Abstract
Both norepinephrine and acetylcholine have been shown to be critically involved in mediating attention but there remains debate about whether they serve similar or unique functions. Much of what is known about the role of these neurochemicals in cognition is based on manipulations done at the level of the cell body but these findings are difficult to reconcile with data regarding the unique contribution of cortical subregions, e.g. the dorsolateral prefrontal cortex, to attention. In the current study, we directly compared the effects of noradrenergic and cholinergic deafferentation of the rat medial prefrontal cortex, the homologue of primate dorsolateral prefrontal cortex, using an intradimensional/extradimensional attentional set shifting task, a task previously shown to be able to dissociate the function of the primate dorsolateral prefrontal cortex from orbitofrontal cortex. We found that noradrenergic, but not cholinergic, deafferentation produces specific impairments in the ability to shift attentional set. We also clarified the nature of the attentional deficits by assessing the ability of rats to disregard irrelevant stimuli. Noradrenergic lesions did not alter the ability of rats to ignore irrelevant stimuli, suggesting that the attentional deficit results from an overly focused attentional state that retards learning that a new stimulus dimension predicts reward.
Keywords: DBH saporin, 192 IgG saporin, infralimbic/prelimbic cortices, selective attention
Studies aimed at understanding the neurochemical basis of attention have found that both norepinephrine (NE) and acetylcholine (ACh) mediate aspects of attention (McGaughy and Sarter, 1995b; McGaughy et al., 1996; Rajkowski et al., 2004; Milstein et al., 2007) but some conditions dissociate the functions of these systems. Yu and Dayan (2005) hypothesize that NE mediates unexpected uncertainty while ACh mediates expected uncertainty. In other words, there exists some known ambiguity in many attentional situations, e.g. spatial or temporal unpredictability of targets, and variability in perceptual attributes of target (Chiba et al., 1995; McGaughy and Sarter, 1995a; Bucci et al., 1998; McGaughy et al., 2002; Maddux et al., 2007). After acquisition, this uncertainty though not predictable is known, expected, and requires ACh (Yu and Dayan, 2002, 2005). Additionally, unexpected uncertain events may occur, e.g. changes in reinforcement contingencies in a well-learned task, and recruit NE (Robbins, 2000; Dalley et al., 2001; Bouret and Sara, 2004, 2005; Dalley et al., 2004; Yu and Dayan, 2005).
Though these frameworks are useful there remains ambiguity about the attentional situations that require ACh and those that require NE. This ambiguity may result from a reliance on data obtained from manipulations of the cell bodies of these systems (Aston-Jones et al., 1994, 2000; McGaughy et al., 1996, 2002). Both NE and ACh innervate much of the neocortical mantle so previous frameworks posited a unitary function of these neurotransmitters across diverse cortical subregions and failed to incorporate what is known about functions of cortical subregions in attention (Dias et al., 1996, 1997; McAlonan and Brown, 2003) with what is known about these neurochemicals in attention. The current study investigates the function of ACh and NE within the medial frontal cortex using a task for which the area is critical.
The use of an intradimensional/extradimensional set-shifting task (ID/ED) has shown that attentional set shifts require the dorsolateral prefrontal cortex of monkeys (Dias et al., 1996, 1997) and its rodent homologue, the medial frontal cortex (Birrell and Brown, 2000) while the orbitofrontal cortex is involved in affective shifts in both monkeys and rats (Dias et al., 1997; McAlonan and Brown, 2003). An attentional set is formed when subjects are reinforced for focusing attention on one attribute of a complex stimulus while disregarding others. When a subject is presented with novel stimuli, it attends to the previously reinforced attribute (intradimensional shift; ID) and learns more readily if that attribute still predicts reward. However, if there is a shift in attentional set, e.g. a new attribute predicts reward (extradimensional shift; ED), learning is impaired (Owen et al., 1991, 1993; Dias et al., 1996; Birrell and Brown, 2000). In the current study, we investigated whether depletions of ACh or NE in the medial frontal cortex of rats impaired attentional set shifting. It was hypothesized that NE but not ACh depletion would further impair the performance in ED, as the ED may test unexpected uncertainty.
EXPERIMENTAL PROCEDURES
Apparatus and materials
Training and testing were performed in a wooden box (91 cm×40.5 cm×25.4 cm; L×W×H) covered with self-adhesive, black contact paper (Pliant Solutions Corporation, North Ridgeville, OH, USA). The box was divided lengthwise into three sections so that a divider could be inserted to control the access of the subjects to the test stimuli. Rats were initially trained to dig for reinforcement (1/6 Frosted Cheerio, General Mills, Minneapolis, MN, USA) buried in standard pine chip bedding in a terra cotta flower pot with a height of 3.5 in (outer diameter 4.5 in). The drainage hole of the pot was filled with clay and melted paraffin was allowed to harden such that only a ¾ in space remained from the top of the wax to the top of the pot for the addition of digging media. These pots were used as complex stimuli in the task by varying the material in the pot, the digging media, and the scent on this material (odor) and the material glued to the outside of the pot (texture). Scents were applied by using a tissue saturated with diluted essential oils (1:200 in vegetable oil). The modification with wax minimized the amount of digging medium needed to fill the cup, increased the difficulty of tipping the pot and allowed the re-use of these stimuli with animals fitted with chronic implants without damage to the subject or implant (data not presented here). Animal care and experimentation were performed in accordance with Boston University Institutional Laboratory Animal Care and Use Committee and were in compliance with the NIH Guide for the Care and Use of Laboratory Animals. The number of rats tested was the minimal number necessary to achieve the study aims. Pain and distress to subjects was minimized, but treated by staff, if and when it did occur.
