Abstract
Object
Although long-term neurological outcomes after traumatic brain injury (TBI) sustained early in life are generally unfavorable, the effect of TBI on the development of academic competencies is unknown. The present study characterizes intelligence quotient (IQ) and academic outcomes an average of 5.7 years after injury in children who sustained moderate to severe TBI prior to 6 years of age.
Methods
Twenty-three children who suffered inflicted or noninflicted TBI between the ages of 4 and 71 months were enrolled in a prospective, longitudinal cohort study. Their mean age at injury was 21 months; their mean age at assessment was 89 months. The authors used general linear modeling approaches to compare IQ and standardized academic achievement test scores from the TBI group and a community comparison group (21 children).
Children who sustained early TBI scored significantly lower than children in the comparison group on intelligence tests and in the reading, mathematical, and language domains of achievement tests. Forty-eight percent of the TBI group had IQs below the 10th percentile. During the approximately 5-year follow-up period, longitudinal IQ testing revealed continuing deficits and no recovery of function. Both IQ and academic achievement test scores were significantly related to the number of intracranial lesions and the lowest postresuscitation Glasgow Coma Scale score but not to age at the time of injury. Nearly 50 % of the TBI group failed a school grade and/or required placement in self-contained special education classrooms; the odds of unfavorable academic performance were 18 times higher for the TBI group than the comparison group.
Conclusions
Traumatic brain injury sustained early in life has significant and persistent consequences for the development of intellectual and academic functions and deleterious effects on academic performance.
Keywords: traumatic brain injury, shaken baby syndrome, academic performance, cognitive outcome, pediatric neurosurgery
Pediatric TBI represents a major public health problem. Authors of recent studies of TBI in the US from 1995 to 2001 have determined that infants and children 4 years of age or younger have higher rates of TBI-related mortality, hospitalization, and emergency department visits than children 5 to 9 or 10 to 14 years of age.40 The external cause of TBI in young children varies with age.8 Inflicted neurotrauma is the most common cause in infants younger than 24 months of age; approximately 90% of cases of significant TBI in this age group are caused by physical abuse.13,19,39 The most frequent external causes of injury in preschoolers include falls and motor vehicle accidents.39 Despite the high incidence of serious TBI in infants and young children, very little is known about long-term developmental outcomes and academic course in these cases. Retrospective follow-up studies have identified persistent neurological and cognitive sequelae in children who sustained inflicted TBI as infants, with particular deficits in motor, visual, speech/language, general cognitive, and adaptive behavior areas.9,14,20 Using prospective designs to assess outcome over a period ranging from discharge to 2 years after TBI, investigators have documented poorer functional and cognitive outcomes in young children who suffered inflicted injury compared with those who suffered noninflicted injury.27,35,36 Cognitive outcomes in children who sustained early TBI are predicted by GCS score,51 duration of impaired consciousness, the number of intracranial lesions, and whether the TBI was inflicted.43
Even with the extensive array of changes in neural processes that occur in normal development during infancy and early childhood, accumulating evidence indicates that there is limited recovery after moderate to severe TBI sustained early in life.5,28,43 Recovery from brain injury is often described in terms of plasticity and recovery of function. Plasticity refers to normal, continual changes of the brain in response to modifications in sensory inputs, internal factors such as awareness, and motor outputs.42 In this context, plasticity is not a process that occurs after a brain injury, but rather a reflection of the effect that changes to the brain can have on normal developmental processes. Following the initial resolution of transient changes after TBI, subsequent processes include relearning, which involves initial unmasking and strengthening of existing neural pathways as well as the creation of new ones.42
Recovery of function refers to processes of neural and behavioral adaptation that occur following brain insult.37 In developmental studies, recovery has been viewed in relation to the type of lesion, the age and/or developmental stage at the time of injury and at assessment, and the outcome domain evaluated. Although recovery is generally favorable following focal brain lesions sustained during childhood,46 recovery appears to be less favorable following conditions producing diffuse brain insult, such as TBI.3,24 The apparent impact of a brain injury on a given skill may depend on the developmental stage at which the lesion is sustained and at which the skill is evaluated.31,38 Some skills may show a stable deficit over time, others may show a transient lag and partial catch-up growth, while others may show increasing deficit; when the damaged substrate required for mature expression of specific skills cannot support those skills, children are likely to fall further behind age-level expectations.
Time since injury is a critically important variable. The impact of time since TBI is best characterized using models that depict longitudinal posttraumatic changes in the level, rate of change, and eventual performance level of a given ability or skill and permit assessment of possible moderating effects of socioenvironmental factors. Evaluation of different outcome domains may also yield different findings in relation to early brain injury. For example, early injuries to the orbitofrontal regions in both animals and humans are associated with relatively good recovery of cognitive abilities and poor development of adaptive and social behaviors that becomes increasingly evident with maturation.2,22,38
Little is known about how damage to neural mechanisms sustained early in life interacts with environmental conditions to influence the unfolding of normal developmental processes. The restricted behavioral repertoire of infants limits their “relearning” or “recovery” of skills. Rather, infants must acquire new skills using a damaged or altered substrate. Because infants have only limited behavioral repertoire prior to injury and the substrate they must use to acquire new skills is damaged by the injury, the impact of TBI on skill acquisition is likely to be greater when TBI is sustained in infancy than when it is sustained later in childhood or in adolescence.
Neural processes involved in recovery from brain injury both influence and are influenced by diverse environmental and social factors, including socioeconomic status and psychosocial stressors. Additional studies are needed to examine 1) how brain injury affects the reorganization of existing skills and the acquisition of new skills, and 2) ways that socioenvironmental factors interact with the injury to enhance or disrupt skill development.23
Recovery curves appear to differ in relation to age at TBI. For example, age-adjusted IQ and academic achievement test scores in older children and adults who sustain moderate to severe TBI typically show initial deficit, significant increase during the first 6 months, and then a plateau representing a stable deficit relative to performance of individuals with less severe injuries.3,23,33 However, recovery curves depicting IQ across the 1st year postinjury in infants and children of preschool age who sustained moderate to severe TBI show lower initial scores and less recovery over time. In young children, curves depicting the posttraumatic recovery of IQ across time are either flat, indicating no improvement in scores after the initial injury,23 or show a decline across time,3 indicating failure to develop new skills at age-appropriate rates. The less complete recovery in young children may reflect damage to mechanisms involved in learning and memory, which interferes with the acquisition of new information and skills.47 Therefore, individuals who suffer TBI at a young age are at high risk for lifelong reduction in cognitive abilities. In particular, brain injury sustained early in life may interfere with the acquisition of later-developing academic skills due to the combined impact of lower IQ and impaired learning efficiency.
