Abstract
Background
Interest in combining antiarrhythmic drugs has been prompted by the lack of efficacy of monotherapies and the toxicity resulting from high doses of individual agents.
Objectives
We tested the hypothesis that procainamide and sotalol combined have greater beneficial effects on restitution, on the dispersion of refractoriness, and on decreasing the complexity of ventricular fibrillation (VF) than either drug alone.
Methods
Six open-chest pigs received intravenous procainamide (15 mg/kg load and 50 μg/kg/min maintenance) followed by sotalol (1.5 mg/kg). Another six pigs received sotalol first and procainamide second. Before drugs and after each drug, 20-second episodes of electrically induced VF were recorded from a 21 × 24 unipolar electrode plaque (2 mm spacing) sutured on the lateral posterior left ventricular epicardium. Restitution properties and dispersion of refractoriness were estimated from activation recovery intervals during pacing.
Results
The combination of the two drugs reduced the maximum slope of the restitution curve and during VF reduced the number of wavefronts, the activation rate, the percentage of wavefront families exhibiting reentry, and the conduction velocity more than either drug alone. In addition, in the group that received sotalol first, both drugs together reduced the SD and the coefficient of variation of the spatial dispersion of refractoriness compared with baseline.
Conclusions
Procainamide and sotalol combined have greater beneficial effects on restitution properties, dispersion of refractoriness, and the complexity of VF than either drug alone compared with baseline.
Keywords: ventricular fibrillation, sotalol, procainamide, activation patterns
Introduction
The control of ventricular fibrillation (VF) by electropharmacological means still faces major challenges.1 Clinical antiarrhythmic drugs that focus exclusively on preventing ventricular tachycardia (VT) initiation by decreasing sodium channel activity (class I) have not been effective in reducing sudden cardiac death.2 In contrast, other classes of drugs that are not particularly effective for the suppression of VT/VF reduce mortality from sudden death.3 These drugs have been demonstrated to either prolong refractoriness or reduce the slope of the restitution relation and to prevent the induction of VF and convert existing VF into a periodic rhythm.4,5 Interest in the combination of antiarrhythmic drugs has been prompted by the lack of efficacy of monotherapies and the toxicity resulting from high doses of individual agents.6
Whether it consists of multiple wandering wavelets7 or a single stable mother rotor that generates many daughter waves,8 reentry is generally agreed to be the engine that drives VF. Drugs that reduce the incidence of reentry during VF are considered beneficial for the conversion of VF to VT. In this study we used electrical mapping to assess the effects of procainamide and sotalol, administered separately and in combination, on restitution properties, dispersion of refractoriness, and VF activation patterns, focusing on the incidence of reentry.
Procainamide, a class IA antiarrhythmic drug, has an intermediate effect on sodium channel blockade and generally causes significant prolongation of conduction at more rapid heart rates. Sotalol has class III effects mediated primarily by antagonism of rapid-activating delayed rectifier potassium current (IKr) as well as β-adrenergic antagonist effects, so that it lengthens the action potential duration (APD). The motivations for us to combine these two agents are as follows: (1) procainamide and sotalol are widely used as effective antiarrhythmic monotherapy drugs in the clinical setting; (2) the actions of preventing wave break (shown by procainamide)9,10 and reducing the slope of the restitution curve (shown by sotalol)11 provide some basis for this antiarrhythmic drug combination; (3) the traditional concepts of increasing the wavelength and decreasing the excitable gap to prevent arrhythmia are also fulfilled by the combination of these two drugs; (4) the clinical efficacy of sotalol and a class IA drug combination has been confirmed;12,13 (5) procainamide has been shown to reduce heterogeneity of repolarization without altering restitution properties,5 while high doses of sotalol have been shown to reduce the slope of the restitution curve but increase the dispersion of refractoriness.14
Methods
Animals were managed in accordance with the American Heart Association guidelines on research animal use,15 and the protocol was approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Animal Preparation
Sixteen pigs (42 ± 9 kg, mean ± SD) were injected intra-muscularly with Telazol (4.4 mg/kg), xylazine (2.2 mg/kg), and atropine (0.04 mg/kg) for anesthetic induction. Anesthesia was maintained with isoflurane in 100% oxygen by inhalation. Core body temperature, arterial blood pressure, arterial blood gases, ECG lead II, and serum electrolytes were monitored and maintained within normal ranges throughout the study. The heart was exposed through a median sternotomy and supported in a pericardial sling. In 12 pigs, a custom made plaque containing 504 electrodes (24 × 21) with 2 mm space between adjacent electrodes was sutured to the posterior lateral LV, with one edge of the plaque adjacent to the posterior descending artery. A stainless steel wire was attached to the right leg as the ground for the unipolar mapping electrodes. The mapping electrodes were made from Teflon-coated silver wires with a diameter of 0.003 in. The diameter of the tip of each mapping electrode was approximately 0.2 mm. A catheter (model 6942, Sprint, Medtronic, Minneapolis, MN, USA) with a 479 mm2 surface area electrode in the right ventricle and a 766 mm2 surface area electrode in the superior vena cava was inserted for defibrillation. To pace the heart, a pair of barbed silver wire electrodes insulated except at the tips was inserted into the LV epicardium via 21-G needles. The electrodes were 0.2–0.5 cm away from the edge of the mapping plaque.
Pacing Protocol
The restitution relationship was determined during pacing. Pacing stimuli were delivered at twice diastolic threshold to the pacing wires. Trains of 30 S1 stimuli were repeated at the following intervals. Depending on the intrinsic heart rate, pacing started at 450 or 400 ms and was decreased to 300 ms by 50 ms steps. The pacing interval was decreased by 10 ms steps after 300 ms to an interval that induced VF or lost 1:1 capture. To capture during shorter pacing intervals, the S1–S1 interval was initially 300 ms and then decreased in 10 ms steps to the target interval, and thereafter kept at the target interval for 30 beats. Because blood pressure dropped during the rapid pacing, there was a 30–60 seconds interval after each pacing sequence to let blood pressure return to normal. Previous studies measured the APD restitution properties by pacing 12 to 50 beats with different cycle lengths to secure a stable measurement.16,17 We chose 30 beats in this study to ensure a stable measurement but tried to avoid severe ischemia caused by too many fast pacing beats.
