Abstract
Chronic pediatric traumatic brain injury (TBI) is associated with significant and persistent neurobehavioral deficits. Using diffusion tensor imaging (DTI), we examined area, fractional anisotropy (FA), radial diffusion, and axial diffusion from six regions of the corpus callosum (CC) in 41 children and adolescents with TBI and 31 comparison children. Midsagittal cross-sectional area of the posterior body and isthmus was similar in younger children irrespective of injury status; however, increased area was evident in the older comparison children but was obviated in older children with TBI, suggesting arrested development. Similarly, age was correlated significantly with indices of tissue microstructure only for the comparison group. TBI was associated with significant reduction in FA and increased radial diffusivity in the posterior third of the CC and in the genu. The axial diffusivity did not differ by either age or group. Logistic regression analyses revealed that FA and radial diffusivity were equally sensitive to post-traumatic changes in 4 of 6 callosal regions; radial diffusivity was more sensitive for the rostral midbody and splenium. IQ, working memory, motor, and academic skills were correlated significantly with radial diffusion and/or FA from the isthmus and splenium only in the TBI group. Reduced size and microstructural changes in posterior callosal regions after TBI suggest arrested development, decreased organization, and disrupted myelination. Increased radial diffusivity was the most sensitive DTI-based surrogate marker of the extent of neuronal damage following TBI; FA was most strongly correlated with neuropsychological outcomes.
Keywords: traumatic brain injury, diffusion tensor imaging, fractional anisotropy, corpus callosum, outcome
Introduction
Traumatic brain injury (TBI), which is a leading cause of death and disability during childhood (Langlois et al., 2005), significantly impacts cerebral white and gray matter. Although the primary pathophysiological consequence of TBI is traumatic axonal injury (Gennarelli et al., 1998), diffuse pathologic changes reflect the combined effects of direct and secondary injury to myelinated and unmyelinated axons as well as to cell bodies (Reeves et al., 2005; Singleton and Povlishock, 2004). Secondary injuries are related to multiphasic reactive pathologies, including hypoxic-ischemic, contusion, hemorrhagic, apoptotic, and perfusion/reperfusion mechanisms, as well as to cascades of excitatory neurotransmitters (Povlishock, 2000). Pathologic studies of diffuse TBI have revealed microscopic features corresponding to Wallerian-type axonal degeneration, which involves the slow, progressive degradation and phagocytosis of myelin sheaths (George and Griffin, 1994), that most prominently affects the corpus callosum (CC), subcortical white matter, and dorsolateral aspect of the upper brainstem (Adams et al., 1977).
Callosal damage is viewed as a marker of the severity of diffuse TBI (Bigler, 2001; Levin et al., 2000). Focal lesions of the CC have been reported in 15 to 47% of cases with moderate to severe TBI, with the majority of lesions in the splenium (Gentry et al., 1988; Mendelsohn et al., 1992). Injury to the posterior body and splenium is consistent with axonal shear strain produced by the falx and tentorium (Tasker, 2006). In pediatric TBI, volumetric studies show post-traumatic atrophy is greatest in the posterior body, splenium, and genu (Levin et al., 2000; Yount et al., 2002) and correlates with clinical metrics of traumatic axonal injury and cognition (Benavidez et al., 1999; Johnson et al., 1996; Verger et al., 2001).
Relative to conventional MRI, diffusion tensor magnetic resonance imaging (DTI) may provide improved characterization of post-traumatic sequelae that are either poorly visualized or appear as nonspecific cerebral volume loss (Mukherjee and McKinstry, 2006). DTI allows in-vivo quantification of macro- and micro-structural changes in white and gray matter through examination of the degree and directionality of the diffusion of water in tissues (Basser, 1997; Eluvathingal et al., 2007). DTI provides metrics of diffusivity and anisotropy. The diffusion of water is restricted by a number of variables, including myelin, cell membranes, intracellular microtubules and axonal packing (Barkovich, 2005; Le Bihan, 2003; Moseley et al., 2002). In addition to mean diffusion, the directionality of water diffusion is evaluated using eigenvalues that quantify diffusion in three orthogonal directions. The first eigenvalue, the axial diffusivity, quantifies diffusion parallel to the principal axis of fibers. The second and third eigenvalues, the radial diffusivities, are combined to assess diffusion perpendicular to the principal axis. Fractional anisotropy (FA) reflects the degree of diffusion anisotropy within a voxel, which is related to microstructural tissue characteristics (Pierpaoli and Basser, 1996). Microstructural changes occurring in neurodegenerative disorders, including demyelination or the loss of axonal integrity, are likely to decrease FA (Moseley et al., 2002). Although clarification of the microstructural correlates of these metrics is ongoing, FA is believed to index the integrity and degree of fiber organization; mean diffusion has been related to expansion of extracellular space, possibly attributed to neuronal or glial loss (Pierpaoli et al., 2001; Rugg-Gunn et al., 2001; Tasker, 2006). Separate quantification of the radial and axial directional diffusivities may provide additional information regarding post-traumatic axonal pathology and disruption of tissue maturation. Human (Arfanakis et al., 2002; Concha et al., 2006; Tasker et al., 2005; Thomalla et al., 2004) and animal studies (Boska et al., 2007; Mac Donald et al., 2007) suggest that radial diffusivity assesses change associated with myelin. Axial diffusivity is more strongly related to axonal morphology and degradation. Factors affecting axial diffusion include buildup of cellular debris from breakdown of axonal structure, disordered microtubule arrangement, aggregation of filaments, and expansion of extracellular space (Sun et al., 2006).
Concha and colleagues identified the temporal relationship between DTI changes and expected histological changes related to Wallerian degeneration in patients undergoing corpus callosotomy. During the acute stage of recovery when axonal degeneration is expected but the axonal membrane and myelin are relatively intact, the axial diffusivity decreased significantly and was accompanied by a slight increase in radial diffusion. The decreased axial diffusion was attributed to fragmentation of axons, which produces barriers to the longitudinal displacement of water molecules. During the chronic stage associated with degradation of the myelin sheath and further degradation of the axonal membrane, the radial diffusivity was significantly elevated and FA was significantly reduced (Concha et al., 2006).
Recent DTI studies of adults with acute moderate to severe TBI reported reduced FA and increased mean and radial diffusivity in whole brain analyses, with increased axial diffusivity in areas of focal injury in the splenium believed to reflect axotomy (Newcombe et al., 2007). In adults with chronic TBI, Kraus and colleagues (2007) noted reduced FA and elevated axial and radial diffusivities in all 13 regions of interest in patients with moderate to severe TBI relative to controls. In patients with mild TBI, FA was reduced in three of 13 regions (corticospinal tract, sagittal stratum, superior longitudinal fasciculus); only the axial diffusivity was elevated in two of the regions of interest. These findings suggest a continuum of widespread neural changes in moderate to severe TBI affecting tissue organization, myelin, and axonal integrity. Following mild TBI, the increased axial diffusivity and relatively normal radial diffusivity may indicate persistent axonal damage in a subset of neural regions (Kraus et al., 2007).
Across studies of adults with TBI of varying severity, DTI metrics have shown inconsistent relations with clinical measures of injury severity, outcome ratings, and cognition. Newcombe and colleagues (2007) found that microstructural metrics did not correlate with either clinical measures of injury severity or global outcome ratings (Newcombe et al., 2007). In contrast, other studies noted that an index of global white matter pathology based on the number of regions with reduced FA correlated significantly with cognitive outcome measures (Kraus et al., 2007), and that mean diffusivity obtained from multiple regions of interest correlated with memory and learning performance (Salmond et al., 2006).
DTI metrics from different regions of the CC may reflect the integrity of white matter that is functionally connected to different cerebral regions subserving neural networks involved in complex cognitive and motor abilities. Callosal fibers are roughly topographically organized in relation to the cortical regions that they connect. The callosal subregions have connections with the following cortical regions: rostrum, caudal/orbital prefrontal and inferior premotor; genu, prefrontal; rostral body, premotor and supplementary motor; anterior midbody, motor; posterior midbody, somaesthetic and posterior parietal; isthmus, superior temporal and posterior parietal; and the splenium, which carries occipital and inferior temporal fibers (Witelson, 1989). Recent studies have documented correlations between specific neuropsychological outcomes and microstructure of callosal regions in healthy persons. For motor abilities, bimanual coordination correlated with the body FA (Johansen-Berg et al., 2007); visually-guided motor task performance correlated with FA from the posterior body and isthmus (Wahl et al., 2007). Based on a modified fiber-tracking parcellation method (Huang et al., 2005), phonological awareness and reading comprehension correlated with FA from the CC region carrying temporal fibers (Dougherty et al., 2007). IQ scores were not significantly correlated with any callosal regions in healthy children (Alexander et al., 2007; Dougherty et al., 2007), but were correlated with FA from the body and splenium in children with autism (Alexander et al., 2007) and TBI (Ewing-Cobbs et al., 2006a).