Despite the high incidence of and significant morbidity from TBI sustained during infancy and the preschool years, there are no prospective longitudinal studies examining cognitive outcomes beyond 2.5 years postinjury and no studies of the impact of early TBI on the development of academic skills and classroom performance. The purpose of this paper is to characterize IQ and academic outcomes an average of more than 5 years postinjury in children who sustained moderate to severe TBI prior to 6 years of age and were followed in a prospective, longitudinal study. Relative to a community comparison group, children with TBI were hypothesized to have: 1) lower IQ, with no evidence of recovery of function, as indicated by stable intelligence test scores from 2 months to at least 3 years postinjury; 2) lower scores on all measures of academic skills; and 3) reduced class-room performance and greater utilization of special education services. In addition, IQ and academic achievement test scores were expected to be moderated by the family’s access to resources and the presence of psychosocial stressors.
Clinical Material and Methods
Study Population and Follow Up
Children who presented with TBI from 1994 to 1998 at either of two children’s hospitals in Houston, Texas were enrolled in a prospective, longitudinal study of outcome after early inflicted or noninflicted TBI. Written informed consent to participate was obtained from the children’s guardians. The protocol was approved by the institutional review boards at the University of Texas Health Science Center at Houston, Baylor College of Medicine, and the affiliated children’s hospitals.
Longitudinal intelligence scores were available from assessments completed at 2, 12, and 24 months after TBI for the injured children and at similar intervals after study enrollment for comparison children. Long-term IQ, academic achievement, and classroom placement variables were assessed an average of 5.7 years (range 3.8–8.3 years) after TBI for the injured children and after study enrollment for the comparison children. Outcomes were examined prospectively at least 3 years postinjury in 23 children who sustained moderate or severe TBI between the ages of 4 and 71 months. The performance of children with TBI was compared with that of children in a community comparison group (21 children from comparable sociodemographic backgrounds). Inclusion criteria were: 1) moderate to severe TBI; 2) sufficient recovery of cognitive skills to permit standardized assessment; 3) no known premorbid neurological or metabolic disorder; 4) no history of prior TBI; and 5) birth after at least 30 weeks of gestation. The community comparison group was composed of children born at the hospitals in which the children with TBI were hospitalized, children who attended federally subsidized well-baby clinics, and children whose parents responded to community notices. Children in the comparison group met criteria 3, 4, and 5 for entry into the study. The two groups did not differ significantly in age at assessment, sex, ethnicity, grade level, or access to financial and social resources (Table 1).
TABLE 1.
Demographic information for the TBI and comparison groups
| TBI Group | Comparison Group | Total Tested | |||
|---|---|---|---|---|---|
| Variable | (23 children) | (21 children) | (44 children) | Statistic (df) | p Value |
| sex (no. of children) | |||||
| female | 11 | 11 | 44 | χ2 = 0.09 (1) | 0.7628 |
| male | 12 | 10 | |||
| ethnicity (no. of children) | |||||
| Caucasian | 9 | 7 | 44 | χ2 = 1.36 (3) | 0.7141 |
| African-American | 8 | 9 | |||
| Hispanic | 3 | 4 | |||
| other/multiethnic | 3 | 1 | |||
| age at testing in mos* | 89.6 ± 26.2 | 101.0 ± 29.0 | 42 | t = 1.38 (42) | 0.1763 |
| Family Resource Scale score* | 118.4 ± 20.6 | 127.7 ± 14.6 | 42 | t = 1.70 (42) | 0.0959 |
| Parenting Stress Scale score* | −0.16 ± 1.2 | −0.66 ± 0.9 | 41 | t = 1.56 (42) | 0.1264 |
| school grade (no. of children) | |||||
| Kindergarden–1 | 11 | 8 | 44 | χ2 = 0.42 (1) | 0.5151 |
| 2–6 | 12 | 13 |
Scores are given as means ± SDs.
Cause and Severity of TBI
The external cause of injury was inflicted TBI in 10 cases; in these cases, the primary central nervous system insult was extraaxial hemorrhage and/or intracerebral contusion or focal edema rather than global hypoxic–ischemic injury. Noninflicted injuries included motor vehicle accidents (four cases), vehicle/pedestrian collisions (four cases), and falls (five cases).
As depicted in Table 2, severity of TBI was determined by three factors: GCS score,51 the duration of impaired consciousness, and acute computed tomography or brain magnetic resonance imaging findings. The GCS was originally developed for adults. We modified two scales—the verbal and the motor scales—to accommodate the behavioral capabilities of infants and children from birth through 35 months of age. “Following commands” on the original GCS was replaced with spontaneous movement for infants 6 months of age and younger and goal-directed movements for children between the ages of 7 and 35 months. “Confused” and “oriented” on the original scale were replaced with “cries” and “cries to indicate need.” The modified GCS motor scale was used to determine duration of impaired consciousness, which was defined as the number of days a child was unable to follow a one-stage command or engage in goal-directed movements. Moderate TBI was defined as injuries resulting in lowest postresuscitation GCS scores of 9 to 12. Children with GCS scores of 9 to 15 were considered to have moderate TBI if imaging demonstrated extraaxial bleeding, intraparenchymal hemorrhage, or edema; if neurological deficits were present; or if impaired consciousness persisted for less than 24 hours. Children were considered to have severe TBI if their lowest postresuscitation GCS score was between 3 and 8 or their level of consciousness was impaired (GCS motor score < 6) for more than 24 hours. We have used this categorization system successfully in prior studies of infants and young children with TBI.27
TABLE 2.
Neurological information for the TBI group
| Variable | Value |
|---|---|
| age at injury in mos (mean ± SD) | 21.2 ± 21.9 |
| mos from injury to test (mean ± SD) | 68.3 ± 15.4 |
| lowest postresuscitation GCS score (no. of children) | |
| 3–8 | 11 |
| 9–12 | 6 |
| 13–15 | 6 |
| days of impaired consciousness (mean ± SD) | 3.1 ± 5.0 |
| GOS score (no. of children) | |
| discharge | |
| good recovery | 10 |
| moderate/severe disability | 13 |
| follow up | |
| good recovery | 9 |
| moderate/severe disability | 14 |
| acute imaging findings (no. of lesions/no. of children)* | |
| intracerebral lesions | |
| hemorrhage/contusion | 8/6 |
| edema | 10/7 |
| shear injury | 20/6 |
| extraaxial lesions | |
| subdural hematoma | 36/13 |
| epidural hematoma | 1/1 |
| subarachnoid hemorrhage | 8/6 |
| chronic findings | |
| subdural hygroma | 4/3 |
| ex vacuo ventriculomegaly | 3/3 |
| total lesions/no. of children | 90/21* |
Abnormalities were noted on acute neuroimaging studies obtained in 21 of 23 children with TBI.