VF Induction
Any VF induced during the restitution protocol was used for VF analysis. If VF was not induced twice during the restitution protocol, it was induced by a 9 V battery held briefly to the right ventricle. Each VF episode was mapped for 20 seconds before it was halted by a 400–600 V biphasic shock (6/4 ms) delivered from a defibrillator (Ventritex, HVSO2, St. Jude Medical Inc., CA, USA) via the catheter electrodes.
Procainamide and Sotalol Administration
The 12 pigs were equally divided into two groups. After the baseline pacing protocol and the induction of two episodes of VF, 15 mg/kg procainamide was infused intravenously at a rate no faster than 50 mg/min in six pigs. After the initial load, a maintenance infusion of 50 μg/kg/min procainamide was started and the pacing protocol and the induction of two episodes of VF were again performed. Sotalol was infused intravenously at a dose of 1.5 mg/kg to achieve class III action following procainamide measurement. The same pacing protocol and two more episodes of VF induction were repeated. The infusion of procainamide was maintained until the end of the experiment. In the other six pigs, the order of procainamide and sotalol was reversed and the same pacing and VF induction protocols were performed at baseline and after each drug administration.
Activation Recovery Interval Measurements
The activation recovery interval (ARI) was determined as an estimate of the APD and, hence, the refractory period.18 A 15-point quadratic differentiating filter was applied to determine the first derivatives of the extracellular potentials and to minimize the interference from noise. Activation time in the unipolar electrograms was defined as the steepest downslope of the QRS complex and recovery time as the fastest upslope of the T wave.19 The ARI was defined as the interval between the activation time and the recovery time. Less than 5% of the unipolar recordings were discarded because of electrograms in which the fastest upslope of the T wave was ambiguous.
Restitution Properties
An exponential function, ARI = a + b × e−DI/c, was used to fit a restitution curve for each mapping electrode recording.20–22 DI (diastolic interval) is the difference between the pacing cycle length and the last ARI of the last paced beat, and a, b, c are model constants that are fit by a least-square procedure, which halts when the difference in calculated ARI between the last two iterations converges to <10–8. Only those restitution curves for which the square of the correlation coefficient (R2) was > 0.8 were included in the analysis. Restitution slopes were calculated as the first derivatives of the exponential function at each DI, and the maximum slope was identified within the range of the exponentially determined DIs.
Quantitative Analysis of VF Activation
Quantitative analysis of VF activation patterns was performed on a Linux system computer using algorithms discussed in detail elsewhere.23–27 During the first 20 seconds of each VF episode, we computed the following descriptors of the VF wavefronts for each 1 second of data: (1) number of wavefronts, (2) mean area swept out by the wavefronts, (3) fractionation incidence, (4) collision incidence, (5) block incidence, (6) breakthrough or focal incidence, (7) multiplicity, a parameter that measures the number of distinct wavefront activation pathways, (8) repeatability, the average number of wavefronts that traverse each distinct pathway, (9) activation rate, (10) propagation velocity, (11) negative peak dV/dt of VF activations, and (12) incidence of reentry.
Verification of the Relationship of APD and ARI Before and After Drug Administration
To verify whether the drugs affected the relationship of APD and ARI, in another four pigs, an epicardial monophasic action potential (MAP) catheter was placed at the center of the area where the plaque of electrodes was located in the first 12 pigs. Four unipolar electrodes were placed, one each on all four sides of the MAP recording site about 1 cm away. We simultaneously determined the APD90 from the MAP recording as described previously28 and the mean of the ARIs at the four surrounding electrodes before and after each drug and their combination after 30 beats of pacing at the rates of 300, 250, and 200 ms.
Statistical Analysis
Results are expressed as mean ± SD. All VF variables were compared before and after procainamide or sotalol administration and after the combination of these two agents using multivariate analysis of variance (MANOVA) with repeated measures to test for differences between baseline and treatments. Dispersion of refractoriness was quantified using the SD of the ARIs across all of the plaque electrodes and using the coefficient of variation (CV, the SD divided by the mean of the ARIs, in percent). For all analyses, P ≤ 0.05 was considered statistically significant. Least squares linear regression was used to measure the relationship of APD and ARI before and after drug administration.
Results
Effects of Drugs on Electrical Restitution Properties
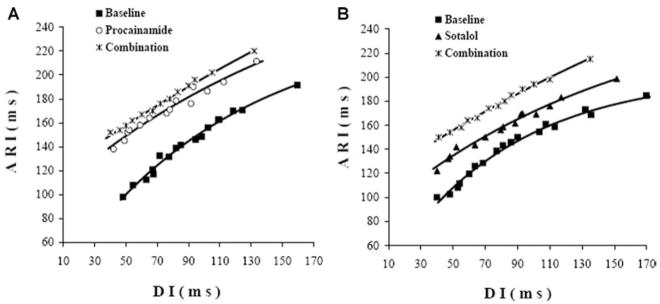
Of the 504 electrodes in the plaque, 315 ± 87 had restitution curve recordings that could be fit with an exponential function with an R2 greater than 0.8 and were, therefore, used for analysis. At baseline (before either drug was given in the two groups), the mean maximal slope of the restitution curves was >1 (Table 1, Fig. 1A). Compared with baseline in the procainamide-first group (1.15 ± 0.37), pro-cainamide alone did not significantly decrease the maximum slope (0.94 ± 0.38, P = 0.06). Instead, it shifted the curve upward because of the prolongation of APD as estimated from the ARI (Fig. 1A). After combination with sotalol, the maximum slope of the restitution curve was significantly decreased compared with baseline and with procainamide alone by 35% and 20% (P < 0.01, P = 0.02, respectively). In the sotalol-first group, sotalol alone significantly decreased the maximum slope of the restitution curve by 16% (P = 0.01, Table 1, Fig. 1B) compared with baseline. The combination of sotalol with procainamide further decreased the maximum slope of the restitution curve to 27% less than the baseline value (P < 0.01, Table 1). In the four pigs in which a MAP was recorded, procainamide, sotalol and their combination did not change the relationship between APD90 and ARI at each pacing rate (R = 0.95).
TABLE 1.