Presurgical training
Rats were placed in the larger portion box with the dividing wall in place. The baited pot was placed behind a divider out of the view of the rat. Upon removal of the divider, the rat was given 90 s to retrieve the reward. Over successive trials, the reward was increasingly hidden. After rats were reliably retrieving 10 consecutive, fully buried reinforcements, there were considered eligible for surgery.
Post-surgical testing
After 2 weeks of surgical recovery with ad libitum food and water, testing began. On each of three sequential mornings, rats were presented with pairs of testing pots that were scented with either lemon or almond and filled with either shredded envelope or shredded manila folder. On the first day, subjects were trained on three consecutive simple discriminations (SDs) of odor, digging media or texture with pairs of pots that differed on only one of these dimensions (e.g. Lemon/Envelope vs. Lemon/Folder+ or Almond/Envelope+ vs. Lemon/Envelope or Lemon/Fuzzy+ vs. Lemon/Smooth). Throughout all stages of testing the correct pot was baited with 1/6 Frosted Cheerio (General Mills) while the incorrect pot contained an equal amount of crushed Cheerio to prohibit animals from using the scent of the cereal as a cue to indicate the correct pot. For each trial the animal was contained in the larger portion of the box behind a divider that prohibited access to the test stimuli. The trial began when the divider was removed. The texture used in the exemplar was tan fake fur with Fuzzy indicating the furry side was out and smooth indicating the reverse of the material was outward. The order of these discriminations was counterbalanced across subjects. This training allowed the subjects to gain familiarity with response rules on all types of trials without the distraction of other stimulus dimensions. Rats were given four discovery trials (90 s) to explore and dig in both pots until they received reinforcement. An omission at this stage was not punished but resulted in a repetition of the trial until a response was emitted. Subsequent to the discovery period, trials were continued until the subject responded correctly for six consecutive trials. These exemplars were not reused.
Table 1 shows the details of the ID/ED task used. Comparisons were made for textures with one raised side of the material facing outward on one pot e.g. the fuzzy side of the black fake fur or the smooth reverse side on its comparator pot to minimize the use of visual and olfactory cues. Subsequent tests of texture discrimination used velvet, bubble wrap and corduroy. Subsequent test pairs of odors used after the exemplar stage were: nutmeg vs. clove, vanilla vs. jasmine, and cinnamon vs. patchouli. Digging media pairs were: navy vs. white tissue paper, thick diameter vs. thin diameter wool cut into 5 cm strips, small gold beads vs. large gold beads.
Table 1.
Testing stimuli in the ID/ED task
| Task | Testing pair 1 | Testing pair 2 | Never relevant attribute |
|---|---|---|---|
| SD | Clove/navy paper+ vs. nutmeg/navy paper |
Clove/white paper+ vs. nutmeg/white paper |
Texture e.g. All bubble wrap |
| CD | Clove/navy paper+ vs. nutmeg/white paper |
Clove/white paper+ vs. nutmeg/navy paper |
Bubble wrap |
| ID | Cinnamon/thick yarn+ vs. patchouli/thin yarn |
Cinnamon/thin yarn+ vs. patchouli/thick yarn |
Corduroy |
| REV1 | Patchouli/thick yarn+ vs. cinnamon/thin yarn |
Patchouli/thin yarn+ vs. cinnamon/thick yarn |
Corduroy |
| ED | Large beads/vanilla+ vs. small beads/jasmine |
Large beads/jasmine+ vs. small beads/vanilla |
Velvet |
| REV2 | Small beads/vanilla+ vs. large beads/jasmine |
Small beads/jasmine+ vs. large beads/vanilla |
Velvet |
| Learned irrelevance | Small beads/vanilla+ vs. large beads/jasmine |
Small beads/jasmine+ vs. large beads/vanilla |
Satin |
An example of a testing schedule for a subject whose initial, relevant stimulus dimension was odor for the SD, CD, ID and REV1 is shown. There are two additional stimulus attributes present for every trial, digging media and texture. Testing pairs 1 and 2 are randomly alternated at all stages of testing. During testing of the SD, the stimulus pairs varied on a single dimension e.g. odor. Subsequent to the SD, the stimulus pairs vary on two dimensions e.g. odor and digging media. The alternation of stimulus pairs trains the animals to focus attention on one stimulus attribute and disregard the variable, irrelevant attribute e.g. digging media as well as the never relevant attribute. The variable, irrelevant attribute e.g. digging media in the initial stages of testing becomes the relevant attribute in the ED. A novel exemplar of the never relevant dimension is introduced subsequent to REV2 to insure that rats learned to successfully ignore this stimulus attribute e.g. texture. The order of testing of the stimulus pairs was constant across subjects but the relevant, variable irrelevant and constant irrelevant dimensions were counterbalanced across subjects as described in the Experimental Procedures.