Impaired consciousness persisted for an average of 3.1 days (range 0–20 days). Glasgow Outcome Scale34 scores indicated that fewer than half of the children were rated as having a good recovery at discharge. At the late follow-up examination, the GOS score had changed one category in seven of 23 children. Scores were as likely to improve (three cases) as to decline (four cases). Neuroimaging studies indicated that extraaxial hemorrhages were the most frequently occurring acute finding. Parenchymal hemorrhage and focal regions of edema/infarction were noted less frequently. Chronic conditions included subdural hygroma and ex-vacuo ventriculomegaly; findings of these conditions on scans obtained in the acute phase were interpreted as evidence of abnormalities that existed before the TBI. The distribution of GOS scores and data regarding neurological status and acute imaging findings was similar in children with inflicted and noninflicted TBI (all p > 0.15). The children with inflicted TBI were injured at significantly younger ages than those with noninflicted TBI (df = 21, t = 3.25, p = 0.0038).
Examination of Attrition
To ascertain comparability of the present sample to the original sample of children with TBI who were enrolled in our study between 1994 and 1999, we examined demographic and neurological variables for the 23 children with TBI who returned for long-term follow up and the 37 children who were lost to follow up. The groups did not differ on age at injury (df = 58, t = 0.0, p = 0.9809), sex (60 children, df = 1, chi square = 0.9040, p = 0.3417), ethnicity (60 children, df = 3, chi square = 3.5892, p = 0.7517), or socioeconomic status (60 children, df = 2, chi square = 4.0915, p = 0.1293). Neurological variables assessing the severity of TBI were also comparable in children who returned and in the original sample; GCS scores (df = 58, t = 0.30, p = 0.7619), duration of impaired consciousness (df = 58, t = 0.54, p = 0.5948), the presence of hemiparesis (58 children, df = 1, chi square = 0.9741, p = 0.3237), and the proportion of children with GOS scores34 indicating a good recovery versus moderate or severe disability (60 children, df = 1, chi square = 0.1001, p = 0.7517) were comparable.
Outcome Measures
The SB454 was administered. This scale yields a standard age score reflecting the level of general cognitive function with a mean of 100 and an SD of 16; the scores were restandardized to a mean of 100 and an SD of 15 to make them comparable to other general cognitive scores. The test is composed of scales reflecting verbal reasoning, visual reasoning, and short-term memory. The Vocabulary, Pattern Analysis, Memory for Sentences, and Bead Memory subtests were examined (mean 50, SD = 8).
Selected subtests from the WJ–III56 were administered to assess basic skill development, skill application, and fluency in reading, mathematics, and language areas. For reading, the Word Attack, Letter–Word Identification, Reading Fluency, and Passage Comprehension subtests were administered. Mathematics subtests included Calculation, Applied Problems, and Math Fluency. Language subtests included Spelling, Writing Fluency, and Oral Comprehension. Fluency subtests were only given to children who were at least 7 years of age.
The GORT–455 was administered to children who were at least 7 years of age at follow up to examine their reading speed, decoding accuracy, and comprehension of connected text (mean 10, SD = 3). Age-based scores were used for all academic tests.
Academic performance was categorized as favorable or unfavorable. Outcome was classified as unfavorable if a child either failed a grade, required support services in a self-contained special education program (partial- or full-day), or both.
Family Environment
At the same time as the IQ and academic achievement tests were administered to the children, data pertaining to the family environment were collected. These data were obtained from a biological parent in all cases of noninflicted TBI and in five of 10 cases of inflicted TBI. In the remaining five cases of inflicted TBI, the data were obtained from biological relatives who had adopted the children.
The adequacy of access to financial, physical, and social resources was measured using the Family Resource Scale.21 Possible scores on this scale range from 0 to 150, with higher scores indicating more favorable access.
The level of parental stress was assessed using the Total Stress scale from the Parenting Stress Inventory (mean 0.0, SD = 1.0).1 This scale provides an index of the overall level of parenting stress and incorporates measures of parental distress, stress emanating from the parent’s interaction with the child, and stress related to the child’s behavioral characteristics. Lower scores indicate lower levels of parent stress.
Design and Analyses
The design includes one between-subjects factor, membership in the TBI or comparison groups. Group differences on the outcome variables were examined using t-tests for symmetrically distributed variables and the chi-square test for categorical variables. Pearson product-moment correlation coefficients were used to examine relationships of both age at injury and severity of TBI to outcome scores. Multiple regression models were used to examine the degree to which general cognitive and academic achievement test scores were predicted by family environment and neurologic variables. The models were constructed to test hypotheses in a hierarchical fashion rather than for variable selection or model building. Separate analyses were performed for each academic outcome as expressed by achievement test results. To address whether the effect of each predictor was moderated by group, the model for each outcome was constructed to contain all the variables, including the interaction terms. In each model, the interaction terms were tested first to examine possible moderating effects and were then trimmed if nonsignificant. One-tailed significance tests were used to test hypotheses regarding the direction of group differences on outcome measures. Logistic regression models were used to examine predictors of academic placement.
Growth-curve analysis allows modeling of the processes of change that underlie development and recovery of function.30 Individual growth-curve analyses were used to model the level of IQ, change in IQ over time, and the rate of change in IQ change from the initial assessment 2 months postinjury to subsequent evaluations at 1 year, 2 years, and 3–7 years postinjury. The growth curve analyses examined longitudinal cognitive scores from the Bayley Scales of Infant Development12 for evaluations performed prior to age 3; all other evaluations used the SB454 composite score. The IQ standard scores were analyzed using linear mixed models by allowing the scores to be expressed as functions of the time since the injury. A three-parameter polynomial function of time was used, with intercept, slope, and curvature as the parameters. The time since injury was centered at 48 months to minimize multicollinearity between the linear and quadratic terms and because 4 years postinjury represents a reasonable point at which to compare the TBI group and the control group. Thus, the intercept represented the level of outcome at 4 years postinjury, the slope represented the rate of growth in the outcome at 4 years postinjury, and the curvature (quadratic term) represented the acceleration or deceleration of the curve, that is, the rate at which the slope was changing.
Results
Group Differences on General Cognitive and Academic Achievement Test Scores
General Cognitive Scores
The descriptive and inferential statistics for the IQ and academic achievement test scores for the TBI and comparison groups are provided in Table 3. Preliminary analyses revealed no significant differences in cognitive or academic achievement test scores between children with inflicted TBI and those with noninflicted TBI. Therefore, in subsequent analyses we examined the performance of all children with TBI in relation to the comparison group.
TABLE 3.