Effects of Procainamide and Sotalol on Restitution Slope and Dispersion of Refractoriness
| Procainamide-First Group (n = 6)
|
Sotalol-First Group (n = 6)
|
|||||
|---|---|---|---|---|---|---|
| Baseline | Procainamide | Combination | Baseline | Sotalol | Combination | |
| Slope | 1.15 ± 0.37 | 0.94 ± 0.38 | 0.75 ± 0.29**† | 1.11 ± 0.17 | 0.93 ± 0.18** | 0.81 ± 0.10**† |
| ARI (ms) | 190 | 198** | 200** | 187 | 194** | 197** |
| SD (ms) | 8.2 | 7.4 | 7.6 | 8.1 | 5.6** | 6.0** |
| CV (%) | 4.3 | 3.7 | 3.8 | 4.7 | 2.9** | 3.1** |
P < 0.05,
P < 0.01 compared to baseline,
P < 0.05, compared to monotherapy.
Slope is the maximum slope of the restitution curve; ARI, SD, CV are at a pacing cycle length of 300 ms.
Figure 1.
Effects of drugs on restitution properties. The restitution curves from one animal for the same electrode at baseline (solid square), procainamide (open circle), and combined with sotalol (star, Fig. 1A) and another animal at baseline (solid square), sotalol (solid triangle), combined with procaimamide (star, Fig. 1B). The maximum slopes of the restitution curves for this animal are 1.2 (Baseline in panel A), 0.95 (procainamide), 0.8 (procainamide + sotalol), 1.3 (Baseline in panel B), 0.92 (sotalol), and 0.82 (sotalol + procainamide).
ARI and Dispersion of Refractoriness
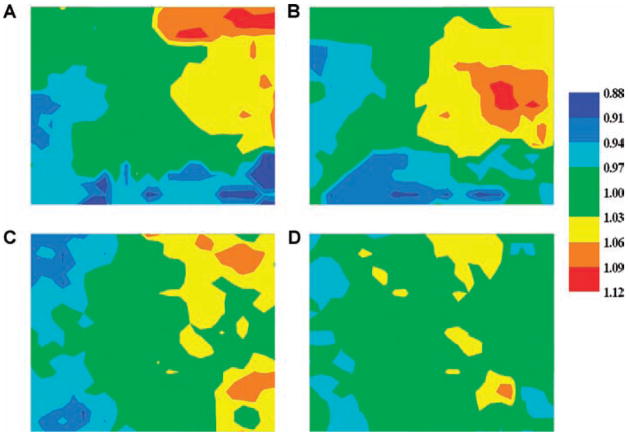
To estimate the dispersion of refractoriness, ARIs were measured from each recording electrode at a pacing cycle length of 300 ms (Table 1). Compared with baseline, both procainamide and sotalol significantly prolonged the ARI (190 ± 8.2 ms vs 198 ± 7.4 ms, P < 0.01; 187 ± 8.1 ms vs 194 ± 5.6 ms, P < 0.01, respectively). In the procainamide-first group, procainamide alone or in combination with sotalol did not significantly reduce the dispersion of refractoriness as estimated from the SD and CV of the ARIs throughout the mapping plaque (Table 1, Fig. 2A,B). In contrast, in the sotalol-first group, sotalol alone or in combination with pro-cainamide significantly reduced the dispersion of refractoriness compared with baseline by reducing both the SD and the CV (Fig. 2C,D).
Figure 2.
Representative maps of ARIs before and after drugs. Representative maps of ARIs at a 300 ms CL in one animal at control (panel A) and after procainamide (panel B) and in another animal at control (panel C) and after sotalol (panel D). The ARI at each electrode is represented by a color corresponding with the value ranges shown on the bar. The ARIs have been normalized by dividing the ARI at each electrode by the mean ARI for all electrodes.
Quantitative Analysis of VF Activation Patterns
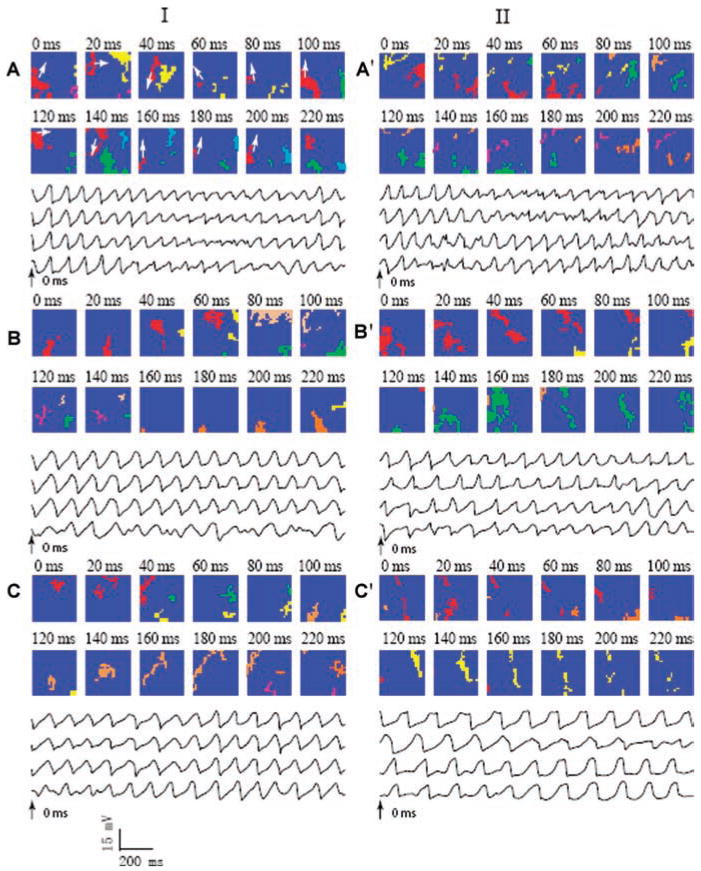
Quantitative data from all 1-second intervals of VF were pooled for the procainamide-first group in Table 2 and for the sotalol-first group in Table 3. The changes in VF activation patterns caused by each drug and by the combination of drugs were observed in all pig hearts, as were the beneficial effects of the combination of drugs. Examples of VF activation sequences at baseline and after procainamide and sotalol and their combination are shown in Figure 3.
