, erythema, swelling; nasopharyngeal = discharge, congestion, sore throat; other = lymphadenitis, epistaxis, other viral illnesses; pulmonary = cough, chest pain, pneumonia, asthma; skin = rash, folliculitis, boil, cellulitis.
Worst grade counted per subject. Parentheses indicate per cent of subjects with events of the grade indicated.
Grade from DAIDS Toxicity Manual
Grade 3 Events after LAIV were malaise; finger pain; and leg boil.
Grade 3 Events after TIV were fever and 1 subject with an injection site lump.
Brackets indicate the number of ungraded diagnoses designated possibly or probably vaccine-related. Subject who had graded and ungraded diagnoses were counted separately in this category. These included - LAIV: Possibly = presumed pneumonia; conjunctivitis (2); sinusitis; Probably = pharyngitis (2); asthma. TIV; Possibly = conjunctivitis; Probably = otitis (2); pharyngitis (2); pneumonia.
Table 2b indicates the occurrence of grade 2 and grade 3 adverse events after each vaccine. No grade 4 events were reported. Grade 2 events occurred in 16–31% of subjects in individual immunological Groups, but there were no statistically significant differences between these Groups, regardless of the vaccine administered. Grade 2 events were more common with TIV as a result of injection site reactions. There were 3 subjects with Grade 3 events following LAIV (one considered vaccine-related) and 2 Grade 3 events following TIV (both considered vaccine-related). The number of ungraded diagnoses were similar in the vaccine Arms. Two cases of pneumonia were reported and radiographically confirmed. One subject who received TIV had a RML pneumonia that occurred 17 days after vaccination and was treated successfully as an outpatient with Azithromycin. Another subject received LAIV 13 days before developing a non-productive cough. Right-sided infiltrates were noted on chest X-ray; white blood cell count was 34,500/µl (74 polymorphs; 5 band forms), and a low-grade fever was present. Nasal wash was negative for influenza by rapid assay and culture, but the PCR demonstrated vaccine strain influenza A (H3N2). The patient improved within 24 hours after an injection of Ceftriaxone.
There were no significant increases from baseline in median/mean plasma HIV viral load after either vaccine in any of the immunological Groups. Similarly, median CD4% did not change significantly at any time point as a result of vaccination.
The immune response to each vaccine was measured by serum HAI assays. Table 3 indicates the proportion of subjects with HAI titer ≥40 analyzed with the immunologic Groups combined. The proportion with post-vaccination titers of this magnitude against the H1N1 antigen and H3N2 antigen was similar for both LAIV and TIV recipients. TIV recipients were roughly twice as likely to have responses of this magnitude to the B antigen, although the proportion of subjects with this baseline titer was higher in the TIV Arm. A similar proportion of LAIV or TIV recipients also achieved a 4-fold increase in HAI titer against the H1N1 antigen, whereas significantly more TIV recipients achieved a 4-fold increase in HAI titer against the H3N2 antigen at week 4 post-vaccination (but not at week 24) and against the B antigen at both post-vaccination time points. The geometric mean titer (GMT) of specific antibody responses to either vaccine is shown in Table 4. The GMT after either vaccine is similar against the H1N1 antigen, but is significantly higher after TIV against the H3N2 and B antigens. Antibody responses to a given antigen in each Arm were similar regardless of the immunological Group (data not shown).
Table 3.