Rats were then given two new pairs of pots and trained on a new SD for odor, digging media or texture (counterbalanced across subjects). Though each pair of stimuli differed on one dimension, all stimulus attributes were present. Alternate stimulus pairs were used on each trial, e.g. Clove/Navy paper+ vs. Nutmeg/Navy Paper alternated with Clove/White Paper+ vs. Nutmeg/White Paper, to train the rats to focus attention on the relevant dimension. During this testing the third stimulus dimension, e.g. texture, was the same for all pots. As before, rats were given four discovery trials with a 90 s limited hold. On all trials subsequent to these discovery trials, the limited hold period was abbreviated to 60 s until criterion performance (six consecutive correct responses) was achieved. On day 2, rats received training on the compound discrimination (CD) using the same pots from the SD, with the same dimension reinforced but stimuli differed on two dimensions on each trial (e.g. Clove/Navy Paper+ vs. Nutmeg/White Paper randomly alternated with Clove/White Paper+ vs. Nutmeg/Navy Paper). After subjects achieved criterion performance on CD, they were tested on the ID. During the test of the ID, rats were given a novel set of stimuli where they were to attend to the same stimulus dimension as reinforced in the SD and CD e.g. attend to scent when presented with Cinnamon/Thick Yarn + vs. Patchouli/Thin Yarn alternated with Cinnamon/Thin Yarn+ vs. Patchouli/Thick Yarn. On day 3, rats received the reinforcement reversal (REV1) for the ID stimuli, e.g. Patchouli was reinforced regardless of the digging media with which it was paired followed by testing in the ED. At the ED stage of testing rats were again given a new set of stimulus pairs e.g. Large Beads/Vanilla+ vs. Small Beads/Jasmine alternated with Large Beads/Jasmine+ vs. Small Beads/Vanilla but had to learn to inhibit responding on the previously relevant perceptual dimension (e.g. odor) and learn to attend to the previously irrelevant dimension (e.g. digging medium). This stage was followed by a reversal (REV2) where the alternate member of the pair e.g. small beads would be reinforced regardless of scent. Subsequent to REV2, the animal's ability to disregard a stimulus dimension that had never predicted reinforcement was assessed in a test of learned irrelevance (L-IRR). The stimulus pairs differed on three dimensions throughout testing, but only two were required for the assessment of attentional set shift. The third dimension e.g. texture was kept constant among all pots (e.g. all covered in velvet) and the final test introduced a new exemplar of this dimension (e.g. satin) to determine if either lesion impaired the ability of the rat to filter irrelevant sensory information (L-IRR). Though in the above example texture was the completely irrelevant dimension, both odor and digging media were also tested across subjects.
Training of the attentional set shift was completed using four of six possible combinations of stimulus dimensions for the ID/ED shifts (odor to texture, texture to odor, digging media to texture, texture to digging medium). The use of four possible combinations allowed six subjects to be assigned to each shift and allowed an assessment of the influence of modality on the ID/ED shifts. The dependent measure was the number of trials required to reach criterion (six consecutive, correct responses), correct and incorrect response latencies. Response latencies were measured as the amount of time between removal of the barrier and the displacement of digging media by the rat with its forepaw.
Surgery
Subjects were pretreated with 0.25 mg/kg/ml dose of atropine to suppress mucosal secretions and clear the airway for inhalation anesthesia, halothane (2%) was delivered in 30:70 oxygen/nitrous oxide gas mixture. Rats were randomly assigned to one of three groups: noradrenergic deafferentation of infralimbic/prelimbic cortices (IL/PL; noradrenergically lesioned, NE-LX), cholinergic deafferentation of IL/PL (cholinergically lesioned, ACh-LX) or sham lesioned (SHAM-LX). Selective noradrenergic deafferentation was produced by infusion of dopamine beta hydroxylase (DBH) saporin (0.01 μg/μl; 0.5 μl/hemisphere; Advanced Targeting Systems, San Diego, CA, USA) while cholinergic deafferentation was produced by an infusion of the selective cholinotoxin 192 IgG saporin (0.01 μg/μl; 0.5 μl/hemisphere; Advanced Targeting Systems). SHAM-LX rats received infusions of vehicle, Dulbecco's saline, into IL/PL. All infusions were made using a 10 μl, 26 gauge Hamilton syringe attached to an infusion pump (World Precision Instruments; Sarasota, FL, USA) with the needle left in place for 4 min prior and subsequent to the controlled infusion of the toxin (125 nl/min) to minimize unintended damage to nearby regions. Coordinates were from Bregma AP: +2.8 mm; ML: ±0.6; DV: −5.2 from skull. Rats were allowed 1 week of free food and water prior to testing to allow sufficient time for the toxin to be retrogradely transported and produce selective deafferentation.
Histological procedures for DBH staining
Following the completion of behavioral testing, rats were deeply anesthetized with sodium pentobarbital (200 mg/kg/ml) and exsanguinated and perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS). Perfused brains were then placed in 30% sucrose to provide cryoprotection prior to sectioning (50 μm) with a cryostat. Alternate sections were stained for DBH positive fibers or Nissl bodies using Thionin. To prevent uneven staining, all rinses and incubations were performed using an orbital shaker. Sections were initially placed in a solution of 1% H2O2 and 3% normal goat serum in PBS. Without rinsing, sections were transferred to the primary antibody solution (1:1000 rabbit anti-DBH, Chemicon; Temecula, CA, USA) in PBS with 0.2% Triton × (Tx-PBS) and incubated overnight. Subsequent to 3×10 min rinses in PBS, sections were incubated in biotinylated secondary antibody (goat anti-rabbit, Vector Laboratories, Burlingame, CA, USA) for 2 h. After rinsing 3×10 min in PBS, sections were incubated in the avidin–biotin complex solution (ABC; Vector Laboratories) for 1.5 h. Subsequent to rinsing, 3×10 min PBS, visualization was accomplished with a solution of nickel enhanced 3,3-diaminobenzidine (Vector Laboratories) using an incubation time of 5 min. Finally sections were rinsed with PBS (3×10 min) prior to mounting on gelatin-coated slides. Sections were dried overnight in a 37 °C oven prior to dehydration, defatting and coverslipping.