TABLE 2.
Effects of Procainamide and Sotalol on Activation Patterns During VF in Procainamide-First Group (n = 6)
| Baseline | Procainamide | Baseline vs Procainamide %change | Combination Procainamide + Sotalol | Baseline vs Combination %change | Procainamide vs Combination %change | |
|---|---|---|---|---|---|---|
| Number of wavefronts | 71 ± 23 | 42 ± 10 | 41%↓** | 35 ± 11 | 51%↓** | 17%↓‡ |
| Area swept out (mm2) | 228 ± 36 | 234 ± 49 | 3%↑ | 275 ± 71 | 21%↑** | 18%↑ ‡ |
| Fractionation incidence | 0.11 ± 0.04 | 0.09 ± 0.06 | 18%↓** | 0.11 ± 0.06 | → | 18%↑ ‡ |
| Collision incidence | 0.12 ± 0.05 | 0.10 ± 0.05 | 17%↓** | 0.11 ± 0.05 | 8%↓** | 10%↑ |
| Block incidence | 0.34 ± 0.08 | 0.37 ± 0.12 | 9%↑ | 0.35 ± 0.13 | 3%↑ | 3%↓ |
| Breakthrough incidence | 0.29 ± 0.07 | 0.30 ± 0.12 | 3%↑ | 0.27 ± 0.09 | 7%↓* | 10%↓ ‡ |
| Multiplicity | 12.5 ± 4.2 | 6.9 ± 2.1 | 45%↓** | 6.0 ± 2.1 | 52%↓** | 13%↓ ‡ |
| Repeatability | 5.5 ± 10. | 5.8 ± 1.0 | 5%↑* | 6.0 ± 1.7 | 9%↑** | 3%↑ |
| Activation rate (s−1) | 9.4 ± 1.4 | 6.8 ± 1.1 | 28%↓** | 6.7 ± 1.0 | 29%↓** | 1%↓ |
| Velocity (m/s) | 0.46 ± 0.07 | 0.38 ± 0.05 | 17%↓** | 0.39 ± 0.05 | 15%↓** | 3%↑ |
| Peak-dV/dt (V/s) | −1.0 ± 0.09 | −0.97 ± 0.06 | 3%↓** | −0.99 ± 0.14 | 1%↓** | 2%↑ |
| Reentry incidence | 1.2 ± 1.1 | 1.0 ± 1.5 | 17%↓ | 0.3 ± 0.5 | 75%↓** | 70%↓ ‡ |
P < 0.05,
P < 0.01 compared to baseline,
P < 0.05,
P < 0.01 compared to monotherapy.
TABLE 3.
Effects of Sotalol and Procainamide on Activation Patterns During VF in Sotalol-First Group (n = 6)
| Baseline | Sotalol | Baseline vs Sotalol %change | Combination Procainamide + Sotalol | Baseline vs Combination %change | Sotalol vs Combination %change | |
|---|---|---|---|---|---|---|
| Number of wavefronts | 90 ± 18 | 68 ± 21 | 24%↓** | 31 ± 19 | 66%↓** | 54%↓‡ |
| Area swept out (mm2) | 225 ± 26 | 230 ± 32 | 2%↑ | 322 ± 142 | 43%↑** | 40%↑‡ |
| Fractionation incidence | 0.16 ± 0.03 | 0.14 ± 0.04 | 13%↓** | 0.12 ± 0.06 | 25%↓** | 14%↓‡ |
| Collision incidence | 0.16 ± 0.03 | 0.14 ± 0.04 | 13%↓** | 0.11 ± 0.06 | 31%↓** | 21%↓‡ |
| Block incidence | 0.28 ± 0.08 | 0.30 ± 0.10 | 7%↑ | 0.31 ± 0.14 | 11%↑* | 3%↑ |
| Breakthrough incidence | 0.27 ± 0.08 | 0.29 ± 0.10 | 7%↑ | 0.30 ± 0.11 | 11%↑ | 3%↑ |
| Multiplicity | 14.3 ± 4.1 | 11.1 ± 4.1 | 22%↓** | 5.0 ± 3.2 | 65%↓** | 55%↓‡ |
| Repeatability | 6.0 ± 1.2 | 5.8 ± 1.0 | 3%↓ | 5.8 ± 1.9 | 3%↓ | → |
| Activation rate (s-1) | 9.7 ± 0.9 | 7.9 ± 1.2 | 19%↓** | 5.6 ± 1.4 | 42%↓** | 29%↓‡ |
| Velocity (m/s) | 0.45 ± 0.05 | 0.41 ± 0.05 | 9%↓* | 0.31 ± 0.06 | 31%↓** | 24%↓‡ |
| Peak-dV/dt (V/s) | −1.1 ± 0.09 | −1.1 ± 0.11 | → | −1.0 ± 0.11 | 9%↓** | 9%↓‡ |
| Reentry incidence | 3.3 ± 3.2 | 1.6 ± 2.1 | 52%↓** | 0.5 ± 1.1 | 85%↓** | 69%↓‡ |
P < 0.05,
P < 0.01 compared to baseline;
P < 0.05,
P < 0.01 compared to monotherapy.
Figure 3.
Snapshots of activation of VF before and after drugs. Column I represents snapshots of activation of VF in one animal at control (panel A), after procainamide (panel B) and combined with sotalol (panel C). Column II represents snapshots of activation of VF in another animal at control (panel A’), after sotalol (panel B’) and combined with procainamide (panel C’). The numbers show the time with time 0 beginning 2 s after VF onset. Each color indicates an individual wavefront, except in panel A where all of the wavefronts that are part of a reentrant pathway are shown in red. Each colored pixel indicates an electrode site at which the rate of voltage change (dV/dt) was less than or equal to 0.5 V/s sometime during the 5 ms interval represented by each frame. The arrows in panel A indicate the direction of wavefront movement in the clockwise reentrant circuit. Recordings from the same four electrodes are shown below the activation maps for each panel for 2 s beginning at time 0 ms (arrows). In column I, the broad picture of the dynamics are as follows: Panel A, large spatial and temporal variation; Panel B, more organized and repeatable activation patterns with less fractionation and collision; Panel C, even more organized and repeatable activation patterns with larger wavefronts. In column II, after sotalol, activation patterns are more organized and repeatable (panel B’). The combination of the two drugs shows changes similar to that shown in panel C of column I.