Percentage of Subjects with Titer ≥40 and Percentage with four-fold increase over baseline in HAI Assay
| Percentage of Subjects with Titer ≥40 | ||||
|---|---|---|---|---|
| Influenza Antigen | Week | LAIV | TIV | (TIV-LAIV)1 |
| A H1N1 | 0 | 42% (49/116) | 46% (55/119) | 0.04 (−0.09, 0.17) |
| 4 | 63% (72/114) | 67% (75/112) | 0.04 (−0.09, 0.16) | |
| 24 | 45% (50/110) | 55% (64/116) | 0.10 (−0.03, 0.23) | |
| A H3N2 | 0 | 78% (90/116) | 88% (105/119) | 0.11 (0.01, 0.20)* |
| 4 | 92% (105/114) | 96% (108/112) | 0.04 (−0.02, 0.12) | |
| 24 | 95% (104/110) | 97% (113/116) | 0.03 (−0.03, 0.09) | |
| B | 0 | 23% (27/116) | 37% (44/119) | 0.14 (0.02, 0.25)* |
| 4 | 33% (38/114) | 69% (77/112) | 0.35 (0.23, 0.47)* | |
| 24 | 32% (35/110) | 63% (73/116) | 0.31 (0.18, 0.43)* | |
| Percentage of Subjects with four-fold increase over baseline | ||||
| Influenza Antigen | Week | LAIV | TIV | (TIV-LAIV)1 |
| A H1N1 | 4 | 32% (37/114) | 33% (37/112) | 0.01 (−0.12, 0.13) |
| 24 | 22% (24/110) | 16% (19/116) | −0.05 (−0.16, 0.05) | |
| A H3N2 | 4 | 14% (16/114) | 44% (49/112) | 0.30 (0.18, 0.41)* |
| 24 | 29% (32/110) | 34% (39/116) | 0.05 (−0.08, 0.17) | |
| B | 4 | 11% (12/114) | 34% (38/112) | 0.23 (0.13, 0.34)* |
| 24 | 11% (12/110) | 22% (25/116) | 0.11 (0.01, 0.20)* | |
The difference in rate between TIV and LAIV and its 95% confidence interval.
Indicates significant difference with p<0.05
Table 4.
Geometric mean antibody responses to LAIV and TIV
| Influenza Antigens | Week | N | LAIV GMT (CI)1 | N | TIV GMT (CI) | GMT ratio [LAIV/TIV] (CI) |
|---|---|---|---|---|---|---|
| A H1N1 | 0 | 116 | 17.7 (13.2,23.8) | 119 | 20.8 (15.9,27.1) | 0.85 (0.58,1.26) |
| 4 | 114 | 34.9 (28.6,42.6) | 112 | 47.3 (36.1,61.5) | 0.74 (0.53,1.03) | |
| 24 | 110 | 25.1 (19.8,32) | 116 | 30.9 (24.0,39.8) | 0.81 (0.57,1.15) | |
| A H3N2 | 0 | 116 | 78.0 (63.6,96.1) | 119 | 89.7 (74.2,108.1) | 0.87 (0.66,1.15) |
| 4 | 114 | 102.4 (84.8,123.2) | 112 | 265.8 (215.3,328.0) | 0.39* (0.29,0.51) | |
| 24 | 110 | 139.0 (112.0,172.2) | 116 | 224.9 (186.9,270.7) | 0.62* (0.46,0.82) | |
| B | 0 | 116 | 17.1 (14.5,20.1) | 119 | 20.9 (17.8,24.3) | 0.82 (0.65,1.03) |
| 4 | 114 | 20.0 (17.2,23.3) | 112 | 41.0 (34.3,48.7) | 0.49* (0.39,0.62) | |
| 24 | 110 | 19.8 (16.7,23.4) | 116 | 36.9 (31.8,42.7) | 0.54* (0.43,0.67) |
GMT = geometric mean titer; CI = 95% confidence intervals
Indicates significant difference with p<0.05
Univariate linear regression analysis of antibody titers in subjects prior to vaccination indicated that HAI GMTs were not significantly different between the three immunological Groups, and that prior to vaccination there was little consistent relationship of HAI GMT to age, CD4% or CD8%, or entry HIV RNA plasma levels. However, the baseline HAI GMT for 3 of 6 baseline comparisons was significantly higher in girls than in boys. Univariate linear regression analysis demonstrated that with all strains in both vaccines the HAI GMT four weeks after vaccination correlated directly with the HAI GMT titer prior to vaccination (P<0.0001). Also at four weeks post-vaccination in LAIV recipients, there was an inverse relationship between entry HIV RNA plasma levels and HAI GMT against one strain (H3N2, P=0.02). In TIV recipients this inverse relationship was observed for all strains (H1N1, P=0.03; H3N2, P=0.05; and B, P=0.004). Age, gender, and number or type of circulating lymphocytes had no consistent effect on HAI GMT after either vaccine.