Descriptive and inferential statistics for intelligence and academic achievement test scores for the TBI and comparison groups*
| Measure | TBI Group (23 children) |
Comparison Group (21 children) |
t Statistic (df) | p Value |
|---|---|---|---|---|
| SB4 | ||||
| composite score | 83.0 ± 14.0 | 95.7 ± 10.5 | 3.39 (42) | 0.0008 |
| component subtests | ||||
| Vocabulary | 44.5 ± 7.5 | 48.9 ± 7.1 | 1.96 (42) | 0.0284 |
| Pattern Analysis | 44.2 ± 7.2 | 49.5 ± 5.6 | 2.82 (42) | 0.0037 |
| Memory for Sentences | 44.2 ± 7.3 | 50.0 ± 8.0 | 2.60 (42) | 0.0065 |
| Bead Memory | 44.1 ± 9.1 | 46.1 ± 8.7 | 0.77 (42) | 0.2235 |
| WJ–III reading subtests | ||||
| Letter–Word Identification | 92.8 ± 12.4 | 108.9 ± 13.1 | 4.21 (42) | 0.0001 |
| Word Attack | 93.9 ± 10.4 | 108.4 ± 11.7 | 4.12 (39) | 0.0001 |
| Reading Fluency | 91.0 ± 13.3 | 99.9 ± 17.6 | 1.28 (28) | 0.1051 |
| Passage Comprehension | 93.0 ± 12.9 | 100.5 ± 13.2 | 1.82 (41) | 0.0340 |
| GORT–4 | ||||
| Fluency | 6.5 ± 3.6 | 10.1 ± 4.3 | 2.39 (32) | 0.0119 |
| Accuracy | 7.5 ± 3.2 | 10.3 ± 3.6 | 2.13 (32) | 0.0208 |
| Comprehension | 8.1 ± 3.9 | 10.9 ± 2.6 | 2.19 (32) | 0.0185 |
| WJ–III mathematics subtests | ||||
| Calculation | 94.1 ± 13.2 | 104.2 ± 12.2 | 2.56 (34) | 0.0076 |
| Applied Problems | 94.2 ± 12.2 | 107.2 ± 11.5 | 3.49 (42) | 0.0005 |
| Math Fluency | 85.5 ± 13.5 | 102.3 ± 18.8 | 2.56 (30) | 0.0080 |
| WJ–III oral and written language subtests | ||||
| Oral Comprehension | 94.2 ± 14.6 | 107.1 ± 11.6 | 2.96 (38) | 0.0027 |
| Spelling | 91.6 ± 13.7 | 109.1 ± 14.1 | 3.83 (37) | 0.0003 |
| Writing Fluency | 85.6 ± 17.4 | 104.7 ± 17.4 | 2.90 (29) | 0.0036 |
Scores are presented as means ± SD.
As indicated in Table 3, the SB4 composite score was significantly reduced in the TBI group relative to the comparison group. Forty-eight percent of the children in the TBI group and 19% of those in the comparison group had composite scores below the 10th percentile (chi square = 4.05, df = 1, 44 children, p = 0.0443). On the component subtests, the children in the TBI group scored significantly lower than those in the comparison group on the Pattern Analysis, Memory for Sentences, and Vocabulary subtests. These subtests identified reductions in visual–spatial reasoning, phonological short-term memory, and oral definition of word meanings, respectively. The performance of the two groups was comparable on the Bead Memory subtest, which evaluates storage of information in visual short-term memory.
To compare changes in IQ over time between the children with TBI and the comparison group, a general linear mixed modeling procedure was used. Specifically, the model was set up as a random-coefficients, or growth-curve, model. Intelligence scores were modeled as a function of time since the baseline evaluation. Because IQ was not assessed in all children at the same time or even the same number of times, a mixed modeling procedure was used. Mixed models rely on maximum likelihood rather than least squares and do not require listwise deletion of data. The time variable was centered at 48 months the approximate mean age across all assessments. Random intercept and slope were modeled in addition to a fixed quadratic (curvature) term to account for potential nonlinearity. Group membership was added as a between-subjects variable. There are several methods available to estimate df for mixed models. We estimated the model using the containment method and the Satterthwaite and Kenwood–Rogers procedures as well as the traditional between- and within-subjects df from a general linear model. Although the df varied somewhat across models, the results were consistent. We report df from the Satterthwaite procedure.
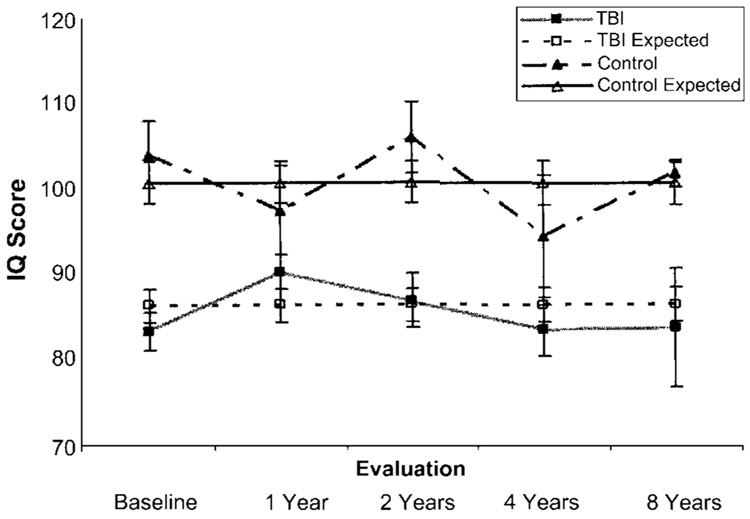
The model was based on 145 observations from 44 children; most children contributed IQ scores from three or four different testing periods to the analysis. The results indicated that the intercepts (levels at 48 months) differed significantly between the groups (df = 53, t = 4.51, p < 0.0001), with almost a 14-point difference in IQ (TBI group 85.3, comparison group 99.1). However, the slope (rate of change) and quadratic (curvature) terms were not significantly different from zero or from each other, indicating that the rate of change in each group was similar and did not differ with respect to the norm group. The actual and expected mean IQ values are shown by group across time in Fig. 1.
FIG. 1.
Graph depicting growth-curve analysis of longitudinal IQ scores from 2 months to an average of 5.7 years after TBI indicates a persistent deficit relative to the comparison group. Nonsignificant slope and curvature terms indicate that there was no recovery of IQ scores across the follow-up period for the TBI group. Mean and standard error values are provided for actual and expected scores.
Academic Achievement Test Scores
As indicated in Table 3, children with TBI scored significantly lower than children in the comparison group on tests of reading, mathematics, and language. Scores on the WJ–III reading subtests examining phonological word decoding, word identification, and reading comprehension for sentence-level text were significantly reduced in the TBI group. Reading fluency, which could only be evaluated in older children, did not differ between the two groups. The GORT–4 subtests examine reading performance for connected text. Scores on these subtests were significantly lower in the TBI group than in the comparison group for decoding accuracy, reading speed, and comprehension of written text. The TBI group scored lower than the comparison group in written calculation, solving word problems, and the speed of retrieval of math facts from long-term memory. Performance on oral and written language subtests indicated that the TBI group had significantly lower auditory comprehension of word and sentence meanings, less accurate written spelling, and reduced speed of production of written text (Table 4).