Number of wavefronts
Compared with baseline, procainamide alone and in combination with sotalol significantly reduced the number of wavefronts by 41% and 51%, respectively (Table 2, Fig. 3B, C). Sotalol alone and in combination with procainamide significantly reduced the number of wavefronts by 24% and 66%, respectively (Table 3, Fig. 3B,C).
Area swept out by wavefront
Neither procainamide (Fig. 3B) nor sotalol (Fig. 3B) alone significantly altered the area swept out by each wavefront compared with baseline. However, the combination of pro-cainamide followed by sotalol or sotalol followed by pro-cainamide significantly increased the area swept out compared with baseline (21% and 43%, respectively).
Fractionation and collision incidence
Procainamide alone significantly reduced the incidence of wavefront fractionations and collisions by 18% and 17%, respectively, compared with baseline, sotalol alone significantly reduced the incidence of wavefront fractionations and collisions by 13% each compared with baseline. Sotalol followed by procainamide significantly reduced these two measures compared with sotalol alone (14% and 21%, respectively). Procainamide followed by sotalol significantly increased wavefront fractionations (18%) but not collisions.
Block and breakthrough incidence
Neither procainamide nor sotalol alone significantly altered the incidence of conduction block or breakthrough to the epicardium. However, in the sotalol-first group the drug combination significantly increased the incidence of block compared with baseline, while in the procainamide-first group the drug combination significantly decreased the incidence of breakthrough.
Multiplicity and repeatability
Multiplicity was significantly decreased by procainamide or sotalol alone by 45% and 22%, respectively, and was decreased further when the two drugs were combined in either order compared with baseline (52% and 65%). In the procainamide-first group, repeatability was significantly increased by the drug combination compared with baseline, while in the sotalol-first group neither sotalol alone nor in combination with procainamide significantly changed repeatability.
Activation rate
Both procainamide and sotalol alone significantly reduced the activation rate (28% and 19%, respectively). The combination of procainamide followed by sotalol did not significantly alter the activation rate compared with procainamide alone. However, sotalol followed by procainamide further significantly decreased the activation rate by 29% compared with sotalol alone.
Conduction velocity and Peak-dV/dt
Procainamide significantly decreased conduction velocity by 22% and Peak-dV/dt by 3%. Sotalol significantly decreased conduction velocity by 9% but did not significantly change Peak-dV/dt. Yet the combination of sotalol with pro-cainamide in either order significantly reduced both conduction velocity and peak dV/dt by 31% and 9%.
Reentry incidence
Sotalol alone significantly reduced the incidence of reentry within the mapped region by 52%, but procainamide alone did not. The combination of these two drugs in either sequence significantly and markedly reduced reentry incidence within the mapped region compared with baseline (75% with procainamide first and 85% with sotalol first).
Discussion
This study demonstrates that procainamide and sotalol combined have greater beneficial effects on reducing the incidence of reentry, dispersion of refractoriness, complexity of VF and flattening of the restitution curve than either drug alone. Reentry incidence within the mapped region during VF was significantly reduced 75% to 85% by the combination of the two drugs. The drug combination synergistically affected most measured VF parameters, causing changes indicating greater organization during VF. As discussed below, several mechanisms may have accounted for the striking reduction in reentry by the two drugs together.
Electrophysiologic Effects of the Combination of Sotalol and Procainamide
The prolongation of APD by sotalol is often decreased at higher stimulation rates29 implying that there may be less antiarrhythmic effectiveness during the rapid activation of VF. Some of the rate-related shortening of APD and loss of drug-related APD prolongation may be partially due to a relatively greater contribution of IKs, which is not affected by sotalol.30 Procainamide also exhibits a class III effect by inhibiting IKr in a time- and voltage-dependent manner, thus prolonging the APD,31 whereas sodium channel blockade occurs to a greater degree at shorter cycle lengths.32 The combination of procainamide and sotalol may eliminate the rate-dependent effect of sotalol by the increased class III effect of procainamide and the maintenance of some class I effect at higher heart rates.33 In addition, the prolonged APD by sotalol alone could increase the risk for early afterdepolarizations and torsade de pointes (TdP). However, several clinical studies have revealed that the combination of class IA and class III antiarrhythmic agents rarely result in TdP or prolonged QT interval.34–36 Interestingly, Frederique et al. documented that sotalol combined with a class IA drug was no more arrhythmogenic than either of the drugs alone, and significantly decreased the incidence of TdP in the early stage of drug administration in dogs,37 suggesting there may be a complex relationship between these antiarrhythmic drugs. Consistent with those results, the combination of sotalol and procainamide in our study did not significantly prolong the ARI compared with monotherapy (Table 1).
Alteration of VF by Procainamide and Sotalol
Several mechanisms for block that can lead to reentry have been proposed. The classical mechanism is the nonuniform dispersion of refractoriness.7 An increase in the nonuniformity of dispersion of refractoriness increases both the inducibility of VF38 and the duration of sustained VF in animals in which VF tends to stop spontaneously.39 More recently, mechanisms for block and reentry have been suggested that can occur even in tissues whose properties, including refractoriness, are totally homogeneous. In computer and chemical models of excitable media, alternans in recovery time and/or cycle length has been shown to be a precursor to block.40–42 Computer simulations indicate that reentrant spiral waves become unstable and break down into multiple spiral waves when the slope of the restitution curve for refractoriness is greater than 1.43
Procainamide prevents wave break during VF, which is thought to be the main mechanism for maintenance of VF.10 Consistent with this finding, this study showed that pro-cainamide reduced the incidence of fractionation and collision. The reduction of fractionation and collision is commonly thought to be a result of reduced wave block caused by many antiarrhythmia drugs.5 However, procainamide did not significantly change the incidence of block in this study. This finding is consistent with our findings that neither restitution properties nor dispersion of refractoriness was changed by procainamide, which are generally agreed to be the main factors that cause wave block.