Histological procedures for acetylcholinesterase (AChE)
Following the completion of behavioral testing, rats were deeply anesthetized with sodium pentobarbital (200 mg/kg/ml) and exsanguinated and perfused with 10% formalin (vol/vol solution). Perfused brains were then placed in 30% sucrose to provide cryoprotection prior to sectioning (50 μm) with a cryostat. Alternate sections were stained to identify AChE positive fibers (Tago et al., 1986) or Nissl bodies using Thionin. To prevent uneven staining, all rinses and incubations were performed using an orbital shaker. Sections were initially placed for 30 min in 0.1% H2O2 and rinsed with 0.1 M maleate buffer (pH=6.0; 3×3 min) and incubated in 5 mg acetylthiocholine iodide, 0.147 g sodium citrate, 0.075 g cupric sulfate, 0.0164 g potassium ferricyanide in maleate buffer. Sections remained in this solution for 45 min and were rinsed in 50 mM Tris buffer (pH=7.6; 3×3 min). The second incubation of 0.05 g 3,3-diaminobenzidine with 0.375 g nickel ammonium sulfate/per 125 ml 50 mM Tris buffer continued for 10 min prior to the addition of 12 drops of 0.1% H2O2 which caused visualization. Visualization was terminated when cortical layers could be discerned in the neocortex. Finally sections were rinsed with 5 mM Tris buffer (3×3 min) prior to mounting on gelatin coated slides. Sections were dried overnight in a 37 °C oven prior to dehydration, defatting and coverslipping.
Histological analyses
As the fixation procedures to optimize staining for AChE and DBH-positive fibers differed, it was not possible to stain tissue for both markers. As a result the SHAM group was stained for DBH and an additional seven animals were killed to serve as histological controls for the AChE staining. Both AChE-positive and DBH-positive fibers were quantified in cortical areas in a manner modified from that described by McGaughy et al. (1996). Sections were analyzed using an Olympus B×51 microscope attached to a Nikon DXM 1200 camera in conjunction with Image Pro Plus v. 4.5.1.23 software to count fibers in a 50 μm area that crossed a grid imposed over the area. Counts were obtained for both hemispheres in the area of the IL/PL at the levels of Bregma: +3.7; +2.7 and +2.2, in the anterior cingulate cortex at Bregma +2.2 and +0.7 and at Bregma +4.7, +3.7 and +2.7 in orbitofrontal cortex as noted in Paxinos and Watson (1986). Histological data were lost from two animals, one from the DBH-SHAM group and one from the NE-LX group due to an error in visualization that prevented discrimination of NE fibers from background staining.
Statistical analyses
All statistical analyses were performed with SPSS v. 14.0 (SPSS, Chicago, IL, USA). The number of trials to criterion from the two lesion groups were compared with SHAM-LX rats in separate analyses of variance (ANOVAs). Histological data were analyzed using a mixed factor ANOVA for each cortical subregion with the between-subjects factor of Lesion (three) and the within-subjects factor of Rostral to Caudal (three levels in counts from IL/PL; two levels in counts from cingulate cortex). The initial acquisition of the task was analyzed in a separate ANOVA with Test (two levels, SD, CD) as a within subjects factor and Lesion (three levels) and Shift (four levels) as between subject factors. Note that four levels of the variable Shift represent the possible modality shifts over the course of testing with one stimulus dimension the focus of attention prior to the ED and another after the ED e.g. texture to digging media (see full description above in behavioral testing). In the second ANOVA, the effects of reversals after the ID (REV1) and (REV2) were analyzed, Reversal (two)×Shift (four)×Lesion (three). Performance in the ID was compared with the ED in a Test (two)×Shift (four)×Lesion (three) mixed factor ANOVA. The effects of prefrontal lesions on L-IRR were assessed in a univariate ANOVA with the between subjects factors of Shift (four)×Lesion (three).
RESULTS
Histological analyses
DBH staining
NE-LX rats had significantly fewer noradrenergic fibers in the IL/PL subregion than did SHAM (F(1,12)=105.03, P<0.001; Fig. 1). The effects of the lesion did not differ along the rostral caudal plane (LESION×-ROSTRAL-CAUDAL (F(2,24)=1.83, P=0.18). There were no significant differences between the groups in the cingulate or orbitofrontal cortex (all P>0.09). The average percentage of loss of these fibers was 66% relative to SHAM-LX rats in the IL/PL, 19% loss relative to SHAM-LX rats in the cingulate cortex and a 3% loss relative to SHAM-LX rats in lateral orbitofrontal cortex (Table 2).
Fig. 1.

Schematic diagram of the areas of the IL/PL deafferented in the current study. The inset box shows the region of IL/PL magnified below for both NE and ACh fibers. Though lesions were bilateral, for illustration purposes, deafferentation in lesioned and sham-lesioned rats is shown side by side. The first row of photographs depicts the extent of loss of NE fibers in the cortex in NE-LX (left) and SHAM-LX (right) rats. Sections from Ach-LX rats are depicted in the second row from ACh-LX (left) and SHAM-LX (right) rats. Both neurochemical lesions produced similar levels of deafferentation in IL/PL (∼60%) and in cingulate cortex (∼18%).
Table 2.
Fiber counts
| IL/PL | CG1 | ORB | |
|---|---|---|---|
| DBH SHAM-LX | 128.2±3.2 | 103.2±8.0 | 115.8±2.1 |
| NE-LX | 43.3±7.6*** | 83.4±7.2 | 112.4±1.9 |
| AVG. LOSS | 66.2% | 19.2% | 3.8% |
| IL/PL | CG1 | ORB | |
| AChE SHAM-LX | 182.0±6.5 | 122.0±3.6 | 140.29±7.4 |
| ACh -LX | 66.5±9.4*** | 101.0±4.4* | 130.11±12.5 |
| AVG. LOSS | 63.5% | 17.2% | 7.0% |
Mean fiber counts and S.E.M.s per region in ACh-LX and NE-LX rats are shown relative to the appropriate histological control group. While the extent of damage to the cingulate cortex was similar in both lesion groups relative to SHAM-LX rats, this difference was statistically different in only the ACH-LX rats and may be related to the smaller variability in AChE fiber counts in this region.
P<0.05, significant difference relative to control rats.
