Abstract
Methanol, ethylene glycol, and diethylene glycol intoxications can produce visual disturbances, neurological disturbances, acute renal failure, pulmonary dysfunction, cardiac dysfunction, metabolic acidosis, and death. Metabolic acidosis and an increased serum osmolality are important clues to their diagnosis. The former reflects the organic acids produced by metabolism of the parent alcohol, while the latter is due to accumulation of the offending alcohol. However, neither the clinical nor the laboratory findings are specific for toxic alcohol ingestions.
The definitive diagnosis of the alcohol intoxications is commonly based on detection of the alcohol or its metabolites in blood. Early diagnosis is important, because initiation of appropriate treatment can markedly lessen their morbidity and mortality.
At present detection of the parent alcohol in body fluids is inferred from its measurement in blood. This measurement is often performed by specialty laboratories using expensive equipment, and a long delay between obtaining the specimen and getting the results is not unusual. In this report, we describe liquid- based tests that detect methanol, ethylene glycol, diethylene glycol, and ethanol in saliva. The tests are sensitive and they have different specificity for each of the alcohols facilitating distinction among them. The relatively high sensitivity and specificity of the tests as a whole will facilitate the rapid diagnosis of each of these alcohol intoxications.
Keywords: methanol, ethylene glycol, diethyelene glycol, alcohol dehydrogenase, alcohol oxidase, sodium periodate, potassium permanganate
Introduction
Toxic alcohol intoxications including methanol, ethylene glycol, and diethylene glycol can lead to altered mental status and other neurological abnormalities, severe visual disturbances including blindness, acute renal failure, and death (1,2). Intoxications with one of these alcohols may be suspected in the presence of one or more of these clinical findings; however, none of the clinical findings are specific (2).
The coexistence of a high anion gap metabolic acidosis and hyperosmolality, producing a so-called osmolal gap are findings which can further support the diagnosis of a toxic alcohol intoxication (3). However, a high anion gap metabolic acidosis or hyperosmolality are not specific for these intoxications (2). Furthermore, patients with any of the intoxications can present with either a high anion gap metabolic acidosis or serum osmolal gap alone (2).
Definitive diagnosis of toxic alcohol ingestions is usually made by detection of the parent alcohol or one of its byproducts in blood (2,4,5). These studies often use sophisticated laboratory techniques such as gas chromatography (4,5,6), which are labor intensive and relatively expensive. Because of their cost, they are often not available in many hospital clinical laboratories (6,7). As a consequence, even when the diagnosis of one of the toxic alcohol ingestion is suspected, confirmation of the diagnosis can take as long as 48 hours, placing the patient at risk for many of the complications including death (2,8).
This delay in diagnosis is important, since early initiation of treatment such as hemodialysis to remove the alcohol and its byproducts and/ or administration of inhibitors of the critical enzyme alcohol dehydrogenase to prevent generation of its toxic byproducts has been shown to be one the most important factors in determining the clinical outcome (2,9). Given, the potential for high mortality with these intoxications and the difficulty in identifying them, it would be valuable to have available a screening test which is simple to use, relatively inexpensive, and the results of which can be obtained relatively rapidly.
The Alco-Screen (Chematics Inc, North Webster, IN) dipstick uses alcohol oxidase and peroxidase to detect ethanol in saliva. It is rapid and simple to use. However, although it can detect ethanol and methanol in blood and saliva (6,7), it cannot detect ethylene glycol at low enough concentrations to be clinically useful and presumably can not detect diethylene glycol, although this has not been examined.
In the present study, we report a group of liquid-based methods using easily obtainable reagents capable of detecting methanol, ethylene glycol, diethylene glycol, and ethanol in saliva. Using saliva eliminates the trouble of obtaining blood. Since the alcohols are lipid soluble, not protein-bound, non-ionized and have relatively low molecular weight, their concentration will be relatively close to that of blood as has been demonstrated for ethanol and methanol (10).
Thus, it should be possible to estimate the concentration of these substances in blood from their concentration in saliva. However, most importantly once identified in saliva, treatment can be initiated while awaiting results of more sophisticated tests to determine the precise concentration of the alcohols.