TABLE 4.
Predictors of cognitive and academic achievement test scores for the total sample*
| Predictors | |||||
|---|---|---|---|---|---|
| TBI or Comparison Group | Family Resource Scale | ||||
| Variable | t Statistic (df) | p Value | t Statistic (df) | p Value | Model R2 |
| SB4 composite score | 2.90 (41) | 0.0030 | 1.79 (41) | 0.0403 | 0.272 |
| Letter–Word Identification score† | 3.65 (41) | 0.0004 | 2.60 (41) | 0.0065 | 0.394 |
| Applied Problems score‡ | 3.38 (41) | 0.0008 | NS | NS | 0.239 |
NS = not significant.
Woodcock–Johnson III reading subtest.
Woodcock–Johnson III math subtest.
Academic Performance
Children were enrolled in academic programs ranging from kindergarten to the sixth grade. Forty-eight percent of the TBI group and 38% of the comparison group were enrolled in kindergarten or first grade at the time of assessment. Children with TBI were significantly more likely than the children in the comparison group to experience an unfavorable academic outcome, defined as failing a grade or requiring education in a self-contained special education classroom for at least two major academic subjects (chi square = 10.26, df = 1, 44 children, p = 0.0014). Forty-eight percent of the children with TBI and 5% of the children in the comparison group had an unfavorable academic outcome. Of the 11 children with TBI who had an unfavorable outcome, four failed a grade, four were in partial- or full-day self-contained classes, and three had failed a grade and required self-contained placement. An additional four children received content-mastery services or instructional modifications within the regular classroom setting. Thirty-nine percent of the children with TBI had required extensive rehabilitation services during their preschool years and continued to require speech/language, occupational, and/or physical therapies in elementary school.
Neurological and Environmental Predictors of General Cognitive and Academic Outcomes
For the total sample, multiple regression analyses were performed to analyze whether IQ, reading, and math outcomes were predicted by 1) the presence or absence of TBI, 2) family environment variables reflecting concurrent measures of family access to financial, social, and personal resources, and 3) the degree of parenting stress. Tests of moderating effects revealed that the group × family resources and group × parent stress interaction terms were not significant, so these terms were trimmed from each model. The level of parenting stress also did not contribute to the prediction of outcomes and this variable was therefore also trimmed. Variables remaining in the model were found to be significant at a probability level of 0.05 using a one-tailed test of directional hypotheses. (Refer to Table 4 for statistics for regression analyses.)
The SB4 composite score was predicted by the presence or absence of TBI and the level of family resources. The model accounted for 27.2% of the variance in cognitive scores. Word decoding scores also were predicted by group membership and family resources; the model R2 value was 0.394. Mathematical reasoning was predicted only by the presence or absence of TBI; the model accounted for 23.9% of the variability in math scores.
Logistic regression analysis was used to examine predictors of performance in school. Poor academic performance was predicted solely by group membership. Family environment variables did not contribute to prediction of academic performance. For children with TBI, the odds of failing a grade and/or requiring placement in a self-contained special education program were 18 times greater than for the children in the comparison group (44 children, df = 1, chi square = 11.68, p = 0.0006; point estimate = 0.055; confidence interval 0.006–0.477).
Relationships of Age at TBI and Severity of TBI with Outcome Variables
Relationships of age at the time of injury and severity of TBI with outcome variables in the 23 children with TBI were examined initially using Pearson product-moment correlation coefficients. Age at the time of TBI was not significantly correlated with any of the SB4 intelligence scores. For academic achievement test scores, age at TBI was negatively correlated only with scores on the WJ–III Letter–Word Identification (r = −0.451, p = 0.0306) and Reading Fluency subtests (r = −0.569, p = 0.0268); older age at TBI was associated with less adequate performance. Neither math nor language scores showed significant relationship with age at injury.
Outcome scores were more consistently related to the lowest postresuscitation GCS score than to the duration of impaired consciousness. The GCS scores were positively correlated with scores on the SB4 Vocabulary subtest (r = 0.452, p = 0.0304) and the WJ–III Word Attack (r = 0.482, p = 0.0316), Reading Fluency (r = 0.730, p = 0.0020), Applied Problems (r = 0.448, p = 0.0318), Spelling (r = 0.682, p = 0.0018), and Oral Comprehension (r = 0.693, p = 0.0007) subtests. In contrast, the duration of impaired consciousness was negatively correlated with performance on the WJ–III Math Fluency (r = −0.713, p = 0.0019), Reading Fluency (r = –0.650, p = 0.0088), and Oral Comprehension (r = −0.460, p = 0.0412) subtests. Longer duration of impaired consciousness was associated with poorer performance.
Multiple regression analyses were used to assess potential predictors of neurological function in children with TBI. Factors examined included the lowest postresuscitation GCS score, external cause of TBI, and the number of intracranial lesions. Prediction of scores was not enhanced by inclusion of the external cause of TBI, which was trimmed from the models. Regression analyses revealed that the GCS score and the number of lesions shared substantial variance. The GCS score contributed to prediction of performance on the Letter–Word Identification subtest of the WJ–III, but not to prediction of other outcomes. With both variables in the equation, the number of lesions significantly predicted general cognitive performance (the SB4 composite score) and the performance on the Applied Problems subtest of the WJ–III math battery (Table 5). The models accounted for 37.8% of the variability in the general cognitive scores, 17.3% of variability in the reading scores, and 34.5% of the variability in the math scores.
TABLE 5.
Neurological predictors of cognitive and academic scores for the TBI group
| Predictors | |||||
|---|---|---|---|---|---|
| GCS Score | No. of Lesions | ||||
| Variable | t Statistic (df) | p Value | t Statistic (df) | p Value | Model R2 |
| SB4 composite score | NS | NS | −2.42 (19) | 0.0138 | 0.378 |
| Letter–Word Identification score | 1.86 (19) | 0.0391 | NS | NS | 0.173 |
| Applied Problems score | NS | NS | −2.02 (19) | 0.0288 | 0.345 |
Discussion
Moderate-to-severe TBI sustained prior to the age of 6 years has adverse and persistent consequences for intellectual and academic development. When IQ was assessed an average of 5.7 years after moderate-to-severe TBI, injured children scored significantly lower than healthy children from similar sociodemographic backgrounds. Low scores on subtests evaluating vocabulary, verbal short-term memory, and visual–spatial reasoning indicated areas of particular vulnerability. As predicted, examination of the longitudinal course of recovery of IQ, based on testing performed between 2 months and an average of 5.7 years after TBI revealed neither acceleration nor deceleration in the rate of change in IQ over the first 3 to 7 years after injury. The lack of change in IQ over the protracted recovery period indicates a persistent deficit with no evidence of catch-up growth. Even though 43% of the children with TBI received long-term rehabilitation therapies extending into the preschool and school years, the performance gap between their scores and those of the children in the comparison group persisted. These findings are consistent with previous studies showing limited recovery of diverse cognitive abilities after TBI sustained early in life.5,10,15,16,24,53
Academic achievement test scores were reduced in the TBI group relative to the comparison group. Academic skills were reduced in most areas evaluated, including word decoding, reading fluency and comprehension, mathematical calculation, solving mathematical word problems, and speed of retrieval of mathematical facts. Language scores were reduced on indices of spelling, writing fluency, and oral comprehension. Across content areas, the children in the TBI group had difficulty with basic academic skills, such as word decoding, spelling single words, and performing mathematical calculations. They also showed difficulty on academic reasoning tasks requiring reading and language comprehension as well as mathematical problem solving. Fluency was reduced in all academic areas, indicating slowed processing speed for decoding and producing written text as well as for retrieval of mathematical facts.