Multivariate linear regression analyses included the following variables: age, entry log2 (HAI titer), entry CD8%, immunologic Group, and entry HIV RNA. Separate analyses were performed for each strain and each vaccine. These analyses demonstrated direct relationships between the HAI GMT at four weeks after vaccination and entry HAI GMTs for all three influenza strains in both LAIV and TIV vaccines (P<0.0001), and an inverse relationship between entry HIV RNA in plasma and HAI GMT against only two viruses in TIV (H3N2, P=0.04, B, P=0.04). Age, entry CD8%, and immunologic Group were not consistently found in the multivariate regression analyses to correlate with GMT at four weeks after vaccination.
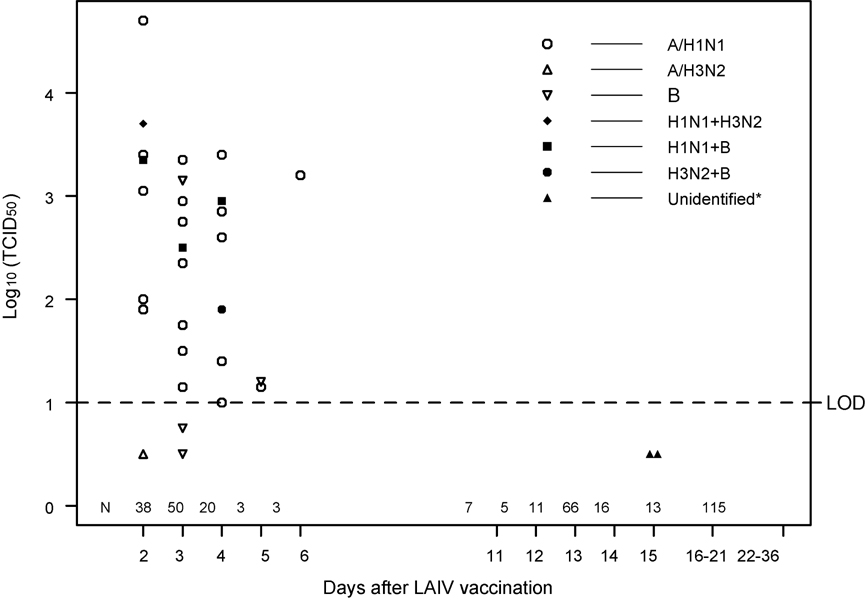
Figure 1 indicates the frequency that vaccine strain influenza was isolated from LAIV recipients. Vaccine strain H1N1 was recovered from 23 of 347 specimens (6.6 %); vaccine strain B was recovered from 11 (3.2 %); and vaccine strain H3N2 was recovered from 3 (<1%). Five specimens contained two vaccine strains. Twenty-five of 30 positive specimens were obtained on days 2 to 4 after LAIV (from 108 specimens). Three of 6 specimens from days 5 and 6 contained vaccine virus. The mean titer of shed virus for both H3N2 and B strains was approximately 2.0 log10 TCID50, and was 2.6 log10 TCID50 for H1N1. Two of 105 specimens taken on days 11 to 15 were positive for type B in a primary isolation at the U. of Colorado lab, but could not be identified as vaccine strain or wild type viruses due to insufficient virus in the sample. Neither of these isolates came from a subject that had virus isolated at an earlier time. None of 128 additional specimens obtained after day 15 were positive for vaccine virus. One wild-type virus was isolated on day 14 and another on day 28; both were H3N2 (data not shown).
Figure 1. Vaccine virus shedding.
N indicates the number of specimens obtained on the days indicated. LOD indicates the level of detection. Five specimens on days 2 to 4 contained two vaccine virus strains. Mean GMT for H1N1 = log10 TCID50 of 2.6; for H3N2 = 2.0; for B = 2.0. Replicates of samples below the limit of detection (<1 log10 TCID50/mL) were reported as 0.5 log10 TCID50/mL.
*”Unidentified” indicates that the origin (vaccine strain vs wild type) of these two primary isolates on day 15 post-vaccination could not be identified due to insufficient virus in the sample. Neither of these isolates came from a subject that had virus isolated at an earlier time.