P<0.001, significant difference relative to control rats.
AChE staining
ACh-LX rats had significantly fewer cholinergic fibers in the region of the IL/PL relative to SHAM rats (LESION F(1,13)=96.49, P=0.001; Fig. 1) and the extent of the loss did not vary across the rostral to caudal plane (all P>0.06). Unlike the noradrenergic lesions, the effects of the cholinergic lesions did extend to the cingulate (LESION F(1,13)=13.31; P=0.003) but this effect was consistent regardless of how far rostral or caudal the section (all P>0.09). Cholinergic fiber counts in the orbitofrontal cortex did not differ between the groups (all P>0.39). The average percentage of loss of these fibers was 64% relative to SHAM-LX rats in the IL/PL, a 7% loss relative to SHAM-LX rats in orbitofrontal cortex and a 17% loss relative to SHAM-LX rats in the cingulate cortex. It should be noted that relative fiber loss is similar in both groups but concurrent cingulate deafferentation was more consistent in the ACh-LX rats and produced a statistically significant difference between the groups though the actual damage was small.
Behavioral analyses
Noradrenergic deafferentation of the medial frontal cortex caused impairments in ED shifts but did not impair performance on other aspects of the behavioral task. In contrast, cholinergic deafferentation of the IL/PL failed to impair performance on any aspect of the behavioral test.
Effects of frontal lesions on attentional set shift
Noradrenergic lesions but not cholinergic lesions impaired the ability of subjects to shift attention to a novel stimulus dimension subsequent to training on an attentional set (LESION×TEST: F(2,12)=18.10, P<0.001). A series of planned comparisons showed that SHAM-LX and NE-LX rats required fewer trials to perform the ID than the ED as predicted and supports the hypothesis that normal rats form an attentional set (SHAM-LX: t(7)=−2.63; P=0.02; NE-LX: t(7)=−7.00; P<0.001). In contrast, ACh-LX rats did not show differential performance on the ID vs. ED (t(7)=0.27; P=0.79). As shown in Fig. 2, rats with NE lesions required significantly more trials to reach criterion performance than either SHAM-LX (t(14)=3.28; P=0.005) or ACh-LX rats (t(14)=3.92, P=0.002). This effect was consistent regardless of which stimulus attributes were used (LESION×TEST×SHIFT: F(6,12)=1.20, P=0.37). There was an overall increase in the number of trials needed to reach criterion performance on tests with novel stimuli when the attentional set was shifted (ED) compared with trials in which the attentional set was maintained (ID vs. ED: TEST F(1, 12)=45.38, P=0.001) regardless of the stimulus attribute (TEST×SHIFT F(3,12)=1.54, P=0.26). There was a main effect of lesion with noradrenergic lesioned rats requiring more trials to reach criterion performance than either cholinergic or sham-lesioned rats (LESION F(2,12)=5.60, P=0.02). There were no other statistically significant effects in the analysis of trials to criterion (all P>0.11) or in the analyses of correct or incorrect response latencies (all P>0.12).
Fig. 2.
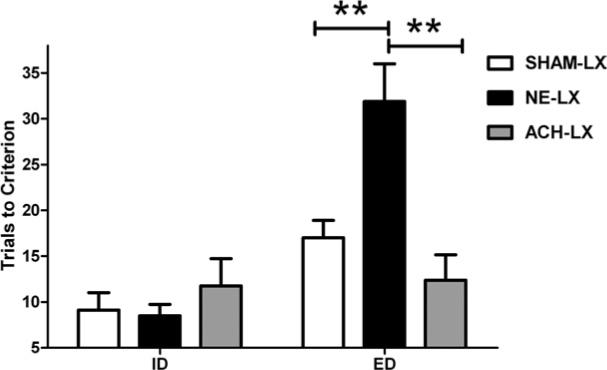
NE-LX rats (black bars) require more trials to reach criterion performance on the ED (right side of graph) than SHAM-LX (white bars) or ACh-LX rats (gray bars). ** Indicates significant group differences with a P value <0.01. Performance on the ID shown on the left side of the graph does not differ among the groups. All bars show group means±S.E.M. As reported in the text, a planned comparison showed that SHAM-LX rats require more trials to achieve criterion performance on the ED relative to the ID suggesting that they successfully form an attentional set and that focus of attention impedes learning when a new dimension predicts reinforcement.
Effects of lesions on acquisition
Neither noradrenergic or cholinergic lesions impaired performance on SD or CDs (LESION: F(2,12)=3.22, P=0.08; TEST×LESION: F(2,12)=1.89. P=0.19; Fig. 3) regardless of which attribute predicted reward (TEST×LESION×SHIFT: F(6,12)=1.91, P=0.16). Performance on the SD and CD did not differ regardless of the stimulus attribute used (TEST×SHIFT: F(1,3)=1.22; P=0.33. The effects of the lesion produce no changes in correct or incorrect response latencies (all P>0.35).
Fig. 3.
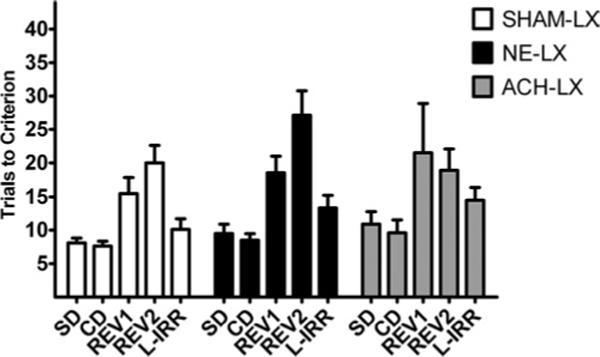
Means±S.E.M. are shown for SHAM-LX rats in white, NE-LX rats in black and ACh-LX rats in gray. There was no difference among the groups in the acquisition of the task on the SD, CD, reinforcement reversals (REV1 and REV2) or the test of L-IRR.