Compared with procainamide, sotalol alone significantly flattened the slope of the restitution curve, decreased the dispersion of refractoriness, decreased the incidence of reentry and also decreased fractionation and collision. As a class III antiarrhythmia agent, the main electrophysiological feature of sotalol is prolongation of refractoriness, which is beneficial in suppression of VT.44 While the effect of sotalol on the prolongation of refractoriness is beneficial, the percentage of decrease in activation rate during VF is less than that caused by procainamide (19% vs 28%, Tables 2 and 3). This may be explained by reverse use dependency of sotalol on the APD. Sotalol selectively blocks the rapid component of Ik, producing a progressive prolongation of APD at slower heart rates.45,46 Many class I antiarrhythmic agents show a use dependency, whereby a progressive increase in sodium channel blockade is seen with a faster heart rate.47,48
Study Limitations
We only investigated the effects of procainamide and sotalol at a fixed dosage. Since our goal is to increase the antifibrillation effect and reduce the toxicity of each drug, a further study should be done to explore different dosages in the combination to see if a lower dose can achieve the same effective antiarrhythmia result. While the defibrillation threshold (DFT) after many single drug administrations has been investigated, the effect of combinations of drugs on the DFT also should be investigated in future studies. Many pathological changes will impact the effect of antiarrhythmic drugs on the heart. Our current study only focused on the normal heart in the pig. Whether the same effects are associated with myocardial infarction or heart failure should be examined in future studies. We did not directly measure the APD but estimated it from the ARI. However, we found a high correlation between the ARI and the APD recorded with a MAP electrode both before and after administering the drugs.
Clinical Implications
Multiple classes of effects working together, such as the prolongation of repolarization, β-adrenergic blockade, and modest slowing of conduction observed with amiodarone, are likely to be successful in the prevention of arrhythmia. The combination of procainamide and sotalol may be effective in preventing arrhythmia through the complementary mechanisms of class IA effects (prolongation of refractoriness and the modest slowing of cardiac conduction) and class III effects (prolongation of ventricular repolarization and β-blocking properties).49,50 This study showed that the combination of the two drugs flattened the restitution slope, decreased the dispersion of refractoriness, and organized VF significantly more than either procainamide or sotalol alone. These synergistic effects suggest that it is important to seek new combinations of drugs to treat arrhythmias more effectively as well as to reduce side effects that are common for many drugs. For example, the drug combination in this study significantly reduced the incidence of reentry. If reentry is the mechanism for maintaining VF, then if a combination of drugs could completely eliminate reentry during VF, VF should terminate.
Besides these antiarrhythmic effects, high-dose class IA drugs also have proarrhythmic effects.18 This might be due to slowing of conduction that would widen the excitable gap and predispose the heart to reentrant arrhythmias. As described above, sotalol has shown reverse use dependence by a progressive loss of effect at higher stimulation rates.29 In this study, we did not observe reverse use dependence by the combination of these two drugs during rapid VF. Thus, the findings of this study provide efficacy as well as some safety information for the combination of procainamide and sotalol.
Because of anatomical and ionic channel differences between humans and animals, no good animal VF model exists that closely models human VF.51 Generally, the VF activation patterns in humans are less complex and the VF activation rate is slower than in swine or canine VF. The combination of drugs made the VF in this porcine model more closely resemble human VF.52 For example, the combination of pro-cainamide and sotalol reduced the activation rate, one of the most important parameters of VF, from 9.7 ± 0.9/s to 5.6 ± 1.4/s, which is close to the rate of human VF reported by Nash et al. (5.11 ± 0.25/s)53 and by Nanthakumar et al. (5.8 ± 1.8/s).52 As with all animal studies, further studies in humans are required before the results of this study are applied clinically.
Acknowledgments
The work was supported in part by an American Heart Association Scientist Development Grant and The National Institutes of Health Research Grants HL-28429, HL-66256, and HL-85370.
References
- 1.Weiss JN, Garfinkel A, Karagueuzian HS, Qu Z, Chen PS. Chaos and the transition to ventricular fibrillation: A new approach to antiarrhythmic drug evaluation. Circulation. 1999;99:2819–2826. doi: 10.1161/01.cir.99.21.2819. [DOI] [PubMed] [Google Scholar]
- 2.The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 3.Held PH, Yusuf S. Impact of calcium channel blockers on mortality in survivors of acute myocardial infarction. In: Singh BN, Wellens HJJ, Hiraoka M, editors. Electropharmacological Control of Cardiac Arrhythmias: To Delay Conduction or to Prolong Refractoriness? Mount Kisco, NY: Futura Publishing Company; 1994. pp. 399–412. [Google Scholar]
- 4.Chorro FJ, Canoves J, Guerrero J, Mainar L, Sanchis J, Such L, Lopez-Merino V. Alteration of ventricular fibrillation by flecainide, verapamil, and sotalol: An experimental study. Circulation. 2000;101:1606–1615. doi: 10.1161/01.cir.101.13.1606. [DOI] [PubMed] [Google Scholar]