In children with chronic TBI, recent DTI studies reveal reduction in FA and increased mean diffusivity in all CC regions (Ewing-Cobbs et al., 2006a; Wilde et al., 2006). However, separate quantification of the radial and axial directional diffusivities has not been reported in pediatric studies. In addition, there are no studies relating multiple DTI metrics to neuropsychological outcomes in children sustaining TBI. Detailed analysis of tissue microstructure may provide additional information regarding post-traumatic pathology and disruption of callosal maturation. Therefore, the aim of this study was to examine the impact of TBI on multiple DTI metrics from well-defined CC regions and to characterize their relation to age and neuropsychological outcomes. We hypothesized that relative to a healthy comparison group, children with TBI would have reduced growth and microstructural development of the posterior CC. Secondly, due to its relation to myelination status, we hypothesized that radial diffusivity would be more sensitive to the presence of chronic TBI than FA. Thirdly, we expected that both FA and the radial diffusivity would be correlated with global outcome ratings and specific neuropsychological outcomes in the TBI group due to disruption of myelination and interference with tissue organization. Particularly in the TBI group, motor scores were expected to correlate with DTI metrics from anterior to mid CC regions; IQ and academic scores were expected to correlate with metrics from mid to posterior CC regions.
Material and Methods
Participants
Participants included 41 children who sustained TBI at ages 0 to 15 years and 31 community comparison children. . Written informed consent for MRI and behavioral assessments was obtained from guardians and adolescents. Assent was obtained from children per institutional review board regulations for the protection of human research subjects. Inclusionary criteria for children in the TBI group were: 1) complicated mild (n=6), moderate (n=8) or severe TBI (n=27), 2) sufficient recovery of cognitive skills to permit standardized assessment, 3) no known premorbid neurologic or metabolic disorder, 4) no history of prior TBI, and 5) gestational age ≥ 30 weeks. Severe TBI was defined as a post-resuscitation Glasgow Coma Scale (Teasdale and Jennett, 1974) score from 3–8. Moderate TBI was defined as a Glasgow score from 9–12. Complicated mild TBI was defined as Glasgow Coma Scale scores from 13–15 with acute neuroimaging evidence of extraaxial hemorrhage or parenchymal injury (Levin et al., 2008; Williams et al., 1990). The community comparison “control” group was recruited from the hospital at which the TBI children were hospitalized and from community notices. Children in the comparison group met criteria 3–5 above for entry into the study and received both imaging and neuro-psychological assessments. Demographic and neurologic variables are provided in Table 1. Children with TBI received neuropsychological evaluations and underwent DTI at least three months after TBI (mean ± SD = 39.1 ± 46.4 months) at ages 7–17 years. The CC was read as normal in 19 cases; Table 2 provides clinical findings from the remaining 22 scans. The 31 community comparison children had normal medical histories and MRI scans read as “normal” by a board certified radiologist.
Table 1.
Demographic and Injury Information for the TBI and Comparison Groups
| TBI (n=41) | Comparison (n=31) | |
|---|---|---|
| Years of Age at Scan (M (SD)) | 12.3 (2.7) | 11.3 (2.7) |
| Gender (% Male) | 80 | 48* |
| Handedness (% Right) | 95 | 100 |
| Glasgow Coma Scale Score (n) | ||
| 3–8 | 27 | -- |
| 9–12 | 8 | -- |
| 13–15 | 6 | -- |
| Glasgow Outcome Scale Score (n)† | ||
| Good Recovery | 11 | -- |
| Mild Disability | 11 | -- |
| Moderate Disability | 16 | -- |
| Severe Disability | 3 | -- |
| Years of Age at Injury (M (SD)) | 9.0 (4.1) | -- |
| Months from Injury to Scan (M (SD)) | 39.1 (46.4) | -- |
Note: p <.001.
Scale modified for children.
Table 2.
Number of TBI Cases with Clinical Findings in Callosal Regions
| Clinical Finding |
|||
|---|---|---|---|
| Callosal Region | Atrophy | Encephalomalacia/Gliosis | Shear |
| Genu | 1 | 1 | 0 |
| Rostral/Anterior Midbody | 5 | 0 | 0 |
| Posterior Body, Isthmus, Splenium | 7 | 4 | 4 |
| Generalized | 4 | 0 | 0 |
Conventional MRI and Diffusion Tensor Data Acquisition
We acquired whole-brain data using a Philips 3.0 T Intera system with a SENSE parallel imaging receive head coil. The MRI protocol included (a) conventional MRI (3D spoiled gradient-echo (SPGR), field-of-view=240×240 mm2 (isotropic voxel size = 0.9375 mm), (b) 2D dual spin-echo images TE1/TE2/TR=10/90/5000 ms, in the axial plane (3mm slice thickness, 44 sections) (c) and a phase-sensitive MRI in the sagittal and axial planes, in addition to a matching volume of diffusion-weighted data as described below.
The diffusion-weighted data were acquired using a single-shot spin-echo diffusion sensitized echo-planar imaging (EPI) sequence with the balanced Icosa21 encoding scheme (Hasan and Narayana, 2003; Hasan and Narayana, 2006), a diffusion sensitization of b=1000 sec.mm−2, a repetition and echo times of TR=6100 ms, TE= 84 ms, respectively. EPI distortion artifacts were reduced by using a SENSE acceleration factor or k-space undersampling R=2 (Bammer et al., 2002; Hasan and Narayana, 2006; Jaermann et al., 2004). The Field-of-view was 240×240 mm2, and slice thickness was 3 mm with 44 axial slices covering the whole-brain (foramen magnum to vertex); the in plane voxel size was 0.9375 mm. Spatial coverage matched the conventional MRI sequences described above. DTI acquisition time was approximately 7 minutes and resulted in SNR-independent DTI-metric estimation (Hasan et al., 2007).
DTI Data Processing and Metrics
The diffusion-encoded volumes were acquired with fat suppression and semi-automatically stripped to remove non-parenchymal tissue. The details of the DTI processing and computation of the DTI metrics are provided elsewhere (Hasan and Narayana, 2006). The DTI-derived rotationally-invariant metrics include the mean diffusivity, which is divisible into the axial (first eigenvalue) and radial (mean of the minor (third) and medium (second) eigenvalues) diffusivities (Beaulieu, 2002; Hasan, 2006; Sun et al., 2006). FA ranges from 0 to 1; higher scores indicate greater tissue integrity and fiber organization. For the radial and axial diffusivities, higher scores indicate greater diffusion of water, suggesting less well defined tissue organization, reduced myelination, and/or axonal pathology.
DTI based Segmentation of the Corpus Callosum
The midsagittal CC was identified based on the appearance of the interthalamic mass and the fornix on the isotropically (0.9375 ×0.9375 ×0.9375 mm3) interpolated DTI maps (Hasan et al., 2008), then segmented on the mid-sagittal slice using FA, mean diffusivity, and principal diffusion tensor eigenvector (Pvec). Thresholds are applied on the FA, mean diffusion, and the Pvec maps which were combined to obtain the structures with high fractional anisotropy (FA ≥ 0.25), medium mean diffusivity (0.8±0.2) × 10−3 mm2 sec−1, high orientation along the right-left direction and high local principal vector coherence. The mid-sagittal CC was brought into a standard frame of reference so that the line joining the maximum anterior–posterior points of the CC was horizontal. We used the partitioning scheme developed by Witelson (1989; see Figure 1) to create seven CC segments: rostrum, genu, rostral midbody, anterior midbody, posterior midbody, isthmus and splenium. The callosal subdivision thresholds were optimized by comparing the segmented CC areas on a cohort of healthy controls with the respective areas obtained by manual boundary selection of the CC on corresponding mid-sagittal phase sensitive T1-weighted images acquired in the same scan session. This procedure yields the area and DTI metrics for the seven segments. Because significant anatomic variation reduced reliability of rostrum measures, we report findings for the genu through the splenium. Although recent studies using DTI tractography to parcellate CC segments identified differences relative to the Witelson subdivisions (Hofer and Frahm, 2006; Huang et al., 2005), the Witelson divisions were used in the present study due to the body of literature that relates behavioral outcomes to these parcellation units and the limited information concerning brain-behavior correlations based on DTI-derived parcellation units. We examined the mid-sagittal area, FA, radial diffusion, and axial diffusion from each of the CC segments.
Figure 1.