Materials and Methods
In preliminary studies serum was utilized. However, factors in the serum interfered with several of the determinations requiring processing of the serum before further study, a step which significantly extended the time required for completion of the study. In addition, it has been shown for several of the alcohols that their concentration in saliva approximates that in blood. Therefore, all subsequent studies were performed using saliva. Saliva was purchased from Innovative Research, Inc (Kalamazoo, Mi). All reagents used were of analytic-reagent grade or higher and were purchased from Sigma-Aldrich (St Louis, MO.). Distilled water and saliva served as controls. For studies examining detection of the alcohols in saliva, each of the alcohols was added to the saliva at known concentrations.
The studies described above were performed in accord with the ethical principles of research.
Methods for detection of alcohols
Identification of each of the alcohols in saliva is based on the detection of a product formed from reaction of the particular alcohol with either the enzymes alcohol oxidase or alcohol dehydrogenase or the oxidizing agents sodium periodate, or potassium permanganate as described below. The products formed from these reactions were readily detected by visual inspection and/ or spectrophotometric analysis after addition of one of the following colorimetric agents: (E) 2-hydrazone-3-methyl-2,3 dihydrobenzo[d]thiazole, HMDT; MBTH, 3- methyl-2-benzothiazoline hydrazone, or Purpald®, 4-amino-3-hydrazino-5- mercapto-1,2,4-triazole. For most of the methods, HMDT was used. In addition, Purpald was used as an alternative colorimetric agent with the alcohol oxidase method. Intensity of the color formed with all the methods was proportional to the concentration of the reaction product as described below. In all studies, color was examined separately by two individuals and the relative intensity scores were based on at least two separate observations.
Alcohol dehydrogenase method (ADH method)
β-Nicotinamide adenine dinucleotide (NAD) and yeast alcohol dehydrogenase (ADH) were purchased from Sigma. For each experiment, distilled H2O, or 0.1 ml of saliva, or 0.1 ml of saliva containing the appropriate alcohol at concentration ranging from 1 mg/dl to 100 mg/dl was first added to 0.7 ml of 20 mM sodium phosphate buffer (pH 8.8) followed by the addition of 0.1 ml of 15 mM NAD. To this mixture, the enzyme alcohol dehydrogenase (ADH)(45 units/0.1 ml) was added, incubated at room temperature for 20 min followed by addition of 1 ml 0.4% 3-methylbenzothiazolone hydrazone and a further 10 minute incubation. Finally a 1 ml of a solution containing 1% FeCl3 and 1.6% sulfamic acid was added. After 10 minutes the sample was examined visually and its absorbance was measured at excitation wavelength of 620 nm using a Shimadzu UV-VIS spectrophotometer model UV-1601. With this method, the total time from addition of the sample to completion of the analysis was approximately 40 minutes.
Alcohol oxidase method (ALOx method)
Alcohol oxidase (EC 1.1.3.13) prepared from Hansenula sp. was purchased from Sigma. Distilled H2O, 0.1 ml of saliva, or 0.1 ml of saliva containing the appropriate alcohol at concentration ranging from 1 mg/dl to 100 mg/dl was added to 0.1 ml of 20 mM sodium phosphate buffer (pH 7.5). To this mixture, 0.01 ml of the enzyme alcohol oxidase (0.046 units) was added and incubated at room temperature for 5 min. Deionized water (1 ml) and 0.4% 3-methylbenzothiazolone hydrazone (1 ml) were then added and kept for 5 min. Finally, 1 ml of a solution of 1% FeCl3 and 1.6% sulfamic acid was added and the reaction mixture was kept at room temperature for 10 min. The solution was then examined by visual inspection and the absorbance at an excitation wavelength of 620 nm was measured. Total time required for completion of analysis was 20 minutes.