Shedding of the H1N1 LAIV at day 3 was significantly associated with low baseline HAI antibody against that virus (p<0.01), and there was also a trend for this association with shedding of the H3N2 virus (p = 0.07). Shedding, examining all viruses combined or each individually, did not correlate with age, CD4 count or CD4%, or HIV viral load at the time of vaccination, and did not correlate with the subsequent boost in any specific antibody measured at 4 weeks post-vaccination.
DISCUSSION
The safety profile of LAIV and TIV was similar in HIV-infected children. This was true regardless of the immunologic Groups analyzed. No serious adverse events that were judged by the investigators to be treatment-related occurred during the study, although one LAIV recipient and one TIV recipient developed probable bacterial pneumonia within 2 weeks after vaccination. Overall the frequency of grade 2 and grade 3 signs and symptoms, including pulmonary disease and asthma or wheezing, were equally distributed between the two treatment Arms. Injection site reactions for TIV were observed in 23% of recipients, including one grade 3 event, which is similar to that previously reported (23–26). Mild upper respiratory symptoms were more commonly reported after LAIV (52%) than after TIV (31%), which has also been previously reported (23–26). Pulmonary signs and symptoms were of similar frequency after either vaccine. Although previously observed in young children receiving LAIV (34), we did not find more fever in the LAIV recipients, possibly because subjects in this study were 5 to 17 years of age. Almost all of the injection site and nasopharyngeal symptoms were minor and of short duration. The unblinded nature of this trial, together with more frequent clinic visits required for LAIV recipients, as well as prior reports associating LAIV with oropharyngeal symptoms in HIV-uninfected vaccinees, could have contributed to a reporting bias with respect to these symptoms. Neither LAIV nor TIV administration significantly altered HIV RNA viral load or CD4% at 4 weeks and 28 weeks. The absence of an effect of LAIV on HIV progression confirms earlier small trials in HIV-infected children and adults (29, 30), and extends to LAIV the same safety profile with respect to HIV disease progression reported after administration of other live attenuated or inactivated vaccines (35–38).
As expected in already primed individuals with previous exposure to influenza, TIV induced higher serum HAI titers to H3N2 and B virus compared to LAIV, and a higher proportion of TIV recipients had high antibody levels ≥40 or ≥4-fold increases in titer to these viruses (39). It is important to note that HAI responses to A/H1N1 were assessed using cold-adapted antigen. Subsequent to the conduct of our study, it was reported that due to amino acid differences between cold-adapted and CDC reference A/H1N1/New Caledonia antigen, use of cold-adapted antigen underestimates the post-vaccination HAI response in TIV recipients (40). As a result, TIV recipient responses to A/H1N1 would likely have been higher if tested against CDC reference antigen. Previous studies in children without HIV infection have failed to establish a general immune correlate of protection for LAIV. While the presence of a certain level of serum HAI antibody response is predictive of protection, the absence of such responses following LAIV administration does not correlate with lack of protection, as high levels of efficacy and effectiveness occur with low serum HAI responses elicited by LAIV (41). The explanation for this imperfect correlation between serum antibody and protection may be because LAIV induces protection through other mechanisms, such as by stimulating mucosal immune responses in the respiratory tree (20–22). While efficacy of any type of influenza vaccine in HIV-infected children remains unproven, we observed that the relative antibody responses to LAIV and TIV in HIV-infected children were similar to those reported in children without HIV infection (42, 43).
Regression analyses indicated that the ability to develop influenza-specific immune responses with either vaccine were similar for the three immunologic Groups, which spanned a range of 15% to >25% CD4 cells. Adequate responses to vaccines, both live and inactivated, have been documented in HIV-infected children, suggesting that both of these influenza vaccines will be effective for the population included in this study. Post-vaccination antibody responses were strongly influenced by the baseline titer of specific antibody. Since there did not appear to be a strong effect of immune status on response to influenza LAIV in these children with treated HIV infection, these results taken together suggest that the antibody titer at four weeks after vaccination reflected immunologic memory. This conclusion is consistent with our trial design that required subjects to have recent prior experience with TIV. Although within the immunologic Groups studied the lymphocyte phenotype and number were not associated with response to vaccination, HIV RNA plasma level was negatively correlated with serum HAI responses. An independent effect of HIV in plasma on immune response has been reported previously with both live and inactivated vaccines administered to HIV-infected children (38, 39, 44).