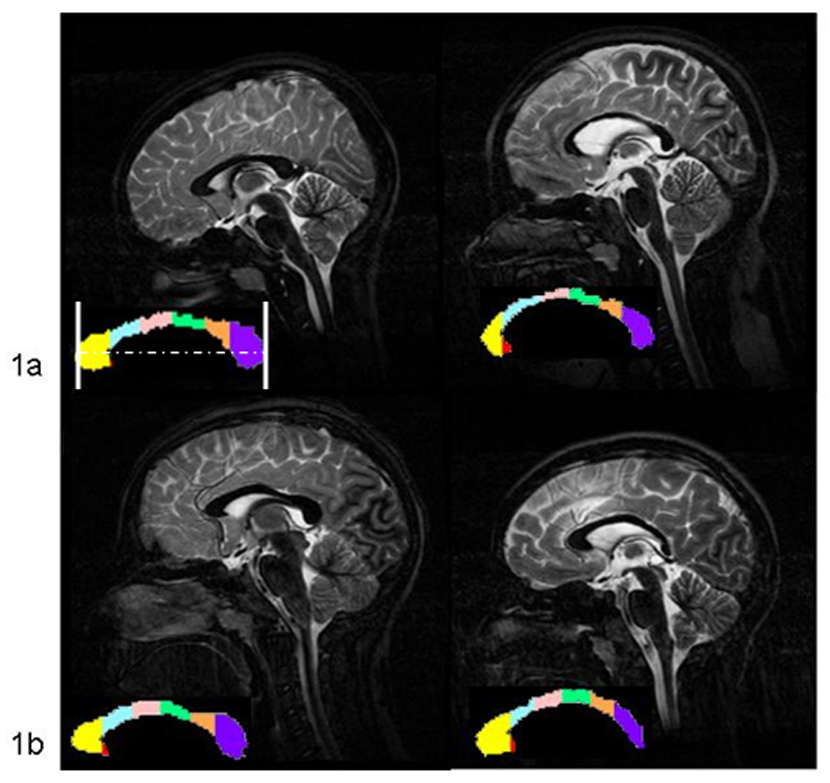
DTI maps of mid-sagittal callosal regions from T2 images in a) 10 year old and b) 13 year old comparison (left) and TBI cases (right). In a, note the mild thinning in the genu and splenium and moderate thinning in the body. Case b shows regional thinning in the posterior body and splenium. The standard frame of reference is depicted for image 1a. Callosal regions are indicated on the color map; genu=yellow, rostral midbody=blue, anterior midbody=pink, posterior midbody=green, isthmus=orange, splenium=purple.
Global Outcome and Neuropsychological Scores
Global Outcome Rating
Glasgow Outcome Scale scores (Jennett and Bond, 1975)] were recorded at the time of MRI acquisition. This widely used outcome scale evaluates outcome on a 5 point scale ranging from 1=Good Recovery to 5=Death.
Neuropsychological Outcome Scores
Neuropsychological tests assessing aspects of cognition related to IQ, motor performance, processing speed, and academic skills were administered individually to each study participant. Testing occurred on the day of scanning for most participants; a subset received the majority of testing on the day of scan and were administered the IQ test within 3 months of the scan.
IQ
The Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) composite score was a standard score reflecting verbal and nonverbal abilities based on performance on the vocabulary and matrix reasoning subtests.
Fine Motor and Processing Speed
The Grooved Pegboard Test (Klove, 1963) assesses manual dexterity based on the time required to insert pegs into a pegboard. To accommodate scores of hemiparetic children, the mean number of pegs placed per second with each hand was calculated. Processing speed was evaluated using the standard score from the Coding subtest from the Wechsler Intelligence Scale for Children-IV (Wechsler, 1991). This paper and pencil task assesses the speed and accuracy for transcribing written symbols.
Verbal Working Memory
Sentence Span (Just and Carpenter, 1992) is a dual task evaluating manipulating information in verbal working memory while also attending to sentence meaning. The child listened to sentences, decided whether they were true or false, and then recalled the last word in each sentence in the order in which they were presented. The dependent variable was the number of last words from each sentence that the child recalled correctly in order.
Reading and Calculation
Reading scores examined the automaticity of retrieving letter names from long-term memory as well as the accuracy of decoding words and comprehending connected text. Dependent variables included the standard score from the Rapid Letter Naming subtest from the Comprehensive Test of Phonological Processing (Wagner et al., 1999) and the Reading Accuracy and Reading Comprehension standard scores derived from the Gray Oral Reading Tests-4 (Wiederholt and Bryant, 2001). Calculation standard scores assessing the ability to complete mixed written math problems in an untimed format were obtained from the Woodcock-Johnson III Tests of Achievement (Woodcock et al., 2001).
Statistical Analyses
Group comparisons
A multivariate approach to repeated measures analysis of variance (ANOVA) was used to examine macro- and micro-structural differences in the 6 CC regions. The within-subjects factor was the 6 CC regions; between-subjects factors included age at scan (treated as a covariate) and group (TBI vs. comparison group). Follow-up comparisons examined differences in DTI metrics in contiguous CC regions. The dependent variables were mid-sagittal regional area, FA, radial diffusivity, and axial diffusivity. Additionally, the influence of age and group on metrics from each region was examined; Bonferroni corrections (p < .01) were employed to control for reported univariate tests.
Within the TBI group, general linear models were employed to examine the effects of TBI severity, age at injury, and time since TBI on the DTI metrics averaged across the mid-sagittal CC regions. Pearson partial correlation coefficients were calculated to examine the effects of gender on regional DTI metrics.
Sensitivity of DTI metrics
As there is limited information regarding the sensitivity of different DTI metrics to the effects of TBI, logistic regression analysis was used to compare the relative sensitivity of FA and radial diffusivity from different CC regions to the effects of TBI while controlling for age. Each model compared the joint contribution of the two DTI metrics; follow-up tests were examined to determine if one metric was more sensitive to TBI above and beyond the influence of the other variable. Odds ratios are reported to provide evidence of effect sizes, with chi-square tests for the direct estimate of how much a change in DTI metrics influenced classification in the TBI versus comparison groups.
Relation of DTI metrics with global and neuropsychological outcomes
CC metrics were examined in relation to the Glasgow Coma Scale and Glasgow Outcome Scale scores, which are global ratings of injury severity and outcome. The mid-sagittal area, FA, radial diffusivity, and axial diffusivity were individually averaged across the entire CC and correlated with the Glasgow scores using Pearson partial correlation coefficients.
Neuropsychological measures were expected to show specific relations with regional callosal DTI-derived metrics. Therefore, Pearson partial correlation coefficients controlling for age at scan examined the relation between regional DTI metrics and neuropsychological outcomes. To reduce the number of statistical tests and minimize Type 1 error, analyses were completed for metrics from four of the six CC regions using a 0.01 significance level. We selected the genu, anterior midbody, isthmus, and splenium to provide sampling of anterior, mid, and posterior regions. The genu and anterior midbody were selected to identify possible correlations of these regions with working memory and motor abilities, respectively. The isthmus and splenium carry temporal and posterior parietal fibers putatively related to reading and math performance; therefore, these regions were selected to examine correlations with academic outcomes.
Results
Group Comparisons of Callosal Macro- and Microstructure
Table 3 contains the results of repeated measures ANOVA. The Age × Group × Region interaction was not significant for either regional CC areas or DTI metrics and was deleted from each model. Table 4 provides the means (SD) and results of group comparisons for each CC region. Nonsignificant Age × Group interactions were trimmed from each overall and follow-up model.
Table 3.
Repeated Measures Analysis of Variance Comparing Corpus Callosum Region, Group, and Age Effects for DTI Macrostructural and Microstructural Metrics
| Area (mm*mm) | FA | Radial Diffusivity | Axial Diffusivity | |||||
|---|---|---|---|---|---|---|---|---|
| Wilks L | Wilks L | Wilks L | Wilks L | |||||
| ANOVA | df(5,64 ) | p | df(5,65) | p | df(5,65) | p | df(5,65) | p |
| Within-subjects effects | ||||||||
| Region | 20.55 | <0.001a | 20.20 | <0.001b | 4.65 | <0.001c | 3.64 | 0.006d |
| Region × age | 3.29 | 0.010 | 1.79 | 0.126 | 0.51 | 0.766 | 0.92 | 0.472 |
| Region × group | 0.66 | 0.659 | 2.72 | 0.027 | 0.74 | 0.596 | 0.93 | 0.465 |
| Between-subjects effects | F(1,68) | F(1,69) | F(1,69) | F(1,69) | ||||
| Group | 4.92 | 0.030 | 17.20 | <0.001 | 14.79 | 0.003 | 3.73 | 0.058 |
| Age | 1.20 | 0.278 | 0.54 | 0.466 | 0.70 | 0.404 | 2.29 | 0.135 |
| Age × group | 7.56 | 0.008 | ||||||
Note: Significant comparisons for adjacent callosal regions:
mm*mm: genu > rostral midbody > anterior midbody; splenium > isthmus
FA: genu > rostral midbody; splenium > isthmus
radial diffusivity: rostral midbody > genu; isthmus > splenium
axial diffusivity: splenium > isthmus
Table 4.