Effects of lesions on L-IRR
Performance between groups did not differ when changes to the stimulus dimension that never predicted reinforcement were tested (LESION F(2,12)=1.85, P=0.19; Fig. 3). The effects of this change were similar regardless of the stimulus dimension that was changed (LESION×SHIFT F(9,12)=1.3 P=0.33).
Effects of lesions on reinforcement reversals
As expected, damage to the IL/PL did not impair performance on reversal trials (LESION: F(2,12)=2.54, P=0.12; LESION×TEST: F(2,12)=1.99, P=0.18; Fig. 3) regardless of which stimulus attribute predicted reinforcement (TEST×SHIFT: F(3,12)=1.64, P=0.23; TEST×LESION×SHIFT: F(6,12)=0.56, P=0.75). All other main effects and interactions also failed to reach statistical significance in the analyses of trials to criterion and response latencies (all P>0.12).
DISCUSSION
The current study shows that NE, but not ACh in the medial frontal cortex is necessary for the shifts of attentional set. Though both NE and ACh have been implicated in attentional control, specifically in the area of sustained attention (McGaughy et al., 1996; Robbins, 1997; Sarter and Bruno, 1999; Robbins, 2000; Milstein et al., 2007), the present data suggest a dissociable role of these neuromodulators in prefrontal processing that contributes to shifts of attentional set. The cognitive effects of noradrenergic deafferentation are selective to the ED and produce overly focused attention. Neither noradrenergic nor cholinergic deafferentation in IL/PL impairs performance on the SD, CD or any of the reversals.
Neuroanatomical selectivity of the lesions
Previous work has shown that catecholaminergic lesions of frontal cortex prevent maintenance of an attentional set but these lesions were to both dopaminergic and noradrenergic terminals (Crofts et al., 2001). The current methodology allows restricted depletion of NE while sparing dopaminergic terminals (Wrenn et al., 1996; Milstein et al., 2007). Though the locus coeruleus cell bodies provide widespread innervations of sub-cortical and cortical targets (Foote et al., 1983), recent data from Robbins and colleagues (Milstein et al., 2007) has shown that lesions produced by intracortical infusions of DBH saporin deplete cortical targets while “subcortical structures” are “spared at all levels.” These data support the hypothesis that cognitive effects in the current task result from a loss of cortical rather than sub-cortical targets. The previous study showed loss in other cortical subregions, but the use of a lower dose of toxin and slower infusion rate in the current study produces lesions that were restricted to the medial prefrontal cortex with no loss in orbitofrontal cortex of NE-LX relative to SHAM-LX rats. Additionally, loss as assessed by immunohistochemistry on DBH positive fibers or neurochemistry showed comparable results suggesting that DBH positive fiber staining is adequate to assess the extent of the lesion (Milstein et al., 2007). Despite the specificity of our lesions, there is evidence that NE neurons may co-release dopamine and NE (Devoto et al., 2001)so even neurochemically specific lesions may decrease cortical dopamine levels. However, data from our laboratory show that systemic administration of a NE reuptake blocker is sufficient to restore the ability to shift attentional set (Newman et al., 2008) at doses of the drug insufficient to increase cortical dopamine levels (Bymaster et al., 2002) suggesting that modulation of the cortical NE without concomitant increases in dopamine is sufficient to restore attentional set shifting. Collectively, these data suggest that NE is necessary and sufficient to mediate attentional set shifting.
Hypoactive or hyperactive NE?
An additional concern may be that the relatively small depletions in the NE system resulted in compensatory increases in neurotransmitter release to produce a hyperactive rather than hypoactive NE system (Hughes and Stanford, 1998). To reduce the likelihood that the system assessed was overactive, behavioral testing was scheduled to coincide with low levels of NE as reported in other studies with similar lesions (Milstein et al., 2007). Though previous work has shown that noradrenergic systems can neurochemically compensate for lesions of 75−90% of the system by increasing LC firing rates (Chiodo et al., 1983), depletions greater than 50% are sufficient to decrease basal and stimulated release (Abercrombie and Zigmond, 1989). This is similar to findings that lesions of the cholinergic system may produce small increases in basal release but this compensation is inadequate in the presence of cognitive testing that taxes the neurochemical system thus exceeding the capacity of the compromised system (McGaughy et al., 2002). A NE reuptake inhibitor restores normal performance in NE-LX rats but impairs performance of normal rats (Newman et al., 2008). Based on these data, we hypothesize that the attentional deficits in the current study result from a decrease in levels of cortical NE but concurrent microdialysis during the behavioral test would be useful in determining how NE levels correlate with performance.
Our findings are in line with recent findings from Robbins and colleagues (Tait et al., 2007) who showed that lesions of the dorsal noradrenergic bundle produce more severe loss of noradrenergic innervation of the cortex and also impair ED. The current findings differ slightly from results produced by Lapiz and Morilak (2006) that failed to find impairments in any aspect of the task following systemic administration of clonidine, an α2 autoreceptor agonist which decreases NE levels. This discrepancy may be due to the inability of a single dose of clonidine to mimic the decrease in NE produced by DBH saporin infusions in IL/PL or it may be that the detrimental effects produced by the α2 agonist on release may have been countered by post-synaptic benefits (Arnsten, 1998).