Sodium periodate method
Distilled H2O, 0.05 ml of saliva sample, or saliva containing 0.05 ml of the appropriate alcohol was combined with 0.74 ml of water and added to 0.01 ml of 16 mM sodium periodate. The samples were kept for 10 min in the dark, followed by addition of 0.4% 3-methylbenzothiazolone hydrazone (0.1 ml) and incubation of the mixture for 5 min. A solution of 1% FeCl3 and 1.6% sulfamic acid (0.1 ml) was then added and the reaction mixture was kept at room temperature for and additional 5 min. The sample was then examined by visual inspection and the absorbance of the mixture was measured with the spectrophotometer at an excitation wavelength of 620 nm as described previously.
Potassium permanganate – PA method (KMnO4-PA method)
Distilled H20, 0.1 ml of saliva, or 0.1 ml of saliva containing the appropriate alcohol at concentrations ranging from 1 mg/dl were combined with 0.2 ml of water. This mixture was then added to 0.1 ml of 2% potassium permanganate solution and 0.05 ml of 5% phosphoric acid. The samples were mixed by vortexing and kept at room temperature for 10 min. The samples were then decolorized by adding 0.16 ml of 2.5% sodium bisulfate solution in water followed by addition of 1 ml of 0.4% 3-methylbenzothiazolone hydrazone and incubated at room temperature for 10 min. One milliliter of a solution of 1% FeCl3 and 1.6% sulfamic acid was added and after 20 minutes at room temperature, the samples were examined by visual inspection or the absorbance at an excitation wavelength of 620 nm was measured with the spectrophotometer.
Results
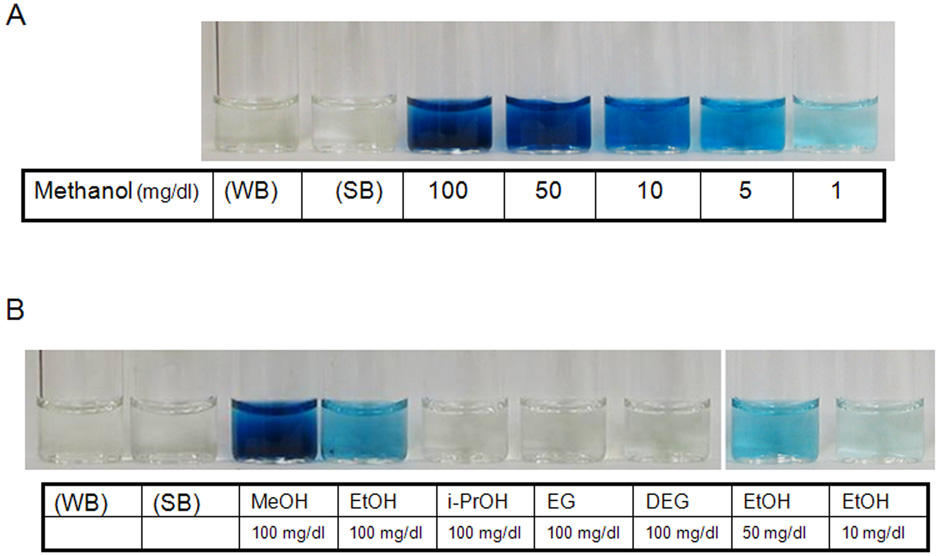
The results of typical experiments in which the alcohols were detected in saliva using the different methods are depicted in Figure 1 – Figure 4. The results of experiments using the alcohol oxidase method, the same method used by the ALCO-Screen are illustrated in Figure 1. As can be seen, addition of only 1 mg/dl of methanol to saliva resulted in a blue hue, while in the absence of the alcohol, water or saliva gave no detectable color change (Figure 1, panel A). Ethanol could also be detected with the alcohol oxidase method, but in contrast to methanol, in our hands significant color was detected only when the concentration was above 10 mg/dl, more than 10 fold greater than that seen with methanol. Ethylene glycol, diethylene glycol, and isopropanol were not detected with this method at concentrations up to 100 mg/dl (Figure 1, panel B).
Figure 1. An example of typical experiments using the alcohol oxidase method to detect the different alcohols in saliva.