Means and Standard Deviations for DTI Metrics by Callosal Region for Traumatic Brain Injury and Typically Developing Comparison Groups
| DTI Metric |
||||||||
|---|---|---|---|---|---|---|---|---|
| Area (mm*mm) |
FA 0–1 |
Radial Diffusivity (× 10−3mm2s−1) |
Axial Diffusivity (× 10−3mm2s−1) |
|||||
| Callosal Region | TBI | TDC | TBI | TDC | TBI | TDC | TBI | TDC |
| Genu | 150.72 | 172.58 | .4802 | .5344a | .7289 | .6391a | 1.658 | 1.642 |
| (43.75) | (34.46) | (.8481) | (.0383) | (.1594) | (.0619) | (.1488) | (.1389) | |
| Rostral Midbody | 102.25 | 88.97 | .3813 | .4169 | .8704 | .7668a | 1.645 | 1.559 |
| (30.40) | (22.55) | (.0730) | (.0432) | (.1724) | (.0761) | (.1907) | (.1287) | |
| Anterior Midbody | 76.51 | 78.11 | .3663 | .3983 | .8770 | .7912 | 1.612 | 1.551 |
| (14.76) | (12.36) | (.0699) | (.0462) | (.1745) | (.0928) | (.1795) | (.1270) | |
| Posterior Body | 75.80 | 80.32 a, b,ab | .3528 | .3919a | .9050 | .8028a | 1.619 | 1.549 |
| (13.82) | (16.74) | (.0740) | (.0523) | (.1877) | (.1435) | (.1497) | (.1698) | |
| Isthmus | 66.22 | 77.85 ab | .3436 | .4151a | .9504 | .8209a | 1.682 | 1.661 |
| (15.97) | (15.21) | (.0948) | (.0605) | (.1906) | (.0992) | (.1685) | (.1403) | |
| Splenium | 173.08 | 188.96 | .4954 | .5834a | .7630 | .6343a | 1.801 | 1.819 |
| (37.95) | (29.72) | (.0911) | (.0420) | (.1534) | (.0614) | (.1714) | (.1319) | |
Results of univariate tests
Group p<0 .01
Age p <0 .01
Age × Group p < 0.01.
TBI=traumatic brain injury; TDC=typically developing comparison group.
The influence of gender was examined within the TBI and comparison groups. Gender was not significantly correlated with DTI metrics reflecting callosal macrostructure in the TBI (all p > 0.11) or in the comparison group (all p > 0.15). Gender did not correlate with measures of tissue microstructure in either group (TBI: all p > 0.17; comparison group, all p > 0.18). Therefore, gender was not considered further.
Mid-sagittal Callosal Macrostructure
Table 3 shows a significant Region × Age interaction; the area of the posterior body and isthmus increased more with increasing age than did other regions. The Age × Group interaction indicated that with increasing age, the areas of the CC regions increased in the comparison but not in the TBI groups (see Figure 2). Follow-up comparisons showed that areas of the posterior body and isthmus were significantly smaller in the TBI relative to the comparison groups at older, but not younger ages (see Table 4).
Figure 2.
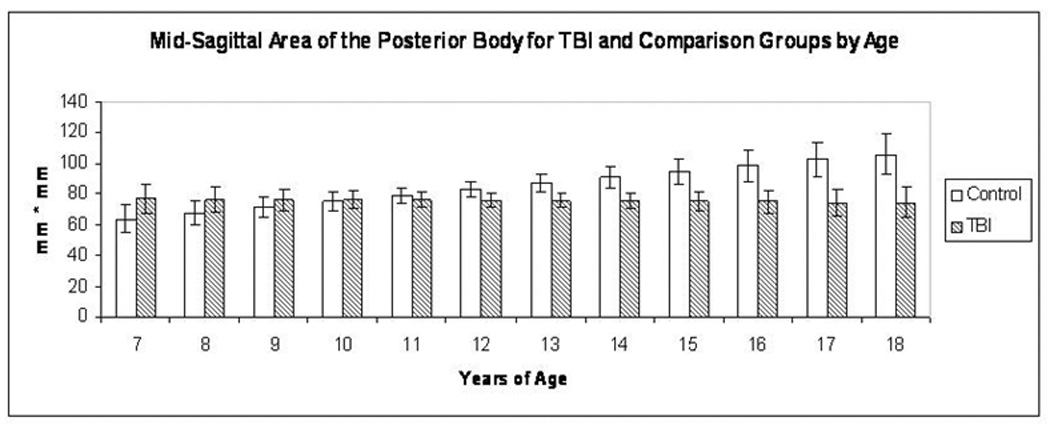
The Age × Group interaction for the mid-sagittal area of the posterior body is depicted. Although the area was similar in the younger TBI and typically developing comparison cases, the posterior body area was significantly smaller in the older TBI than in the older comparison cases, suggesting atrophy and arrested development.
Mid-sagittal Microstructure: Effects of Callosal Region, Group, and Age on DTI Metrics
FA differed in different callosal regions. The significant Region × Group interaction revealed that FA was significantly lower in the TBI than the comparison group in the genu, posterior body, isthmus, and splenium, but not in the rostral or anterior midbody. FA did not vary with age.
The radial diffusivity showed main effects of Region and Group. The radial diffusivity was significantly elevated in TBI versus control groups in 5 of 6 regions and did not vary significantly with age. For the axial diffusivity, only the Region effect was significant. Nonsignificant between-subjects effects revealed that the axial diffusivity was similar in the TBI and comparison groups across age and CC regions.
Pearson correlations assessing the relation of age and DTI metrics from the 6 CC regions show that none of the DTI metrics was significantly related to age for the TBI group. However, in the comparison group, FA increased with age in the posterior midbody; the radial diffusivity decreased with age in the isthmus and splenium (see Table 5).
Table 5.
Pearson Partial Correlation Coefficients Showing Relation of Age at Scan to DTI Metrics for Traumatic Brain Injury and Comparison Groups.
| DTI Metric |
|||||
|---|---|---|---|---|---|
| Callosal Region | Group | Area (mm*mm) | FA | Radial Diffusivity | Axial Diffusivity |
| Genu | TBI | −0.254 | −0.066 | −0.013 | −0.190 |
| Comparison | 0.062 | 0.214 | −0.304 | −0.132 | |
| Rostral Midbody | TBI | −0.023 | 0.044 | −0.078 | −0.086 |
| Comparison | 0.181 | 0.392 | −0.386 | −0.085 | |
| Anterior Midbody | TBI | 0.095 | −0.014 | −0.008 | −0.049 |
| Comparison | 0.555** | 0.421 | −0.305 | −0.001 | |
| Posterior Body | TBI | −0.042 | −0.017 | 0.024 | −0.057 |
| Comparison | 0.622*** | 0.619*** | −0.376 | −0.050 | |
| Isthmus | TBI | −0.232 | −0.117 | 0.186 | 0.110 |
| Comparison | 0.462* | 0.373 | −0.554** | −0.353 | |
| Splenium | TBI | −0.087 | −0.202 | 0.124 | −0.209 |
| Comparison | 0.390 | 0.306 | −0.603*** | −0.411 | |
Note: p<.01
p<.005
p<.001.
Relative Sensitivity to TBI
FA vs. Radial Diffusivity
Logistic regression analysis comparing the sensitivity of FA and the radial diffusivity to the effects of TBI was significant for all callosal segments. Radial diffusivity predicted TBI above and beyond the effects of age and FA for the rostral midbody and splenium; higher radial diffusivity was associated with greater odds of being in the TBI group (see Table 6). Both metrics individually were sensitive to the effects of TBI for the genu, anterior midbody, posterior body, and isthmus; however, when examined together, neither metric was uniquely sensitive to the effects of TBI in these regions due to shared variance.
Table 6.
Logistic Regression Analysis Comparing Relative Sensitivity of Radial Diffusivity and Fractional Anisotropy to Effects of Traumatic Brain Injury in Callosal Regions.
| Radial Diffusivity vs. FA | |||
|---|---|---|---|
| Model | Odds Ratio | ||
| X2 (3, N=72) | (95% CI) | ||
| Callosal Segment | Radial Diffusivity | FA | |
| Genu | 15.24 | 1.05 | 0.42 |
| 0.002 | (0.95–1.16) | (0.09–2.01) | |
| Rostral Midbody | 14.73 | 1.08* | 0.96 |
| 0.002 | (1.01–1.16) | (0.26–3.54) | |
| Anterior Midbody | 10.05 | 1.04 | 0.69 |
| 0.018 | (0.98–1.11) | (0.16–2.92) | |
| Posterior Body | 12.02 | 1.03 | 0.42 |
| 0.007 | (0.96–1.10) | (0.08–2.30) | |
| Isthmus | 19.99 | 1.07 | 0.58 |
| 0.002 | (0.99–1.16) | (0.18–1.89) | |
| Splenium | 41.84 | 1.24** | 0.19 |
| < 0.001 | (1.06–1.44) | (0.02–1.60) | |
X2 test of individual predictors:
p< 0.05
p < .01
p < .001
Relation of TBI Severity, Age at Injury, and Time since Injury to DTI Metrics
Pearson partial correlation coefficients controlling for age at scan were calculated to examine the relation of severity of TBI, as indicated by Glasgow Coma Scale scores, to DTI metrics. For these analyses, the DTI metrics were averaged across the mid-sagittal CC regions. Both FA (r=0.340, p=0.032) and the radial diffusivity (r= −0.315, p=0.048) were significantly correlated with GCS scores. Neither the midsagittal area nor the axial diffusivity was significantly related to GCS scores.