Relatively good intellectual and academic outcomes are often seen after early nonprogressive focal brain injury.6,7,11 In young children who sustain diffuse or multifocal brain injury from TBI or from other causes, such as cranial irradiation, infection, or endocrine disturbance, however, cognitive skills are more vulnerable to disruption.4,44,45,48 Despite the dynamic progressive and regressive neural processes regulating axonal and dendritic development and connectivity during infancy and the preschool years,32 there appear to be significant limits on neural and cognitive plasticity after early TBI. The mechanisms involved in recovery and deficits after early brain injury may be clarified through future studies in which longitudinal neuropsychological follow-up data are integrated with data from enhanced neuroimaging incorporating diffusion tensor and functional imaging methodologies.26,52
In our study, early TBI had an adverse effect on academic performance in the classroom. Forty-eight percent of the children with TBI failed a grade or required special education support services. These findings probably underestimate the total occurrence of academic failure in our group relative to that in groups of older children with TBI, because half of the children in our TBI group were enrolled in kindergarten or first grade at the time of assessment and had limited opportunity to repeat grades. The odds of significant academic performance problems were 18 times greater for children with TBI than for children in the comparison group. The significant effect of early TBI on academic failure and the need for special education services is similar to the effects noted in children who sustain TBI during their elementary school years or adolescence. In older children, achievement test scores suggest generally good recovery of basic academic achievement skills in conjunction with poor functional academic recovery, as demonstrated by failing grades and the need for support services.18,25,29,50 Several factors in a child’s pre- and postinjury environment, including social and economic disadvantages and family climate,49,57 may facilitate or retard recovery. In the present study, neurological variables such as the number of intracranial lesions, and to a lesser degree, the lowest postresuscitation GCS score, were significantly related to development of general cognitive and mathematical skills. The association between indices of injury severity and outcome is quite robust as these indices predicted outcomes across an average span of 5.7 years. As in previous studies,9,24 in the present study, age at injury was not strongly related to outcomes in children who sustained TBI as infants or during the preschool years, most likely due to the restricted age range. Other studies examining the influence of age at injury within the whole pediatric age range did identify poorer recovery of intelligence scores in infants and preschoolers relative to older children and adolescents.23 In our study family environment variables were not strong predictors of IQ and academic performance. The degree of stress experienced by parents did not predict outcomes. In contrast, family access to financial and social resources was related to general cognitive and reading scores but did not significantly affect math scores or academic performance.
Outcome studies are influenced by differences in sampling and inclusion criteria. In general, the distribution of intelligence or developmental quotients in the population of young children with moderate to severe TBI is bimodal, with peaks at about 80 to 90 (the 10th–25th percentiles) in children who recover to testable levels and about 50 (< 1st percentile) in children who develop multiple posttraumatic disabilities and are able to be assessed with functional outcome measures due to severe sensory, motor, and cognitive limitations precluding traditional academic and neuropsychological assessment. The focus of this study was on specific neuropsychological outcomes; therefore, only children who recovered to a testable level were enrolled and followed up. Excluding untestable children yielded a more favorable estimate of outcomes than if the entire spectrum of severe cases had been assessed. However, for the IQ and academic achievement tests administered in this study, scores can only be calculated if the child is able to pass at least one item; zero-base scores are not available for children unable to perform the tests.
Limitations of the present study include the relatively small sample size and resultant reduction in statistical power as well as issues regarding sampling. Although the study was sensitive to differences between the TBI and comparison groups, because of the small sample size it had limited power to detect possible differences between children with inflicted and noninflicted TBI. Sampling differences have a major effect on measured outcomes. The children with inflicted TBI who participated in this phase of the long-term follow-up study had been placed with a nonoffending parent or family member rather than being removed from the extended family following the protective agency’s investigation of the abusive injury. Even though selective attrition was not noted, it is possible that the children who continued to participate had more intact families and better outcomes than those who were lost to follow up.
Strengths of the study include the prospective longitudinal design, inclusion of a community comparison group, detailed information on acute injury variables, long-term follow up based on direct examination of each child’s abilities, and information on actual school performance. Retrospective studies have been important in highlighting the unfavorable outcomes in young children with TBI. However, retrospective studies may not capture the full range of outcomes. Prospective designs may capture a greater range of positive outcomes compared with designs that use retrospective identification of patients. For example, authors of prospective studies27,36 have identified a higher rate of favorable outcomes than have those who identified cases using retrospective methods.20 In addition, this study examined long-term influences of both neural and environmental variables on cognitive and academic outcomes and identified striking needs for continuing special education and rehabilitation services.
Conclusions
The societal costs of TBI are high, not only during the initial hospitalization but also during long-term stages of recovery due to persistent neurobehavioral deficits that often necessitate years of rehabilitation and educational interventions. Children who sustain TBI early in life suffer significant and persistent consequences in the development of intellectual and academic functions as well as deleterious effects on academic performance. In the present sample, long-term cognitive and academic outcomes were unfavorable in children who sustained early TBI. Forty-eight percent of the children with TBI had intelligence test scores below the 10th percentile and experienced academic failure or placement into self-contained special education classrooms. Furthermore, repeated measurement of IQ during the period from 2 months to an average of 5.7 years postinjury showed no change over time, indicating no measurable recovery of function during the lengthy follow-up period. Academic achievement scores were significantly lower in the TBI group than in the comparison group in nearly all areas evaluated, including reading, mathematics, oral language, and written language. Cognitive and academic performance gaps between the TBI and comparison groups may be presumed to represent a lasting deficit in the children with TBI, because there is no evidence of improvement over time or lessening of the performance gap. Nearly half of the children with TBI continued to require rehabilitation therapies to support their academic performance.
Age at the time of injury was not related to developmental outcomes across the infant and preschool age ranges. Acute measures of the severity of TBI, including the GCS score and the number of intracranial and extraaxial lesions, were significantly related to cognitive and academic outcomes.