Though previous data support a role for prefrontal NE in attentional set shifting, we have better elucidated the exact nature of the impairment following lesioning with the assessment of L-IRR (Owen et al., 1991, 1993). The ED requires two simultaneous cognitive skills, attending to a previously irrelevant stimulus attribute that now predicts reward while simultaneously disregarding the dimension that previously predicted reward (Owen et al., 1991, 1993). Performance of all groups was similar on L-IRR which assesses how susceptible the rats are to distraction or previously irrelevant attributes. The ED has the additional demand that rats must disengage attention from the previously relevant dimension. NE-LX rats require many more trials to reach criterion on the ED than the L-IRR suggesting these rats have difficulty inhibiting attentional set. NE-LX animals would often fail to explore the other stimulus attribute in the test of ED for many trials. It is unlikely that the NE-LX rats suffer from a loss of sustained attention as rats with larger NE lesions are unimpaired in standard tests of sustained attention (McGaughy et al., 1997; Milstein et al., 2007). Unpublished data show that similar noradrenergic lesions of the IL/PL produce an insensitivity to cross-modal distraction in a visual sustained, attention task (Newman et al., 2006) supporting the hypothesis that the attentional focus of these rats is abnormally narrow and that they persist in responding to a stimulus that previously predicted reinforcement. This may seem in contrast to the findings by Robbins and colleagues (Carli et al., 1983) who found that dorsal noradrenergic bundle lesions produced an increased sensitivity to cross-modal distraction but these distractors were extremely salient whereas stimulus attributes in the current protocol were selected because they were equal in salience. Similar studies have confirmed that the noradrenergic system is sensitive to exceptionally salient changes in the testing environment but also rapidly adapts to these modifications (Carli et al., 1983; Dalley et al., 2001, 2004). This is in line with Yu and Dayan's (2005) framework that suggests NE is recruited under conditions of unexpected uncertainty.
If one assumes that cortical NE mediates unexpected uncertainty (Yu and Dayan, 2005) then it may be predicted that performance on both the ED as well as the first reversal would be impaired. Lapiz and Morilak (2006) found that increasing NE via systemic administration of an α2-autoreceptor antagonist which acts to increase levels of NE facilitates performance in both the ED and REV1. As reversal learning has been shown to be impaired as a result of damage to the orbitofrontal rather than medial frontal cortex, it is not wholly surprising that no deficits were found in affective shifts in the current study as the method of lesioning prevents damage to orbitofrontal cortex (Dias et al., 1996, 1997; McAlonan and Brown, 2003). The facilitation in REV1 found by Lapiz and Morilak (2006) is likely due to increased NE release in orbitofrontal cortex, not IL/PL. In the present study, fiber counts in the orbitofrontal cortex showed no loss in NE-LX rats relative to SHAM-LX rats even in the region of the infusion site. Future studies will be aimed at assessing the effects of specific NE depletion of orbitofrontal cortex. It is predicted that this damage will produce impairment in the initial test of reversal learning within a stimulus modality but spare performance in cases of expected uncertainty namely subsequent reversals and suggests that unexpected uncertainty may take several forms.
Other studies regarding the role of NE in attention have correlated firing rates and firing patterns of neurons in the locus coeruleus during tests of attentional processing (Aston-Jones et al., 1994, 2000; Bouret and Sara, 2004, 2005; Aston-Jones and Cohen, 2005). Bouret and Sara (2005) have proposed that transient increases in LC firing allow a subject to maintain task-related, within-trial attention while longer lasting LC activation facilitates changes in attention on a larger time scale. Aston-Jones and Cohen (2005) have suggested that phasic firing of the LC related to target stimuli correlates with “exploitation” of current response strategies to maintain attentional focus while tonic firing is correlated with “exploration” of the environment when current response strategies fail to produce reward for longer periods of time. The current behavioral data suggest that animals are able to maintain phasic firing related to transient increases in LC activation as they readily learn all discriminations and are capable of maintaining attentional focus. However, they may be overly focused and less able to engage a new strategy after that strategy becomes ineffective as in the ED.
The increased number of trials to reach criterion performance on the ED relative to the ID in SHAM-LX rats and NE-LX rats suggests both groups form an attentional set and trials that require subjects to shift attentional set are more difficult for these rats. ACh-LX rats do not show any differential performance on the ED relative to the ID. Though this lack of differential performance dissociates performance of this group from all others, the failure to reject the null hypothesis does not elucidate the nature of the cognitive impairments in these rats. We hypothesize that ACh-LX rats fail to form an attentional set. This hypothesis would account for the similar performance on ID, ED and L-IRR despite changes in cognitive demands among these tests, namely the ED requires an inhibition of attentional set. Additionally if ACh-LX rats were given multiple tests of ID, they should show no improvement in performance with additional exposure to the attentional set. In contrast, SHAM-LX rats should show improved performance on subsequent ID tests. Lesions of noradrenergic and dopaminergic inputs to frontal cortex have been shown to produce highly variable performance across multiple tests of ID suggesting they fail to maintain an attentional set (Crofts et al., 2001). Future studies may elucidate the similarities or differences between the effects of cholinergic and catecholaminergic deafferentation on attentional set formation and maintenance.
It may be hypothesized that more diffuse depletions of cortical ACh or greater damage to anterior cingulate cortex would produce impairments in the ED similar to those seen in other tests of attention (Chudasama et al., 2003; Maddux et al., 2007). This seems unlikely as Tait and Brown (2008) have tested the effects of cholinergic depletion of the entire neocortical mantle produced by nucleus basalis magnocellularis infusions of 192 IgG saporin and also failed to produce any changes in task accuracy in this task. Similarly excitotoxic lesions of the cholinergic basal fore-brain in primates also failed to impair attentional set shifting (Roberts et al., 1992). These data contrast with the findings of Baxter and colleagues (Chen et al., 2004) who found that centrally acting scopolamine impaired both attentional and affective shifts. However, the drug administrations in this study were systemic so these data do not elucidate the neuroanatomical substrate of action for these effects and it was hypothesized to be extra-cortical.