Within the TBI group, general linear models were used to examine differences in DTI metrics averaged across the callosal regions in children with complicated mild/moderate versus severe TBI. The severe TBI group had lower FA (1, 37) = 7.84, p = 0.006; and elevated radial diffusion FA (1, 37)= 5.05, p = 0.031 relative to the complicated mild/moderate TBI group. The mid-sagittal area and axial diffusivity were similar in the complicated mild/moderate and severe TBI groups. Neither age at injury, time since injury nor their interaction was significantly related to any of the 4 DTI metrics (df=1, 37 for all tests; F(p) values as follows: age at injury--area: F=1.14 (0.29); FA: F = 0.01 ( 0.94); radial diffusion: F= 0.21(0.65); axial diffusion, F= 0.67 (0.42); time since injury--area: F = 1.14 ( 0.29); FA: F = 0.00 ( 0.98); radial diffusion: F = 0.00 (0.98); axial diffusion: F = 0.07 (0.80).
Correlation of Callosal Metrics with Neurobehavioral Outcomes
Pearson partial correlation coefficients controlling for age at scan were calculated to examine the relation of the DTI metrics averaged across the entire CC to global outcome ratings. The Glasgow Outcome Scale score at the time of assessment was significantly correlated with the radial diffusivity, r=0.348, p =0.023, and with FA, r = −0.391, p = 0.013.
Several neuropsychological outcomes were examined in relation to DTI metrics. The TBI group performed more poorly than the comparison group on each measure, including rapid letter naming, reading accuracy, reading comprehension, fine motor speed, processing speed, verbal working memory, and IQ (all p < .02). The correlation of neuropsychological measures with midsagittal area, FA, radial, and axial diffusivities obtained from the genu, anterior midbody, isthmus, and splenium was examined for the TBI and control groups after controlling for age at scan (see Table 7). For the comparison group, midsagittal area of the isthmus was significantly correlated (p < 0.01) only with rapid letter naming. None of the microstructural indices was correlated with outcomes for the comparison group.
Table 7.
Pearson Partial Correlation Coefficients Indicating Relation of Neuropsychological Outcome Measures to DTI Metrics for Traumatic Brain Injury Group
| Callosal Region | ||||
|---|---|---|---|---|
| Neuropsychological Measure | Genu | Anterior Midbody | Isthmus | Splenium |
| Wechsler Abbreviated Scale of Intelligence | ||||
| Area (mm*mm) | −0.202 | −0.023 | 0.288 | 0.102 |
| FA | 0.043 | 0.233 | 0.322 | 0.438** |
| Radial Diffusivity | −0.232 | −0.349 | −0.404* | −0.406* |
| Axial Diffusivity | −0.049 | −0.222 | −0.037 | 0.191 |
| Coding | ||||
| Area (mm*mm) | −0.121 | 0.007 | 0.257 | −0.034 |
| FA | 0.108 | 0.402 | 0.419* | 0.483** |
| Radial Diffusivity | −0.190 | −0.368 | −0.339 | −0.346 |
| Axial Diffusivity | 0.121 | −0.182 | 0.181 | 0.196 |
| Fine Motor Dominant Hand | ||||
| Area (mm*mm) | −0.226 | 0.319 | 0.383 | 0.128 |
| FA | 0.104 | 0.368 | 0.435 | 0.485* |
| Radial Diffusivity | −0.152 | 0.191 | 0.133 | −0.275 |
| Axial Diffusivity | −0.101 | −0.230 | 0.140 | −0.213 |
| Fine Motor Nondominant Hand | ||||
| Area (mm*mm) | −0.085 | 0.333 | 0.497** | 0.118 |
| FA | 0.172 | 0.406* | 0.490** | 0.581*** |
| Radial Diffusivity | −0.232 | −0.331 | −0.323 | −0.358 |
| Axial Diffusivity | −0.185 | −0.268 | 0.098 | 0.185 |
| Verbal Working Memory | ||||
| Area (mm*mm) | −0.276 | 0.010 | 0.194 | 0.091 |
| FA | 0.307 | 0.338 | 0.272 | 0.452** |
| Radial Diffusivity | −0.217 | −0.298 | −0.233 | −0.302 |
| Axial Diffusivity | −0.239 | −0.176 | −0.064 | 0.138 |
| Rapid Letter Naming | ||||
| Area (mm*mm) | −0.007 | −0.191 | 0.103 | −0.063 |
| FA | 0.201 | 0.312 | 0.331 | 0.423* |
| Radial Diffusivity | −0.181 | −0.316 | −0.281 | −0.265 |
| Axial Diffusivity | 0.212 | −0.136 | 0.126 | 0.310 |
| Reading Accuracy | ||||
| Area (mm*mm) | −0.156 | −0.080 | 0.200 | −0.054 |
| FA | 0.127 | 0.250 | 0.347 | 0.383 |
| Radial Diffusivity | −0.221 | −0.339 | −0.314 | −0.310 |
| Axial Diffusivity | 0.081 | −0.188 | 0.140 | 0.194 |
| Reading Comprehension | ||||
| Area (mm*mm) | −0.161 | −0.118 | 0.270 | 0.131 |
| FA | 0.205 | 0.296 | 0.315 | 0.419* |
| Radial Diffusivity | −0.288 | −0.336 | −0.405* | −0.383 |
| Axial Diffusivity | 0.037 | −0.133 | −0.155 | 0.071 |
| Calculation | ||||
| Area (mm*mm) | −0.134 | 0.025 | 0.309 | 0.175 |
| FA | 0.049 | 0.170 | 0.319 | 0.350 |
| Radial Diffusivity | −0.157 | −0.233 | −0.303 | −0.277 |
| Axial Diffusivity | −0.055 | −0.186 | 0.011 | 0.165 |
Note: p < .01
p < .005
p < .0001.
In contrast, for the TBI group, significant relations were noted between DTI metrics and neuropsychological outcomes. Figure 3 depicts the macro- and microstructural metrics that were significantly correlated with outcomes. Overall, FA from the splenium was significantly related to multiple cognitive and motor outcomes. IQ and reading comprehension scores were significantly correlated with the radial diffusivity in the splenium and/or isthmus. Fine motor speed and coordination were related to FA from the splenium, isthmus, and anterior body. Axial diffusion was not significantly correlated with any of the neuropsychological scores for either group.
Figure 3.
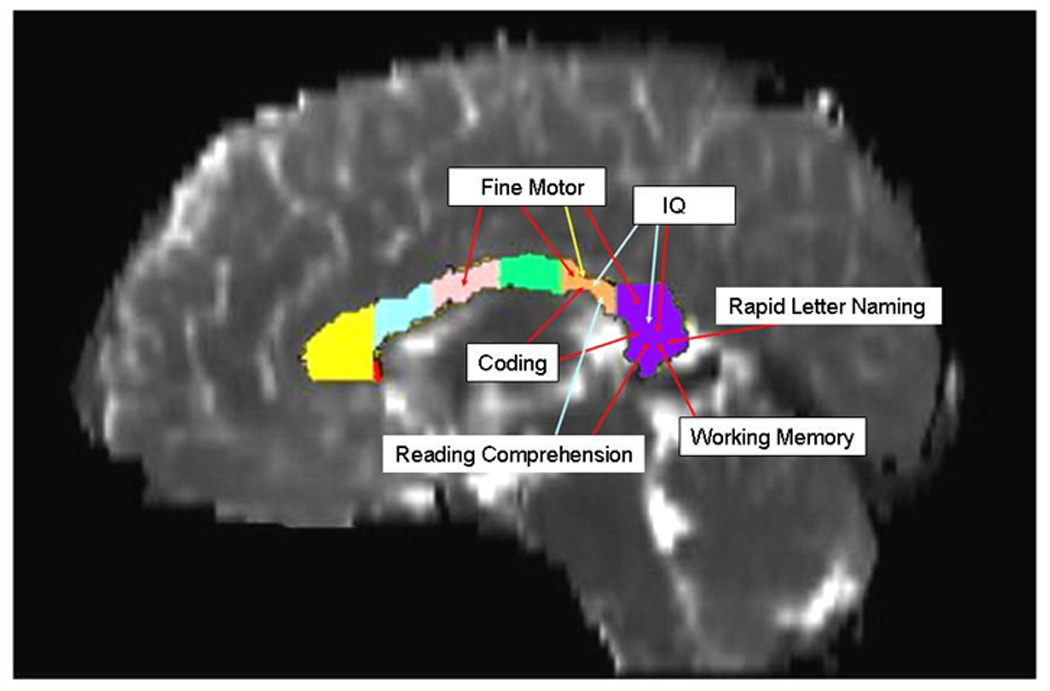
Significant correlation (p < 0.01) of neuropsychological outcomes with DTI macro- and microstructural metrics from the genu, anterior midbody, isthmus, and splenium is depicted for children with TBI. FA (red arrows) was more frequently correlated with motor and cognitive outcomes than the radial diffusivity (blue arrows). The mid-sagittal area (yellow arrow) of the isthmus correlated only with fine motor scores.