The continuing cognitive and academic deficits noted after early TBI have a cumulative, negative impact on academic performance in the classroom. Although nearly half of the children with TBI received intervention services through state-mandated early childhood and preschool programs for children with disabilities, others did not qualify for services because they were performing above the deficient range when screened.
Given the lack of recovery of cognitive functions, high rates of academic failure, and substantial need for special education services in elementary school, greater emphasis should be placed on prevention initiatives as well as on providing additional cognitive and motor rehabilitation services during preschool years for children with a broader range of cognitive disabilities. To reduce the high rate of posttraumatic morbidity, increased attention should be paid to the development of acute-care treatment protocols that minimize secondary brain injury and enhance neuronal repair as well as to prevention efforts, which have demonstrated significant reduction in the occurrence of abusive injuries through parent education programs17 and of motor vehicle injuries through proper use of infant and child restraints.41
Acknowledgments
We thank the families that participated in this study and Harris County Children’s Protective Services.
Preparation of this manuscript was supported in part by a grant from the National Institute of Neurological Disorders and Stroke (No. R01-NS-29462–09, Accidental and Nonaccidental Pediatric Brain Injury) to Linda Ewing-Cobbs.
Abbreviations used in this paper
- df
degrees of freedom
- GCS
Glasgow Coma Scale
- GORT–4
Gray Oral Reading Tests, 4th edition
- GOS
Glasgow Outcome Scale
- IQ
intelligence quotient
- SB4
Stanford–Binet Intelligence Scales, fourth edition
- SD
standard deviation
- TBI
traumatic brain injury
- WJ–III
Woodcock–Johnson III
References
- 1.Abidin RR. Parenting Stress Index. ed 3. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 2.Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 3.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- 4.Anderson V, Smibert E, Ekert H, Godber T. Intellectual, educational, and behavioral sequelae after cranial irradiation and chemotherapy. Arch Dis Child. 1994;70:476–483. doi: 10.1136/adc.70.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson VA, Morse SA, Catroppa C, Haritou F, Rosenfeld JV. Thirty month outcome from early childhood head injury: a prospective analysis of neurobehavioral recovery. Brain. 2004;127:2608–2620. doi: 10.1093/brain/awh320. [DOI] [PubMed] [Google Scholar]
- 6.Aram DM, Ekelman BL. Scholastic aptitude and achievement among children with unilateral brain lesions. Neuropsychologia. 1988;26:903–916. doi: 10.1016/0028-3932(88)90058-9. [DOI] [PubMed] [Google Scholar]
- 7.Ashcraft MH, Yamashita TS, Aram DM. Mathematics performance in left and right brain-lesioned children and adolescents. Brain Cogn. 1992;19:208–252. doi: 10.1016/0278-2626(92)90046-o. [DOI] [PubMed] [Google Scholar]
- 8.Barlow KM, Minns RA. Annual incidence of shaken impact syndrome in young children. Lancet. 2000;356:1571–1572. doi: 10.1016/S0140-6736(00)03130-5. [DOI] [PubMed] [Google Scholar]
- 9.Barlow KM, Thomson E, Johnson D, Minns RA. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics. 2005;116:e174–e185. doi: 10.1542/peds.2004-2739. [DOI] [PubMed] [Google Scholar]
- 10.Barnes MA, Dennis M, Wilkinson M. Reading after closed head injury in childhood: effects on accuracy, fluency, and comprehension. Dev Neuropsychol. 1999;15:1–24. [Google Scholar]
- 11.Bates E. Plasticity, localization, and language development. In: Broman SH, Fletcher JM, editors. The Changing Nervous System: Neurobehavioral Consequences of Early Brain Disorder. New York: Oxford University Press; 2005. pp. 214–253. [Google Scholar]
- 12.Bayley N. The Bayley Scales of Infant Development. ed 2. San Antonio: The Psychological Corporation; 1993. [Google Scholar]
- 13.Billmire ME, Myers PA. Serious head injury in infants: accident or abuse? Pediatrics. 1985;75:340–342. [PubMed] [Google Scholar]
- 14.Bonnier C, Nassogne MC, Evrard R. Outcome and prognosis of whiplash shaken infant syndrome: late consequences after a symptom-free interval. Dev Med Child Neurol. 1995;37:943–956. doi: 10.1111/j.1469-8749.1995.tb11949.x. [DOI] [PubMed] [Google Scholar]
- 15.Chapman SB, Levin HS, Wanek A, Weyrauch J, Kufera J. Discourse after closed head injury in young children. Brain Lang. 1998;61:420–449. doi: 10.1006/brln.1997.1885. [DOI] [PubMed] [Google Scholar]
- 16.Dennis M, Wilkinson M, Koski L, Humphreys RP. Attention deficits in the long term after childhood head injury. In: Broman SH, Michelx ME, editors. Traumatic Head Injury in Children. New York: Oxford University Press; 1995. pp. 165–187. [Google Scholar]
- 17.Dias MS, Smith K, deGuehery K, Mazur P, Li V, Shaffer ML. Preventing abusive head trauma among infants and young children: a hospital-based, parent education program. Pediatrics. 2005;115:e470–e477. doi: 10.1542/peds.2004-1896. [DOI] [PubMed] [Google Scholar]
- 18.Donders J. Academic placement after traumatic brain injury. J Sch Psychol. 1994;32:53–65. [Google Scholar]
- 19.Duhaime AC, Alario AJ, Lewander WJ, Schut L, Sutton LN, Seidl TS, et al. Head injury in very young children: mechanisms, injury types, and ophthalmologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics. 1992;90:179–185. [PubMed] [Google Scholar]
- 20.Duhaime AC, Christian CW, Moss E, Seidl T. Long-term outcome in infants with the shaking-impact syndrome. Pediatr Neurosurg. 1996;24:292–298. doi: 10.1159/000121058. [DOI] [PubMed] [Google Scholar]
- 21.Dunst CJ, Leet HE. Measuring the adequacy of resources in households with young children. Child Care Health Dev. 1987;13:111–125. doi: 10.1111/j.1365-2214.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 22.Eslinger PJ, Biddle KR. Adolescent neuropsychological development after early right prefrontal cortex damage. Dev Neuropsychol. 2000;18:297–329. doi: 10.1207/S1532694203Eslinger. [DOI] [PubMed] [Google Scholar]
- 23.Ewing-Cobbs L, Barnes MA, Fletcher JM. Early brain injury in children: development and reorganization of cognitive function. Dev Neuropsychol. 2003;24:669–704. doi: 10.1080/87565641.2003.9651915. [DOI] [PubMed] [Google Scholar]
- 24.Ewing-Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, Miner ME. Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J Int Neuropsychol Soc. 1997;3:581–591. [PubMed] [Google Scholar]