CONCLUSION
In summary, we have shown that noradrenergic but not cholinergic deafferentation impairs attentional set shifts. These lesions are without effect on other aspects of cognitive control. Specifically, NE-LX rats do not differ from SHAM-LX rats in tests of reversal learning, conditional discrimination, attentional set formation and L-IRR suggesting that noradrenergic deafferentation of the IL/PL produces discrete impairments in executive function that are dissociable from the effects of cholinergic deafferentation.
Acknowledgments
The authors wish to thank Melanie Stollstorff and Stacey Rubin for excellent technical assistance in portions of the behavioral training. Supported by (NIA grant AG09973).
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- ACh-LX
cholinergically lesioned
- ANOVA
analysis of variance
- CD
compound discrimination
- DBH
dopamine beta-hydroxylase
- ED
extradimensional shift
- ID
intradimensional shift
- ID/ED
intradimensional/extradimensional set-shifting task
- IL/PL
infralimbic/prelimbic cortices
- L-IRR
learned irrelevance
- NE
norepinephrine
- NE-LX
noradrenergically lesioned
- PBS
phosphate-buffered saline
- REV
reversal
- SD
simple discrimination
- SHAM-LX
sham-lesioned
REFERENCES
- Abercrombie ED, Zigmond MJ. Partial injury to central noradrenergic neurons: reduction of tissue norepinephrine content is greater than reduction of extracellular norepinephrine measured by microdialysis. J Neurosci. 1989;9:4062–4067. doi: 10.1523/JNEUROSCI.09-11-04062.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Ann Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell J, Brown V. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur J Neurosci. 2004;20:791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gelhert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins T, Evenden J, Everitt B. Effects of lesions to ascending noradrenergic neurons on performance of a 5-choice serial reaction time task in rats: implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Chen KC, Baxter MG, Rodefer JS. Central blockade of muscarinic cholinergic receptors disrupts affective and attentional set-shifting. Eur J Neurosci. 2004;20:1081–1088. doi: 10.1111/j.1460-9568.2004.03548.x. [DOI] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. J Neurosci. 1995;15:7315–7322. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LA, Acheson AL, Zigmond MJ, Stricker EM. Subtotal destruction of central noradrenergic projections increases the firing rate of locus coeruleus cells. Brain Res. 1983;28:123–126. doi: 10.1016/0006-8993(83)91128-9. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Ven Denderen JCM. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and non-contingent performance of a visual attentional task. J Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Flore G, Pani L, Gessa GL. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry. 2001;6:657–664. doi: 10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins T, Roberts A. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins T, Roberts A. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin card sort test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Hughes ZA, Stanford SC. A partial noradrenergic lesion induced by DSP-4 increases extracellular noradrenaline concentration in rat frontal cortex: a microdialysis study in vivo. Psychopharmacology. 1998;136:299–303. doi: 10.1007/s002130050569. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by atentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Maddux J-M, Kerfoot EC, Chatterjee S, Holland PC. Dissociation of attention in learning and action: effects of lesions of the amygdala central nucleus, medial prefrontal cortex, and posterior parietal cortex. Behav Neurosci. 2007;121:63–79. doi: 10.1037/0735-7044.121.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a 5 choice serial reaction time task. J Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sandstrom M, Ruland S, Bruno JP, Sarter M. Lack of effects of lesions of the dorsal noradrenergic bundle on behavioral vigilance. Behav Neurosci. 1997;111:646–652. doi: 10.1037//0735-7044.111.3.646. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine and benzodiazepine receptor ligands. Psychopharmacology. 1995a;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Effects of chlordiazepoxide and scopolamine but not aging on the detection and identification of conditional visual stimuli. J Gerontol Biol Sci. 1995b;50:B90–B96. doi: 10.1093/gerona/50a.2.b90. [DOI] [PubMed] [Google Scholar]
- Milstein JA, Lehmann O, Theobald DEH, Dalley JW, Robbins TW. Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase saporin impairs attentional function and enhances the effects of guanfacine in the rat. Psychopharmacology. 2007;190:51–63. doi: 10.1007/s00213-006-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1097-8. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Moll T, McGaughy JA. Society for Neuroscience, Program No. 369.21. 2006 Neuroscience Meeting Planner. Society for Neuroscience; Atlanta, GA: 2006. Comparison of the effects of selective cholinergic or noradrenergic deafferentation in the medial, prefrontal cortex on sustained attention. 2006. Online. [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain. 1993;116:1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set-shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalohippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. Academic Press; New York: 1986. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty of behavioral performance in a target detection task. J Neurophysiol. 2004;92:361–371. doi: 10.1152/jn.00673.2003. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biol Psychol. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Roberts A, Robbins TW, Everitt BJ, Muir JL. A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience. 1992;47:251–264. doi: 10.1016/0306-4522(92)90241-s. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Abnormal regulation of corticopetal cholinergic neurons and impaired information processing in neuropsychiatric disorders. Trends Neurosci. 1999;22:67–74. doi: 10.1016/s0166-2236(98)01289-2. [DOI] [PubMed] [Google Scholar]
- Tago H, Kimura H, Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem. 1986;34:1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Behav Brain Res. 2008;187:100–108. doi: 10.1016/j.bbr.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Picklo MJ, Lappi DA, Robertson D, Wiley RG. Central noradrenergic lesioning using anti-DBH saporin: anatomical findings. Brain Res. 1996;740:175–184. doi: 10.1016/s0006-8993(96)00855-4. [DOI] [PubMed] [Google Scholar]
- Yu A, Dayan P. Acetylcholine in cortical inference. Neural Netw. 2002;15:719–730. doi: 10.1016/s0893-6080(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Yu A, Dayan P. Uncertainty, neuromodulation and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]