Discussion
As hypothesized, age and TBI had specific relations with regional callosal macrostructure and microstructure. We identified 1) arrested callosal development in children with TBI at both macro and microstructural levels, which was particularly evident in the posterior body, isthmus, and splenium; 2) particular sensitivity of the radial diffusivity to microstructural changes after TBI; and 3) particular sensitivity of regional FA metrics with multiple neuropsychological outcomes following TBI, including fine motor, coding, naming speed, working memory, calculation, IQ, and reading comprehension scores; in contrast, the radial diffusivity was significantly correlated only with IQ and reading comprehension scores. The radial diffusivity may be more sensitive to the effects of TBI; however, FA appears to be the most sensitive index of post-traumatic changes in a wide range of cognitive and motor outcomes.
Callosal Development and Macrostructure
As predicted, callosal growth was attenuated in youth with TBI. Callosal mid-sagittal area is frequently used as an index of developmental change in studies of maturation and as a biomarker of atrophy associated with disease or injury (Barnea-Goraly et al., 2005; Benavidez et al., 1999; Ewing-Cobbs et al., 2006a; Giedd et al., 1999; Hasan et al., 2005; Keshavan et al., 2002; Levin et al., 2000; Levin et al., 2002; Nosarti et al., 2004; Shaw et al., 2006). Callosal axons are largely developed in utero (Rakic and Yakovlev, 1968); therefore, postnatal CC growth is primarily due to myelination-related processes (LaMantia and Rakic, 1990). Callosal development during childhood and adolescence is reflected in increased area of the isthmus and splenium (Giedd et al., 1996; Rajapakse et al., 1996) that are associated with increases in axonal diameter and myelin deposition (Carlson et al., 1988). Oligodendrocytes, the progenitors of myelin, may be particularly vulnerable to insult during stages of rapid myelination during childhood (Back et al., 2002).
Post-traumatic atrophy of the CC may be related to direct injury and to secondary insults related to hypoxic-ischemic injury and metabolic changes. Our DTI-derived metrics of mid-sagittal CC area of the posterior body and isthmus were similar in younger children irrespective of injury status; however, continued development was evident in the older comparison children but was arrested in older children with TBI. Consistent with the finding in developmental pathology that structures in a rapid stage of development are selectively vulnerable to insult (Hebb, 1942), the rapid development of the isthmus and splenium during childhood may confer selective vulnerability of these regions to insult.
TBI obviated developmental changes in both macrostructural and microstructural development of these posterior callosal regions which develop rapidly in healthy older children and adolescents. Our findings are also consistent with previous work reporting decreased area in the posterior body and splenium, and reduced growth in total CC area in children with severe relative to mild TBI from 3 to 36 months after injury (Levin et al., 2000). The posterior CC is selectively vulnerable to injury in children with TBI and in other populations of children with diffuse central nervous system injury sustained early in life or during gestation, including children born prematurely (Anderson et al., 2005; Nosarti et al., 2004; Peterson et al., 2000) and in children with spina bifida myelomeningocele (Klaas et al., 1999).
Callosal Microstructure
Effect of TBI
Characterization of changes in callosal water molecular diffusion tensor anisotropy and directional diffusivity following TBI has been inconsistent. Discrepant results may be attributed in part to differences in patient characteristics (including age, injury severity and chronicity), the DTI metrics evaluated; and extent of parcellation of callosal regions. Studies typically report reduced FA in the genu, body, and/or splenium (Arfanakis et al., 2002; Ewing-Cobbs et al., 2006a; Inglese et al., 2005; Nakayama et al., 2006; Salmond et al., 2006; Wilde et al., 2006); however, mean diffusivity was unchanged in some (Salmond et al., 2006) and increased in other studies (Inglese et al., 2005; Tasker et al., 2005).
In our sample, metrics of tissue microstructure had specific relations with group and CC region. Glasgow Coma Scale scores and Glasgow Outcome Scale ratings were significantly correlated with both the radial diffusivity and with FA averaged across CC regions. Alteration in tissue microstructure in the TBI group was indicated by decreased FA and/or increased radial diffusivity in all CC regions except for the anterior midbody. TBI reduced FA and increased the radial diffusivity in the posterior body, isthmus, splenium, and in the genu. TBI reduced integrity of mid-callosal sagittal fibers, increased extracellular space possibly related to glial loss (Rugg-Gunn et al., 2001; Tasker, 2006), and disrupted myelination (Sun et al., 2006) in these regions. Decreased FA and increased radial diffusivity in the TBI group suggests sensitivity of DTI metrics to disruption in tissue microstructure associated with both the acute and chronic stages of Wallerian-type degeneration (Vargas and Barres, 2007). Several human and animal studies examined changes in both anisotropy and the directional diffusivities following injury. Decreased anisotropy was related to a reduction in the axial diffusivity consistent with axonal fragmentation during the acute stages of recovery; reduced anisotropy during the chronic stages was related to increased radial diffusion consistent with progressive degradation and phagocytosis of the myelin sheath (Concha et al., 2006; George and Griffin, 1994).
Our hypothesis that the radial diffusivity would be the most sensitive metric to the presence of TBI was supported. The radial diffusivity and FA were equally sensitive to TBI effects in four of the six regions evaluated; the radial diffusivity predicted TBI over and above FA for the rostral midbody and splenium. Due to its relation with myelination and sensitivity to myelin damage after TBI, the increased radial diffusivity in the genu and posterior CC may be the most sensitive DTI-based surrogate marker of the extent of myelin damage for assessment of disease impact and monitoring neuroprotective therapies.
Effect of age
Although main effects of age were not identified in ANOVA models, correlational analysis revealed increased FA with age in the posterior midbody and decreased radial diffusivity with age in the isthmus and splenium only in the comparison group. As previously noted, structures in a rapid stage of development appear to be selectively vulnerable to insult (Hebb, 1942). TBI obviated developmental changes in these regions; the size in these posterior regions was similar in the youngest group but was attenuated in the adolescents. The increased FA and reduced diffusion noted in the comparison group is similar to the pattern of findings identified in several association and commissural pathways in typically developing children and adolescents (Alexander et al., 2007; Ashtari et al., 2005; Eluvathingal et al., 2007; Nagy et al., 2004; Neil et al., 2002; Olesen et al., 2003; Schmithorst et al., 2002).
Relation of DTI Metrics with Neurobehavioral and Neuropsychological Outcomes
Metrics of tissue microstructure, especially FA and the radial diffusivity, were related to a variety of neuropsychological outcomes in the children and adolescents with TBI. The weak relationship of callosal macrostructure and neuropsychological outcomes after TBI suggests that microstructural measures of tissue organization and the integrity of myelin may be stronger predictors of outcomes than macrostructural measures of callosal area. The neuropsychological outcomes showed some specific relations with area, FA and the radial diffusivity from CC regions. Overall, FA was more frequently correlated with neuropsychological outcomes than either mid-sagittal area or the radial diffusivity. Mid-sagittal area of the isthmus was the only macrostructural index from the CC that was related to any outcome; it was significantly correlated with fine motor performance. Fine motor speed and coordination were also significantly related to FA from the anterior midbody, isthmus, and splenium. These findings are consistent with recent DTI tractography studies showing that motor and sensory fibers cross more posteriorly through the midbody (Hofer and Frahm, 2006) than suggested in previous studies (Witelson, 1989). Tractography studies also indicate that bimanual coordination tasks correlate with FA from the body (Johansen-Berg et al., 2007). The Coding subtest, which is a visually-guided paper-pencil test of symbol transcription that assesses processing speed, was correlated with FA from the isthmus and splenium. Working memory was specifically correlated with FA from the splenium; other studies also identified significant relations of executive function tasks with FA from the body and splenium following TBI (Kraus et al., 2007). IQ, reading, and calculation scores were also significantly correlated with FA from the splenium. IQ scores were further related to radial diffusion in both the isthmus and splenium, while reading comprehension was related to radial diffusion from the isthmus. This pattern is consistent with the topography of CC tracts, with motor fibers coursing through the body and temporal, parietal, and occipital fibers overlapping in more posterior regions (Hofer and Frahm, 2006). The isthmus and splenium contain interconnecting fibers from posterior association areas involved in distributed networks for reading and academic skill development (Shaywitz et al., 2002; Simos et al., 2002).