- 25.Ewing-Cobbs L, Fletcher JM, Levin HS, Iovino I, Miner ME. Academic achievement and academic placement following traumatic brain injury in children and adolescents: a two-year longitudinal study. J Clin Exp Neuropsychol. 1998;20:769–781. doi: 10.1076/jcen.20.6.769.1109. [DOI] [PubMed] [Google Scholar]
- 26.Ewing-Cobbs L, Hasan KM, Prasad MR, Kramer L, Bachevalier J. Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. AJNR Am J Neuroradiol. 2006;27:879–881. [PMC free article] [PubMed] [Google Scholar]
- 27.Ewing-Cobbs L, Kramer L, Prasad M, Canales DN, Louis PT, Fletcher JM, et al. Neuroimaging, physical, and developmental findings after inflicted and noninflicted traumatic brain injury in young children. Pediatrics. 1998;102:300–307. doi: 10.1542/peds.102.2.300. [DOI] [PubMed] [Google Scholar]
- 28.Ewing-Cobbs L, Miner ME, Fletcher JM, Levin HS. Intellectual, motor, and language sequelae following closed head injury in infants and preschoolers. J Pediatr Psychol. 1989;14:531–547. doi: 10.1093/jpepsy/14.4.531. [DOI] [PubMed] [Google Scholar]
- 29.Fay GC, Jaffe KM, Polissar NL, Liao S, Rivara JB, Martin KM. Outcome of pediatric traumatic brain injury at three years: a cohort study. Arch Phys Med Rehabil. 1994;75:733–741. [PubMed] [Google Scholar]
- 30.Fletcher JM, Ewing-Cobbs L, Francis DJ, Levin HS. Variability in outcomes after traumatic brain injury in children: a developmental perspective. In: Broman SH, Michel ME, editors. Traumatic Head Injury in Children. New York: Oxford University Press; 1995. pp. 3–21. [Google Scholar]
- 31.Goldman PS. An alternative to developmental plasticity: heterology of CNS structures in infants and adults. In: Stein DG, Rosen JJ, Butters N, editors. Plasticity and Recovery of Function in the Central Nervous System. New York: Academic; 1974. pp. 149–174. [Google Scholar]
- 32.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 33.Jaffe KM, Polissar NL, Fay GC, Liao S. Recovery trends over three years following pediatric traumatic brain injury. Arch Phys Med Rehabil. 1995;76:17–26. doi: 10.1016/s0003-9993(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 34.Jennett B, Bond M. Assessment of outcome after severe brain damage. A practical scale. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 35.Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF. A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and non-inflicted traumatic brain injury. Pediatrics. 2004;114:633–639. doi: 10.1542/peds.2003-1020-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keenan HT, Runyan DK, Nocera MA. Child outcomes and family characteristics 1 year after severe inflicted or noninflicted traumatic brain injury. Pediatrics. 2006;117:317–324. doi: 10.1542/peds.2005-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolb B, Gibb R, Gorny G. Cortical plasticity and the development of behavior after early frontal cortical injury. Dev Neuropsychol. 2000;18:423–444. doi: 10.1207/S1532694208Kolb. [DOI] [PubMed] [Google Scholar]
- 38.Kolb B, Pellis S, Robinson TE. Plasticity and functions of the orbital frontal cortex. Brain Cogn. 2004;55:104–115. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- 39.Kraus JF. Epidemiological features of brain injury in children: occurrence, children at risk, causes and manner of injury, severity, and outcomes. In: Broman SH, Michel ME, editors. Traumatic Head Injury in Children. New York: Oxford University Press; 1995. pp. 22–39. [Google Scholar]
- 40.Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Muszynski CA, Yoganandan N, Pintar FA, Gennarelli TA. Risk of pediatric head injury after motor vehicle accidents. J Neurosurg. 2005;102 4 Suppl Pediatrics:374–379. doi: 10.3171/ped.2005.102.4.0374. [DOI] [PubMed] [Google Scholar]
- 42.Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 43.Prasad M, Ewing-Cobbs L, Swank PR, Kramer L. Predictors of outcome following traumatic brain injury in young children. Pediatr Neurosurg. 2002;36:64–74. doi: 10.1159/000048355. [DOI] [PubMed] [Google Scholar]
- 44.Radcliffe J, Bunin GR, Sutton LN, Goldwein JW, Phillips PC. Cognitive deficits in long-term survivors of childhood medulloblastoma and other noncortical tumors: age-dependent effects of whole brain radiation. Int J Dev Neurosci. 1994;12:327–334. doi: 10.1016/0736-5748(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 45.Rovet JF, Ehrlich RM, Sorbara DL. Neurodevelopment in infants and preschool children with congenital hypothyroidism: etiological and treatment factors affecting outcome. J Pediatr Psychol. 1992;17:187–213. doi: 10.1093/jpepsy/17.2.187. [DOI] [PubMed] [Google Scholar]
- 46.Stiles J. Neural plasticity and cognitive development. Dev Neuropsychol. 2000;18:237–272. doi: 10.1207/S15326942DN1802_5. [DOI] [PubMed] [Google Scholar]
- 47.Taylor HG, Alden J. Age-related differences in outcomes following childhood brain insults: an introduction and overview. J Int Neuropsychol Soc. 1997;3:555–567. [PubMed] [Google Scholar]
- 48.Taylor HG, Barry CT, Schatschneider C. School-age consequences of Haemophilus influenzae type b meningitis. J Clin Child Psychol. 1993;22:196–206. [Google Scholar]
- 49.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Burant C. Bidirectional child-family influences on outcomes of traumatic brain injury in children. J Int Neuropsychol Soc. 2001;7:755–767. doi: 10.1017/s1355617701766118. [DOI] [PubMed] [Google Scholar]
- 50.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short-and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16:15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- 51.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 52.Thomas B, Eyssen M, Peeters R, Molenaers G, Van Hecke P, De Cock P, et al. Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain. 2005;128:2562–2577. doi: 10.1093/brain/awh600. [DOI] [PubMed] [Google Scholar]
- 53.Thompson NM, Francis DJ, Stuebing KK, Fletcher JM, Ewing-Cobbs L, Miner ME, et al. Motor, visual-spatial, and somato-sensory skills after closed head injury in children and adolescents: a study of change. Neuropsychology. 1994;8:333–342. [Google Scholar]
- 54.Thorndike RL, Hagen EP, Sattler JM. Stanford-Binet Intelligence Scale. ed 4. Itasca, IL: Riverside Publishing; 1986. [Google Scholar]
- 55.Wiederholt JL, Bryant BR. Gray Oral Reading Tests. ed 4. Austin: Pro-Ed; 2001. [Google Scholar]
- 56.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing: 2001. [Google Scholar]
- 57.Yeates KO, Taylor HG, Drotar D, Wade SL, Klein S, Stancin T, et al. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. J Int Neuropsychol Soc. 1997;3:617–630. [PubMed] [Google Scholar]