- 5.Riccio ML, Koller ML, Gilmour RF., Jr Electrical restitution and spatiotemporal organization during ventricular fibrillation. Circ Res. 1999;84:955–963. doi: 10.1161/01.res.84.8.955. [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof P, Breithardt G. New concepts for old drugs to maintain sinus rhythm in patients with atrial fibrillation. Heart Rhythm. 2007;4:790–803. doi: 10.1016/j.hrthm.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Moe GK, Abildskov JA, Han J. Factors responsible for the initiation and maintenance of ventricular fibrillation. In: Surawicz B, Pellegrino ED, editors. Sudden Cardiac Death. New York, NY: Grune and Stratton; 1964. pp. 56–63. [Google Scholar]
- 8.Chen J, Mandapati R, Berenfeld O, Skanes AC, Jalife J. High-frequency periodic sources underlie ventricular fibrillation in the isolated rabbit heart. Circ Res. 2000;86:86–93. doi: 10.1161/01.res.86.1.86. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, Yashima M, Wu TJ, Doshi R, Chen PS, Karagueuzian HS. Mechanism of procainamide-induced prevention of spontaneous wave break during ventricular fibrillation. Insight into the maintenance of fibrillation wave fronts. Circulation. 1999;100:666–674. doi: 10.1161/01.cir.100.6.666. [DOI] [PubMed] [Google Scholar]
- 10.Kwan YY, Fan W, Hough D, Lee JJ, Fishbein MC, Karagueuzian HS, Chen PS. Effects of procainamide on wave-front dynamics during ventricular fibrillation in open-chest dogs. Circulation. 1998;97:1828–1836. doi: 10.1161/01.cir.97.18.1828. [DOI] [PubMed] [Google Scholar]
- 11.Pak H-NKY-H, Sun K, Shin SH, Lee SJ, Hwang GS, Shim WJ, Ro YM. Anti-ventricular fibrillation and anti-restitution properties of sotalol. Pacing and Clin Electrophys. 2000;23:672. [Google Scholar]
- 12.Lee SD, Newman D, Ham M, Dorian P. Electrophysiologic mechanisms of antiarrhythmic efficacy of a sotalol and class Ia drug combination: Elimination of reverse use dependence. J Am Coll Cardiol. 1997;29:100–105. doi: 10.1016/s0735-1097(96)00423-8. [DOI] [PubMed] [Google Scholar]
- 13.Dorian P, Newman D, Berman N, Hardy J, Mitchell J. Sotalol and type IA drugs in combination prevent recurrence of sustained ventricular tachycardia. J Am Coll Cardiol. 1993;22:106–113. doi: 10.1016/0735-1097(93)90823-j. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade des pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997;96:2038–2047. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- 15.AHA Special Report. Position of the American Heart Association on Research animal use. Circulation. 1985;71:849A–850A. [PubMed] [Google Scholar]
- 16.Mahajan A, Shiferaw Y, Sato D, Baher A, Olcese R, Xie LH, Yang MJ, Chen PS, Restrepo JG, Karma A, Grafinkel A, Qu Z, Weiss J. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophys J. 2007;94:392–410. doi: 10.1529/biophysj.106.98160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koller ML, Riccio ML, Gilmour RF., Jr Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol. 1998;275:H1635–1642. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 18.Dhein S, Muller A, Gerwin R, Klaus W. Comparative study on the proarrhythmic effects of some antiarrhythmic agents. Circulation. 1993;87:617–630. doi: 10.1161/01.cir.87.2.617. [DOI] [PubMed] [Google Scholar]
- 19.Haws CW, Lux RL. Correlation between in vivo transmembrane action potential durations and activation-recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation. 1990;81:281–288. doi: 10.1161/01.cir.81.1.281. [DOI] [PubMed] [Google Scholar]
- 20.Banville I, Gray RA. Effect of action potential duration and conduction velocity restitution and their spatial dispersion on alternans and the stability of arrhythmias. J Cardiovasc Electrophysiol. 2002;13:1141–1149. doi: 10.1046/j.1540-8167.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe MA, Koller ML. Mathematical analysis of dynamics of cardiac memory and accommodation: Theory and experiment. Am J Physiol Heart Circ Physiol. 2002;282:1534–1547. doi: 10.1152/ajpheart.00351.2001. [DOI] [PubMed] [Google Scholar]
- 22.Qin H, Huang J, Rogers JM, Walcott GP, Rollins DL, Smith WM, Ideker RE. Mechanisms for the maintenance of ventricular fibrillation: The nonuniform dispersion of refractoriness, restitution properties, or anatomic heterogeneities? J Cardiovasc Electrophysiol. 2005;16:888–897. doi: 10.1111/j.1540-8167.2005.40650.x. [DOI] [PubMed] [Google Scholar]
- 23.Rogers JM, Usui M, KenKnight BH, Ideker RE, Smith WM. A quantitative framework for analyzing epicardial activation patterns during ventricular fibrillation. Ann Biomed Eng. 1997;25:749–760. doi: 10.1007/BF02684159. [DOI] [PubMed] [Google Scholar]
- 24.Rogers JM, Usui M, KenKnight BH, Ideker RE, Smith WM. Recurrent wavefront morphologies: A method for quantifying the complexity of epicardial activation patterns. Ann Biomed Eng. 1997;25:761–768. doi: 10.1007/BF02684160. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Rogers JM, Kenknight BH, Rollins DL, Smith WM, Ideker RE. Evolution of the organization of epicardial activation patterns during ventricular fibrillation. J Cardiovasc Electrophysiol. 1998;9:1291–1304. doi: 10.1111/j.1540-8167.1998.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 26.Rogers JM, Huang J, Smith WM, Ideker RE. Incidence, evolution, and spatial distribution of functional reentry during ventricular fibrillation in pigs. Circ Res. 1999;84:945–954. doi: 10.1161/01.res.84.8.945. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Walcott GP, Killingsworth CR, Melnick SB, Rogers JM, Ideker RE. Quantification of activation patterns during ventricular fibrillation in open-chest porcine left ventricle and septum. Heart Rhythm. 2005;2:720–728. doi: 10.1016/j.hrthm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Cheng KA, Dosdall DJ, Smith WM, Ideker RE. Role of maximum rate of depolarization in predicting action potential duration during ventricular fibrillation. Am J Physiol Heart Circ Physiol. 2007;293:2530–2536. doi: 10.1152/ajpheart.00793.2007. [DOI] [PubMed] [Google Scholar]
- 29.Hondeghem LM, Snyders DJ. Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation. 1990;81:686–690. doi: 10.1161/01.cir.81.2.686. [DOI] [PubMed] [Google Scholar]