Limitations of the present study include the restricted range of TBI severity; youth with the mildest and most severe injuries were not scanned. Although all scans were obtained at least 3 months after TBI, the time interval from TBI to scan was variable. Initial analyses indicated no difference in DTI metrics in scans obtained from youth sustaining TBI from 3 months to 12 years post injury; therefore, the data were combined into a single TBI group. Future studies should use longitudinal designs to examine changes in DTI metrics in children with TBI and in comparison children to characterize developmental changes and their disruption by neurotrauma.
Conclusions
In the present study, DTI metrics were related to the presence and severity of TBI as well as to outcome ratings and neuropsychological performance. Microstructural metrics were more strongly associated with outcomes than macrostructural metrics and showed some specific relations with TBI variables. The radial diffusivity was sensitive to the effects of traumatic axonal injury and best discriminated TBI and comparison groups. The radial diffusivity may be a surrogate marker of both the impact of TBI and the effect of neuroprotective therapies on myelin. FA, which indexes the integrity of tissue organization, was the most sensitive marker of post-traumatic changes in a wide range of cognitive and motor outcomes. The reduction in FA and increased radial diffusivity in the genu, posterior body, isthmus and splenium suggest disruption in interhemispheric tracts connecting primary and secondary somatosensory fibers and association fibers coursing through temporal and posterior parietal regions. Disruption in these pathways may underlie the significant cognitive and academic skill deficits often noted following TBI (Benavidez et al., 1999; Ewing-Cobbs et al., 2004; Ewing-Cobbs et al., 2006b; Taylor et al., 2002). Alteration of these metrics in the genu implicates loss of prefrontal and frontal fibers involved in motor planning and in regulating cognition and emotion (Levin et al., 2004; Max et al., 2005). Future studies should examine relations of specific neurobehavioral functions to DTI metrics from well-defined callosal regions and distributed neural networks. Studies in healthy children are needed to establish normative values to facilitate examination of alterations in DTI metrics and brain-behavior relations in youth with brain injury and other neurobiological disorders. DTI has the potential to serve as a biomarker of acute changes in axonal integrity and myelination as well as to characterize changes during the chronic stage of recovery that may reflect continued regional demyelination and/or reorganization.
Acknowledgements
This work was funded by the National Institutes of Health-National Institute for Neurological Diseases and Stroke grants R01-NS046308 awarded to LEC and R01-NS052505-03 awarded to KMH. The authors wish to thank Ambika Sankar for assisting in data processing and Vipul Kumar Patel for helping in data acquisition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adams JH, Mitchell DE, Graham DI, Doyle D. Diffuse brain damage of the immediate impact type. Brain. 1977;100:489–502. doi: 10.1093/brain/100.3.489. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray M, Oakes T, Miller J, Lu J, Eun-Kee J, McMahon W, Bigler ED, Lainhart J. Diffusion tensor imaging of the corpus callosum in autism. NeuroImage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Anderson NG, Laurent I, Cook N, Woodward L, Inder TE. Growth rate of corpus callosum in very premature infants. AJNR Am J Neuroradiol. 2005;26:2685–2690. [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ. Diffusion tensor MR imaging in diffuse axonal injury. ANJR Am J Neuroradiol. 2002;53:794–802. [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA. Attention-deficit/hyperactivity disorder: A preliminary diffusion tensor imaging study. Biological Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bammer R, Auer M, Keeling SL, Augustin M, Stables LA, Prokesch RW, Stollberger R, Moseley ME, Fazekas F. Diffusion tensor imaging using single-shot SENSE-EPL. Magnetic Resonance Imaging. 2002;48:128–136. doi: 10.1002/mrm.10184. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ. Magnetic resonance techniques in the assessment of myelin and myelination. J Inherit Metab Dis. 2005;28:311–343. doi: 10.1007/s10545-005-5952-z. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benavidez DA, Fletcher JM, Hannay JH, Bland ST, Caudle SE, Mendelsohn D, Yeakley J, Brunder DG, Harward H, Song J, Perachio N, Bruce DA, Schiebel RS, Lilly MA, Verger-Maestre K, Levin HS. Corpus callosum damage and interhemispheric transfer of information following closed head injury in children. Cortex. 1999;35:315–336. doi: 10.1016/s0010-9452(08)70803-7. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Quantitative magnetic resonance imaging in traumatic brain injury. Journal of Head Trauma Rehabilitation. 2001;16:117–134. doi: 10.1097/00001199-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Boska MD, Hasan K, Kibuule D, Banerjee R, McIntyre E, Nelson JA, Hahn T, Gendelman HE, Mosley RL. Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson's disease. Neurobiology of Disease. 2007 doi: 10.1016/j.nbd.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Earls F, Todd RD. The importance of regressive changes in the development of the nervous system: Towards a neurobiological theory of child development. Psychiatric Developments. 1988;1:22. [PubMed] [Google Scholar]
- Concha L, Gross DW, Wheatley BM, Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy is epilepsy patients. NeuroImage. 2006;32:1090–1099. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proceeding of the National Academy of Sciences. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan K, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cerebral Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Barnes MA, Fletcher JM, Levin HS, Swank PR, Song J. Modeling of longitudinal academic achievement scores after pediatric traumatic brain injury. Developmental Neuropsychology. 2004;25(12):107–133. doi: 10.1080/87565641.2004.9651924. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Hasan K, Prasad M, Kramer L, Bachevalier J. Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. AJNR Am J Neuroradiol. 2006a;27:879–881. [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad M, Kramer L, Cox C, Baumgartner J, Fletcher S, Mendez D, Barnes MA, Zhang X, Swank PR. Late intellectual and academic outcomes following traumatic brain injury sustained during early childhood. Journal of Neurosurgery. 2006b;105 Suppl 4:287–296. doi: 10.3171/ped.2006.105.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE, Graham DI. Diffuse axonal injury: an important form of traumatic brain damage. Neuroscientist. 1998;4:202–215. [Google Scholar]
- Gentry LR, Thompson B, Godersky JC. Trauma to the corpus callosum: MR features. ANJR American Journal of Neuroradiology. 1988;9:1129–1138. [PMC free article] [PubMed] [Google Scholar]
- George R, Griffin JW. Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: The dorsal radiculotomy model. Experimental Neurology. 1994;129:225–236. doi: 10.1006/exnr.1994.1164. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal JD, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Prog.Neuro-Psychopharmacol & Biol.Psychiat. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rumsey JM, Castellanos FX, Rajapakse JC, Kaysen D, Vaituzis AC, Vauss YC, Hamburger SD, Rapoport JL. A quantitative MRI study of the corpus callosum in children and adolescents. Developmental Brain Research. 1996;91:274–280. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Hasan K. Diffusion tensor eigenvalues or both mean diffusivity and fractional anisotropy are required in quantitative clinical diffusion tensor MR reports: Fractional anisotropy alone in not sufficient. Radiology. 2006;239:611–613. doi: 10.1148/radiol.2392051172. [DOI] [PubMed] [Google Scholar]
- Hasan K, Gupta RK, Santos RM, Wolinsky JS, Narayana PA. Diffusion tensor fractional anisotropy of the normal-appearing seven segments of the corpus callosum in healthy adults and relapsing-remitting multiple sclerosis patients. Journal of Magnetic Resonance Imaging. 2005;21:735–743. doi: 10.1002/jmri.20296. [DOI] [PubMed] [Google Scholar]
- Hasan K, Halphen C, Sankar A, Eluvathingal TJ, Kramer L, Stuebing KK, Ewing-Cobbs L, Fletcher JM. Diffusion tensor imaging-based tissue segmentation: Validation and application to the developing child and adolescent brain. NeuroImage. 2007;34:1497–1505. doi: 10.1016/j.neuroimage.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan K, Narayana PA. Computation of the mean diffusivity and fractional anisotropy maps without tensor decoding and diagonalization: Theoretical analysis and experimental validation. Magnetic Resonance Imaging. 2003;50:589–598. doi: 10.1002/mrm.10552. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Ewing-Cobbs L, Kramer LA, Fletcher JM, Narayana PA. Diffusion tensor quantification of the macrostructure and microstructure of human midsagittal corpus callosum across the lifespan. NMR Biomed. 2008;21:1–8. doi: 10.1002/nbm.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan K, Narayana PA. Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magnetic Resonance in Medicine. 2006;56:130–137. doi: 10.1002/mrm.20935. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The effect of early and late brain injury upon test scores, and the nature of normal adult intelligence. Proc of the Am Philosoph Soci. 1942;85:275–292. [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited - Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S. DTI tractography based parcellation of white matter: Application to the mid-sagittal morphology of corpus callosum. NeuroImage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, Grossman RI. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. Journal of Neurosurgery. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Jaermann T, Crelier G, Pruessmann KP, Golay X, Netsch T, Van Muiswinkel AM, Mori S, van Zijl PC, Valavanis A, Kollias S, Boesiger P. SENSE-DTI at 3 T. Magnetic Resonance in Medicine. 2004;51:230–236. doi: 10.1002/mrm.10707. [DOI] [PubMed] [Google Scholar]