The frequency of shedding of vaccine strain virus was similar to that previously reported in both HIV-uninfected and HIV-infected children, as well as in HIV-infected adults (30, 45). Almost 90% of confirmed shedding of vaccine virus occurred within 4 days after vaccination, and 3 additional positive specimens were obtained on day 5 or 6 post-vaccination. Two other positive specimens were obtained at day 15, but the origin (vaccine strain vs wild type) of these could not be identified due to insufficient virus in the sample. Neither of these subjects had positive samples for vaccine virus at any prior time. Wild-type influenza virus was circulating in the community and was detected during the sampling period; two asymptomatic subjects had H3N2 wild-type virus isolated (day 14; day 28). As demonstrated in the figure (and its legend) the titer of vaccine virus isolated was 100 to 10,000 times lower than the inoculum administered (46), and was similar to that previously reported after LAIV administration to children (45). Thus, administration of LAIV to these HIV-infected children did not result in an extended period of vaccine virus shedding to the environment. This statement applies equally to all three of the defined immunologic strata.
We conclude that within this defined population of HIV-infected children the safety, immunogenicity, and pattern of vaccine virus shedding were similar to what has been previously observed in LAIV-vaccinated children without HIV infection. Because LAIV has demonstrated certain potential advantages over TIV in past studies in children, and because the current study supports the safety and immunogenicity of LAIV in HIV-infected children, this vaccine should be further evaluated for a role in immunizing HIV-infected children.
ACKNOWLEDGEMENTS
We wish to acknowledge key laboratory personnel, Julie Patterson, BA at the University of Colorado at Denver School of Medicine, and Gina Lin, BS at the MedImmune Clinical Testing Laboratory.
AIDS CLINICAL TRIALS GROUP P1057 SITES AND CONTRIBUTORS
Site 3701 Johns Hopkins University Hospital (Beth Griffin, RN; Nancy Hutton, MD; Mary Joyner, NP; Andrea Ruff, MD); 4701 Duke University – Pediatric (Joan Wilson, RN; Mary Jo Hassett, RN; Carole Mathison; John Swetnam); 5006 Harlem Hospital (Elaine Abrams, MD; Maxine Frere, RN; LisaGaye Robinson, MD); 5012 NYU Medical Center/Bellevue (William Borkowsky, MD; Sandra Deygoo, BS; Siham Akleh, RN; Aditya Kaul, MD); 5018 University of South Florida Physicians Group (Jorge Lujan-Zilberman, MD; Patricia Emmanuel, MD; Carolyn Graisbery, RN; Carina Rodriguez, MD); 5024 Children's Hospital Kings and Daughters (Randall G. Fisher, MD; Kenji M. Cunnion, MD, MPH; Laura Sass, MD; Donna Sandifer, RN); 5026 Mount Sinai (Mary S. Dolan, RN; Roberto Posada, MD); 5038 Yale University School of Medicine (Warren A. Andiman, MD; Leslie Hurst, BS; Sostena Romano, APRN, MBA); 5040 SUNY Health Science Center (Denise Ferraro, RN; Michele Kelly, PNP; Margaret Oliver, LPN); 5051 University of Florida Health Science Center (Mobeen Rathore, MD; Nizar Maraqa, MD; Kathy Thomas, MA; Angela Lala, LPN); 5052 The Children's Hospital- U. of Colorado; (Mark Abzug MD; Emily Barr CPNP, CNM; Megan Canon; Josephine Greenquist); 5057 University of Rochester - Pediatric Component (Geoffrey A. Weinberg, MD; Francis Gigliotti, MD; Barbra Murante, RNC, PNP; Susan Laverty, RN); 5095 Tulane University (Margarita Silio, MD; Thomas Alchediak, MD; Cheryl Borne, RN; Sheila Bradford, RN); 6701 The Children's Hospital of Philadelphia IMPAACT CTU (Steven D. Douglas, MD; Richard M. Rutstein, MD; Carol A. Vincent, CRNP, MSN; Patricia C. Coburn, RN, BSN); 7301 University of Massachusetts Medical School (Katherine Luzuriaga, MD; Richard Moriarty, MD; William (Jerry) Durbin, MD; Donna Picard, RN); 60336 Baylor College of Medicine (Chivon D. McMullen-Jackson, RN, ADN; Theresa Aldape, LMSW; Mary E. Paul, MD; Heidi