- 30.Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iost N, Virag L, Varro A, Papp JG. Comparison of the effect of class IA antiarrhythmic drugs on transmembrane potassium currents in rabbit ventricular myocytes. J Cardiovasc Pharmacol Ther. 2003;8:31–41. doi: 10.1177/107424840300800i106. [DOI] [PubMed] [Google Scholar]
- 32.Lee RJ, Liem LB, Cohen TJ, Franz MR. Relation between repolarization and refractoriness in the human ventricle: Cycle length dependence and effect of procainamide. J Am Coll Cardiol. 1992;19:614–618. doi: 10.1016/s0735-1097(10)80281-5. [DOI] [PubMed] [Google Scholar]
- 33.Dorian P. Mechanisms of action of class III agents and their clinical relevance. Pacing and Clin Electrophysiol. 2000;1:C6–9. [PubMed] [Google Scholar]
- 34.Marchlinski FE, Buxton AE, Kindwall KE, Miller JM, Rosenthal ME, Gottlieb CD, Bloom RB, Josephson ME. Comparison of individual and combined effects of procainamide and amiodarone in patients with sustained ventricular tachyarrhythmias. Circulation. 1988;78:583–591. doi: 10.1161/01.cir.78.3.583. [DOI] [PubMed] [Google Scholar]
- 35.Toivonen L, Kadish A, Morady F. A prospective comparison of class IA, B, and C antiarrhythmic agents in combination with amiodarone in patients with inducible, sustained ventricular tachycardia. Circulation. 1991;84:101–108. doi: 10.1161/01.cir.84.1.101. [DOI] [PubMed] [Google Scholar]
- 36.Dorian PNDBN. Prolongation of ventricular refractoriness predicts suppression of ventricular tachycardia inducibility. Pacing and Clin Electrophysiol. 1992;15:552. [Google Scholar]
- 37.Chezalviel-Guilbert F, Deplanne V, Davy JM, Poirier JM, Xia YZ, Cheymol G, Weissenburger J. Combination of sotalol and quinidine in a canine model of torsades de pointes: No increase in the QT-related proarrhythmic action of sotalol. J Cardiovasc Electrophysiol. 1998;9:498–507. doi: 10.1111/j.1540-8167.1998.tb01842.x. [DOI] [PubMed] [Google Scholar]
- 38.Han J. Ventricular vulnerability to fibrillation. In: Dreifus LS, Likoff W, editors. Cardiac Arrhythmias. New York: Grune and Stratton; 1973. pp. 87–95. [Google Scholar]
- 39.Tovar OH, Jones JL. Epinephrine facilitates cardiac fibrillation by shortening action potential refractoriness. Moll Cell Cardiol. 1997;29:1447–1455. doi: 10.1006/jmcc.1997.0387. [DOI] [PubMed] [Google Scholar]
- 40.Vinet A, Roberge FA. The dynamics of sustained reentry in a ring model of cardiac tissue. ANBE. 1994;22:568–591. doi: 10.1007/BF02368285. [DOI] [PubMed] [Google Scholar]
- 41.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 42.Cao JM, Qu Z, Kim YH, Wu TJ, Garfinkel A, Weiss JN, Karagueuzian HS, Chen PS. Spatiotemporal heterogeneity in the induction of ventricular fibrillation by rapid pacing: Importance of cardiac restitution properties. Circ Res. 1999;84:1318–1331. doi: 10.1161/01.res.84.11.1318. [DOI] [PubMed] [Google Scholar]
- 43.Karma A. Spiral breakup in model equations of action potential propagation in cardiac tissue. Phys Rev Lett. 1993;71:1103–1106. doi: 10.1103/PhysRevLett.71.1103. [DOI] [PubMed] [Google Scholar]
- 44.Kuhlkamp V, Mewis C, Mermi J, Bosch RF, Seipel L. Suppression of sustained ventricular tachyarrhythmias: A comparison of d,l-sotalol with no antiarrhythmic drug treatment. J Am Coll Cardiol. 1999;33:46–52. doi: 10.1016/s0735-1097(98)00521-x. [DOI] [PubMed] [Google Scholar]
- 45.Strauss HC, Bigger JT, Jr, Hoffman BF. Electrophysiologial and beta-receptor blocking effects of MJ 1999 on dog and rabbit cardiac tissue. Circ Res. 1970;26:661–678. doi: 10.1161/01.res.26.6.661. [DOI] [PubMed] [Google Scholar]
- 46.Schmitt C, Brachmann J, Karch M, Waldecker B, Navarrete L, Montero M, Beyer T, Kubler W. Reverse use-dependent effects of sotalol demonstrated by recording monophasic action potentials of the right ventricle. Am J Cardiol. 1991;68:1183–1187. doi: 10.1016/0002-9149(91)90191-m. [DOI] [PubMed] [Google Scholar]
- 47.Varro A, Nakaya Y, Elharrar V, Surawicz B. Effect of antiarrhythmic drugs on the cycle length-dependent action potential duration in dog Purkinje and ventricular muscle fibers. J Cardiovasc Pharmacol. 1986;8:178–185. doi: 10.1097/00005344-198601000-00026. [DOI] [PubMed] [Google Scholar]
- 48.Campbell TJ. Kinetics of onset of rate-dependent effects of Class I antiarrhythmic drugs are important in determining their effects on refractoriness in guinea-pig ventricle, and provide a theoretical basis for their subclassification. Cardiovasc Res. 1983;17:344–352. doi: 10.1093/cvr/17.6.344. [DOI] [PubMed] [Google Scholar]
- 49.Nademanee K, Feld G, Hendrickson J, Singh PN, Singh BN. Electrophysiologic and antiarrhythmic effects of sotalol in patients with life-threatening ventricular tachyarrhythmias. Circulation. 1985;72:555–564. doi: 10.1161/01.cir.72.3.555. [DOI] [PubMed] [Google Scholar]
- 50.Kuchar DL, Garan H, Venditti FJ, Finkelstein D, Rottman JN, McComb J, McGovern BA, Ruskin JN. Usefulness of sotalol in suppressing ventricular tachycardia or ventricular fibrillation in patients with healed myocardial infarcts. Am J Cardiol. 1989;64:33–36. doi: 10.1016/0002-9149(89)90648-6. [DOI] [PubMed] [Google Scholar]
- 51.Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH. Anatomy of the pig heart: Comparisons with normal human cardiac structure. J Anat. 1998;193(Pt 1):105–119. doi: 10.1046/j.1469-7580.1998.19310105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nanthakumar K, Walcott GP, Melnick S, Rogers JM, Kay MW, Smith WM, Ideker RE, Holman W. Epicardial organization of human ventricular fibrillation. Heart Rhythm. 2004;1:14–23. doi: 10.1016/j.hrthm.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Nash MP, Mourad A, Clayton RH, Sutton PM, Bradley CP, Hayward M, Paterson DJ, Taggart P. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation. 2006;114:536–542. doi: 10.1161/CIRCULATIONAHA.105.602870. [DOI] [PubMed] [Google Scholar]