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–487. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. NeuroImage. 2007;36:T16–T21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Pinkston JB, Bigler ED, Blatter DD. Corpus callosum morphology in normal controls and traumatic brain injury: Sex differences, mechanisms of injury, and neuropsychological correlates. Neuropsychology. 1996;10:408–415. [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: Individual differences in working memory. Psychological Review. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, DeBellis M, Dick E, Kotwal R, Rosenberg DR, Sweeney JA, Minshew N, Pettegrew JW. Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sciences. 2002;70:1909–1922. doi: 10.1016/s0024-3205(02)01492-3. [DOI] [PubMed] [Google Scholar]
- Klaas PA, Hannay JH, Caroselli JS, Fletcher JM. Interhemispheric transfer of visual, auditory, tactile, and visuomotor information in children with hydrocephalus and partial agenesis of the corpus callosum. Journal of Clinical and Experimental Neuropsychology. 1999;21:837–850. doi: 10.1076/jcen.21.6.837.851. [DOI] [PubMed] [Google Scholar]
- Klove H. Clinical neuropsychology. In: Forster FM, editor. The Medical Clinics of North America. New York: Saunders; 1963. [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain. 2007 doi: 10.1093/brain/awm216. In press. [DOI] [PubMed] [Google Scholar]
- LaMantia A-S, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990;10:2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States. Journal of Head Trauma Rehabilitation. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Levin HS, Benavidez D, Verger-Maestre K, Perachio N, Song J, Mendelsohn D, Fletcher JM. Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology. 2000;54:647–653. doi: 10.1212/wnl.54.3.647. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Chang C, Zhang L, Schachar R, Ewing-Cobbs L, Max JE. Working memory after traumatic brain injury in children. Annals of Neurology. 2002;52:82–88. doi: 10.1002/ana.10252. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Roberson GS, Xiaoqi L, Ewing-Cobbs L, Dennis M, Chapman SB, Max JE, Hunter JV, Schachar R, Luerssen TG, Swank PR. Abnormal CT after mild traumatic brain injury predicts cognitive sequelae in school aged children. Journal of Neurosurgery. 2008 doi: 10.3171/PED/2008/1/6/461. In press. [DOI] [PubMed] [Google Scholar]
- Levin HS, Zhang L, Dennis M, Ewing-Cobbs L, Schachar R, Max JE, Landis J, Roberson GS, Scheibel RS, Miller DH, Hunter JV. Psychosocial outcome of TBI in children with unilateral frontal lesions. Journal of the International Neuropsychological Society. 2004;10:305–316. doi: 10.1017/S1355617704102129. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007 doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max JE, Schachar DL, Levin HS, Ewing-Cobbs L, Chapman SB, Dennis M, Saunders AE, Landis J. Predictors of secondary attention deficit/hyperactivity disorder in children and adolescents 6 to 24 months after traumatic brain injury. Journal of the American Academy of Child Psychiatry. 2005;44:1041–1049. doi: 10.1097/01.chi.0000173292.05817.f8. [DOI] [PubMed] [Google Scholar]
- Mendelsohn D, Levin HS, Bruce DA, Lilly MA, Harward H, Culhane KA, Eisenberg HM. Late MRI findings after head injury in children: Relationship to clinical features and outcome. Child's Nervous System. 1992;8:445–452. doi: 10.1007/BF00274405. [DOI] [PubMed] [Google Scholar]
- Moseley M, Bammer R, Illes J. Brain diffusion-tensor imaging of cognitive performance. Cognition. 2002;50:396–413. doi: 10.1016/s0278-2626(02)00524-9. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am. 2006;16:19–43. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Okumura A, Shinoda J, Yasokawa Y-T, Miwa K, Yoshimura S-I, Iwama T. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77:850–855. doi: 10.1136/jnnp.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil JJ, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain - a technical review. NMR in Biomedicine. 2002;15:543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- Newcombe VFJ, Williams GB, Nortje J, Bradley PG, Harding SG, Smielewski P, Coles JP, Maiya B, Gillard JH, Hutchinson PJ, Pickard JD, Carpenter TA, Menon DK. Analysis of acute traumatic axonal injury using diffusion tensor imaging. British Journal of Neurosurgery. 2007;21:340–348. doi: 10.1080/02688690701400882. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin R, Murray RM. Corpus callosum size and very preterm birth: Relationship to neuropsychological outcome. Brain. 2004;127:2080–2089. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Cogn Brain Res. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajecic S, Chen R, Penix LR, Virta A, Basser PJ. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance Medicine. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Povlishock J. Pathophysiology of neural injury: Therapeutic opportunities and challenges. Clinical Neurosurgery. 2000;46:113–126. [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rumsey JM, Vaituzis AC, Hamburger SD, Rapoport JL. Regional MRI measurements of the corpus callosum: A methodological and developmental study. Brain & Development. 1996;18:379–388. doi: 10.1016/0387-7604(96)00034-4. [DOI] [PubMed] [Google Scholar]
- Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. Journal of Comparative Neurology. 1968;132:45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Phillips LL, Povlishock J. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Experimental Neurology. 2005;196:126–137. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn FJ, Symms MR, Barker GJ, Greenwood JS, Duncan JS. Diffusion imaging shows abnormalities after blunt head trauma when conventional magnetic resonance imaging is normal. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70:530–533. doi: 10.1136/jnnp.70.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond CH, Menon DK, Chatfield DA, Williams GB, Pena A, Sahakian BJ, Pickard JD. Diffusion tensor imaging in chronic head injury survivors: Correlations with learning and memory indices. NeuroImage. 2006;29:117–124. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotrophy with age during childhood and adolescence: A cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein DK, Lerch J, Clasen LS, Lenroot R, Gogtay N, Evans AC, Rapoport JL, Giedd JN. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz S, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione K, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Foorman B, Francis DJ, Castillo EM, Davis RN, Fitzgerald M, Mathes PG, Denton CA, Papanicolaou AC. Brain activation profiles during the early stages of reading acquisition. Journal of Child Neurology. 2002;17:159–163. doi: 10.1177/088307380201700301. [DOI] [PubMed] [Google Scholar]
- Singleton RH, Povlishock JT. Identification and characterization of heterogeneous neuronal injury and death in regions of diffuse brain injury: Evidence for multiple independent injury phenotypes. The Journal of Neuroscience. 2004;24:3543–3553. doi: 10.1523/JNEUROSCI.5048-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S-W, Liang H-F, Trinkaus K, Cross AH, Armstrong RC, Song S-K. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magnetic Resonance in Medicine. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Tasker RC. Changes in white matter late after severe traumatic brain injury in childhood. Developmental Neuroscience. 2006;28:302–308. doi: 10.1159/000094156. [DOI] [PubMed] [Google Scholar]
- Tasker RC, Salmond CH, Westland AG, Pena A, Gillard JH, Sahakian BJ, Pickard JD. Head circumference and brain and hippocampal volume after severe traumatic brain injury in childhood. Pediatric Research. 2005;58:302–308. doi: 10.1203/01.PDR.0000169965.08854.25. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: Behavior and achievement. Neuropsychology. 2002;16:15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. NeuroImage. 2004;22:1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annual Review Neuroscience. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Verger K, Junque C, Levin HS, Jurado MA, Perez-Gomez M, Bartres-Faz D, Barrios M, Alvarez A, Bartumeus F, Mercader JM. Correlation of atrophy measures on MRI with neuropsychological sequelae in children and adolescents with traumatic brain injury. Brain Injury. 2001;15:211–221. doi: 10.1080/02699050010004059. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. The comprehensive test of phonological processing. Austin: PRO-ED, Inc.; 1999. [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein J, Steinmetz H, Ziemann U. Human motor corps callosum: Topography, somatotopy, and link between microstructure and function. Journal of Neuroscience. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children - Third Edition. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- Wiederholt JL, Bryant BR. Gray Oral Reading Tests, Fourth Edition. Fourth Ed. Austin: Pro-Ed; 2001. [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, Newsome MR, Scheibel RS, Li X, Levin HS. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2006;23:1412–1423. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum: A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca: Riverside Publishing; 2001. [Google Scholar]
- Yount R, Raschke KA, Biru M, Tate DF, Miller MJ, Abildskov T, Ghandi P, Ryser D, Hopkins RO, Bigler ED. Traumatic brain injury and atrophy of the cingulated gyrus. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:416–423. doi: 10.1176/jnp.14.4.416. [DOI] [PubMed] [Google Scholar]