L. Schwarzwald, MD, MPH); 60422 St. Jude/Memphis (Gregory Storch, MD; Laura Pickering, RN; Katherine Knapp, MD; Jill Utech, RN); 60444 Family Clinical Trials Center (Mavis Dummitt, RN; Caroline Nubel; Stefan Hagmann, MD; Murli Purswani, MD); 2901 Boston Children's Hospital; 3601 UCLA Medical Center; 4001 Children's Hospital of Chicago; 4501 UCSF Medical Center; 4601 UCSD Medical Center; 5008 Children's Hospital at SUNY Downstate; 5013 Jacobi Medical Center; 5031 City Hospital at San Juan; 5041 Children's Hospital of Michigan; 5055 Children's Diagnostic & Treatment Center of South Florida; 5056University of Florida at Gainesville; 6501 St. Jude Children's Research Hospital; 7701 University of Alabama, Birmingham; 60341 Columbia Collaborative - HIV/AIDS; 60349 University of Miami Pediatric Perinatal HIV/AIDS; 60358 NJ Medical School.
This research was supported by grant number 5 MO1-RR00069, General Clinical Research Centers Program, National Center for Research Resources, NIH; N01-HD-3-3162 and by Grant Number U01AI068632 and 1 U01 AI068616 from the National Institute of Allergy and Infectious Diseases and N01-HD-3-3345 from the National Institute of Child Health and Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Morishima T, Togashi T, Yogata S, et al. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002;35:512–517. doi: 10.1086/341407. [DOI] [PubMed] [Google Scholar]
- 2.Siberry GK. Complications of influenza infection in children. Pediatric Annals. 2000;209:683–690. doi: 10.3928/0090-4481-20001101-08. [DOI] [PubMed] [Google Scholar]
- 3.Neuzil KM, Zhu Y, Griffin MR, et al. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J Infect Dis. 2002;185:147–152. doi: 10.1086/338363. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immmunization Practices (ACIP) MMWR. 2004;53(No RR6):1–40. [PubMed] [Google Scholar]
- 5.Rojo J, Ruiz-Contreras J, Fernandez M, Marin M, Folgueira I. Influenza-related hospitalizations in children younger than three years of age. Ped Infect Dis J. 2006;25:596–601. doi: 10.1097/01.inf.0000220208.59965.95. [DOI] [PubMed] [Google Scholar]
- 6.Poehling K, Edwards K, Weinberg G, et al. The underrecognized burden of influenza in your children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 7.Iskander M, Booy S, Lambert S. The burden of influenza in children. Curr Opinions in Infect Dis. 2007;20:259–263. doi: 10.1097/QCO.0b013e3280ad4687. [DOI] [PubMed] [Google Scholar]
- 8.Mahdi SA, Ramasamy N, Bessellar TG, et al. Lower respiratory tract infections associated with influenza A and B viruses in an area with a high prevalence of pediatric human immunodeficiency type 1 infection. Pediatr Infect Dis J. 2002;21:291–297. doi: 10.1097/00006454-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Fine AD, Bridges CB, DeGuzman Am, et al. Influenza A among subjects with human immunodeficiency virus: an outbreak of infection at a residential facility in New York City. Clin Infect Dis. 2001;32:1784–1791. doi: 10.1086/320747. [DOI] [PubMed] [Google Scholar]
- 10.Safrin S, Rush JD, Mills J. Influenza in patients with human immunodeficiency virus infection. Chest. 1990;98:33–37. doi: 10.1378/chest.98.1.33. [DOI] [PubMed] [Google Scholar]
- 11.Klein MB, DelBalso L, Coté S, Boivin G. Influenzavirus infection is a primary cause of febrile respiratory illness in HIV-infected adults, despite vaccination. Clin Infect Dis. 2007;45:234–240. doi: 10.1086/518986. [DOI] [PubMed] [Google Scholar]
- 12.Neuzil KM, Coffey CS, Mitchell EF, Jr, Griffen MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndrom. 2003;34:304–307. doi: 10.1097/00126334-200311010-00008. [DOI] [PubMed] [Google Scholar]
- 13.McFarland EJ. Chapter 37. In: Hay WW Jr, Levin MJ, Sondheimer JM, Deterding RR, editors. Human Immunodeficiency Virus Infection in Current Diagnosis & Treatment. 18th Edition. 2007. pp. 1140–1151. [Google Scholar]
- 14. http://www.cdc.gov/flu/protect/hiv-flu.htm.
- 15.Lyall EG, Charlett A, Watkins P, Zambon M. Response to influenza virus vaccination in vertical HIV infection. Arch Dis Child. 1997;76:215–218. doi: 10.1136/adc.76.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller JD, Craven DE, Steger KA, et al. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: Impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis. 1999;28:541–547. doi: 10.1086/515170. [DOI] [PubMed] [Google Scholar]
- 17.Günthard HF, Wong JK, Spina CA, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis. 2000;181:522–531. doi: 10.1086/315260. [DOI] [PubMed] [Google Scholar]
- 18.Malispina A, Moir S, Orsega SM, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 19.Tasker SA, Treanor JJ, Paxton WB, et al. Efficacy of influenza vaccination in HIV-infected persons. Ann Intern Med. 1999;131:430–433. doi: 10.7326/0003-4819-131-6-199909210-00006. [DOI] [PubMed] [Google Scholar]
- 20.Tomoda T, Morita H, Kurashige T, Maassab HF. Prevention of influenza by the intranasal administration of cold-recombinant, live-attenuated influenza virus vaccine: importance of interferon-gamma production and local IgA response. Vaccine. 1995;13:185–190. doi: 10.1016/0264-410x(95)93134-u. [DOI] [PubMed] [Google Scholar]
- 21.Gorse GJ, Otto EE, Powers DC, et al. Induction of mucosal antibodies by live aattenuated and inactive influenza vaccines in the chronically ill elderly. J Infect Dis. 1996;173:285–290. doi: 10.1093/infdis/173.2.285. [DOI] [PubMed] [Google Scholar]
- 22.Boyce TG, Gruber WC, Coleman-Dockery SD, et al. Mucosal immune response to trivalent live attenuated intranasal influenza vaccine in children. Vaccine. 1999;18:82–88. doi: 10.1016/s0264-410x(99)00183-8. [DOI] [PubMed] [Google Scholar]
- 23.Ashkenazi S, Vertruyen A, Aristegui J, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25:870–879. doi: 10.1097/01.inf.0000237829.66310.85. [DOI] [PubMed] [Google Scholar]
- 24.Fleming DM, Crowari P, Wahn U, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25:860–869. doi: 10.1097/01.inf.0000237797.14283.cf. [DOI] [PubMed] [Google Scholar]
- 25.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. New Engl J Med. 2007;356:685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 26.Belshe RB, Gruber WC, Mendelman PM, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediat. 2000;136:168–175. doi: 10.1016/s0022-3476(00)70097-7. [DOI] [PubMed] [Google Scholar]
- 27.Tam JS, Capeding MRZ, Lum LCS, et al. Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Ped Infect Dis J. 2007;26:619–628. doi: 10.1097/INF.0b013e31806166f8. [DOI] [PubMed] [Google Scholar]
- 28.Rhorer J, Dickinson S, Oleka N, et al. Efficacy in children of live attenuated influenza vaccine: A meta-analysis of 9 randomized clinical trials. Presented at the 48th Annual Meeting of the European Society for Pediatric Research (ESPR); October 6–8, 2007; Prague, Czech Republic. [Google Scholar]
- 29.King JC, Treanor J, Fast PE, et al. Comparison of the safety, vaccine virus shedding, and immungenicity of influenza virus vaccine, trivalent, types A and B, live cold-adapted, administered to human immunodeficiency virus (HIV)-infected and non-HIV-infected adults. J Infect Dis. 2000;181:725–728. doi: 10.1086/315246. [DOI] [PubMed] [Google Scholar]
- 30.King JC, Fast PE, Zangwill KM, et al. Safety, vaccine virus shedding and immunogenicity of trivalent, cold-adapted, live attenuated influenza vaccine administered to human immunodeficiency virus-infected and noninfected children. Pediatr Infect Dis J. 2001;20:1124–1131. doi: 10.1097/00006454-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Dowdle WN, Kendal AP, Noble GR. Influenza viruses. In: Lenette EH, Schmidt NJ, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. 5th ed. Washington, DC: American Public Health Associations; 1979. pp. 603–605. [Google Scholar]
- 32.QIAGEN® OneStep RT-PCR Kit Handbook. Qiagen, Inc.; 2002. May, [Google Scholar]
- 33.Chan ISF, Zhang Z. Test based confidence intervals for the difference of two binomial proportions. Biometrics. 1999;55:1201–1209. doi: 10.1111/j.0006-341x.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 34.Belshe RB, Mendelman PM, Treanor J, et al. The efficacy of live, attenuated, cold-adapted, trivalent, intranasal influenza vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 35.Fuller JD, Craven DE, Steger KA, et al. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: Impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis. 1999;28:541–547. doi: 10.1086/515170. [DOI] [PubMed] [Google Scholar]
- 36.Jackson CR, Vavro CL, Valentine ME, et al. Effect of influenza immunization on immunologic and virologic characteristics of pediatric patients infected with human immunodeficiency virus. Ped Infect Dis J. 1997;16:20. doi: 10.1097/00006454-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Abzug MJ, Pelton SI, Song L-Y, et al. Immunogenicity and safety of and predictors of response to a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Ped Infect Dis J. 2006;25:920–929. doi: 10.1097/01.inf.0000237830.33228.c3. [DOI] [PubMed] [Google Scholar]
- 38.Levin MJ, Gershon AA, Weinberg A, et al. Immunization of HIV-infected children with varicella vaccine. J Pediatr. 2001;139:305–310. doi: 10.1067/mpd.2001.115972. [DOI] [PubMed] [Google Scholar]
- 39.Piedra PA, Glezen PW. Influenza in Children: epidemiology, immunity and vaccines. Semin Pediatr Infect Dis. 1991;2:140–146. [Google Scholar]
- 40.Belshe R, Ambrose C, Wlaker RE, et al. Genetic sequences of circulating 2004–2005 influenza strains and serum antibody responses to LAIV vs. TIV in young children. Joint Meeting of the Pediatric Academic Societies and Asian Society for Pediatric Research; May 2–6, 2008; Honolulu. abstract #4461.2. [Google Scholar]
- 41.Ambrose CS, Walker RE, Connor EM. Live Attenuated Influenza Vaccine in Children. Semin Pediatr Infect Dis. 2006;17:206–212. doi: 10.1053/j.spid.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Edwards KM, Dupont WD, Westrich MK, et al. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza disease. J Infect Dis. 1994;169:68–76. doi: 10.1093/infdis/169.1.68. [DOI] [PubMed] [Google Scholar]
- 43.Treanor JJ, Kotloff, Betts RF, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine. 2000;18:899–906. doi: 10.1016/s0264-410x(99)00334-5. [DOI] [PubMed] [Google Scholar]
- 44.Weinberg A, Gona P, Nachman SA, et al. Antibody responses to hepatitis A virus vaccine among HIV-infected children with evidence of immunologic reconstitution on antiretroviral therapy. J Infect Dis. 2006;193:302–311. doi: 10.1086/498979. [DOI] [PubMed] [Google Scholar]
- 45.Block SL, Ambrose CS, Walker RE. Shedding and immunogenicity of live attenuated influenza vaccine in subjects 5–49 years of age. Vaccine Congress; 9–11 December 2007; Amsterdam, The Netherlands. Abstract P47. [DOI] [PubMed] [Google Scholar]
- 46.FluMist Prescribing Information. MedImmune, Inc.; Issue date: September 2007. [Google Scholar]