Panel A) A light blue hue develops in the sample containing methanol at a concentration of 1 mg/dl. The intensity of the color increases proportionately with the increase in methanol concentration from 1 mg/dl to 100 mg/dl. WB, water negative control; SB, saliva negative control. Panel B) The alcohol oxidase method was used to detect methanol (MeOH), ethanol (EtOH), isopropanol (i-PrOH), ethylene glycol (EG), and diethylene glycol (DEG) in saliva. A strong blue hue was noted with methanol as before. A blue hue developed with ethanol at a concentration of 50 mg/dl. Isopropanol (i-PrOH), ethylene glycol (EG), and diethylene glycol (DEG) were not detected by this method even at concentration of 100 mg/dl.
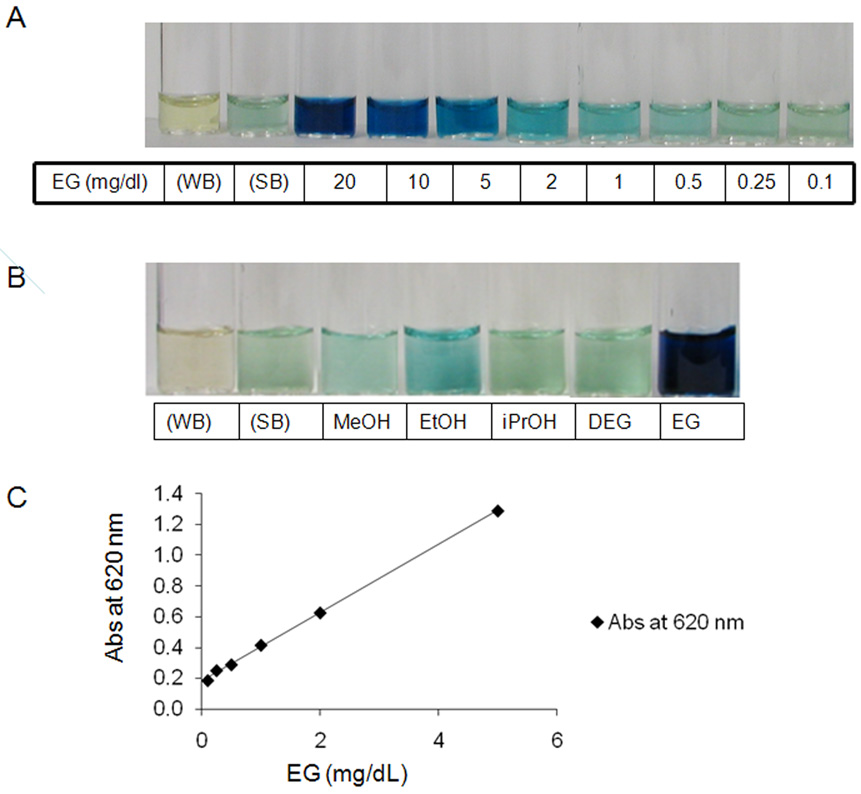
Figure 4. An example of typical experiments to detect the alcohols in saliva using the sodium periodate method.
Panel A) A light blue hue develops in the sample containing ethylene glycol at a concentration of 1 mg/dl. The intensity of the color increases proportionately with the increase in ethylene glycol concentration from 1 mg/dl to 100 mg/dl. WB, water negative control. SB, saliva negative control. Panel B) A light blue hue was noted with 50 mg/dl of ethanol, but methanol (MEOH), diethylene glycol (DEG), and isopropanol (iPrOH) at this same concentration were not different from the saliva control. Panel C) Relationship between ethylene glycol (EG) concentration and absorbance measured with a spectrophotometer. There is good linear relationship between absorbance and ethylene glycol concentration when it is < 10 mg/dl. Absorbance at 620 nm = 0.222 × EG (mg/dl) + 0.181 (R2 = 0.999)
Methanol and ethanol were also detected with the alcohol oxidase method when Purpald was used as the colorimetric agent (data not shown). As with the alcohol oxidase method when HMDT was used as the colorimetric agent, the sensitivity for detection of methanol was much greater than for ethanol.
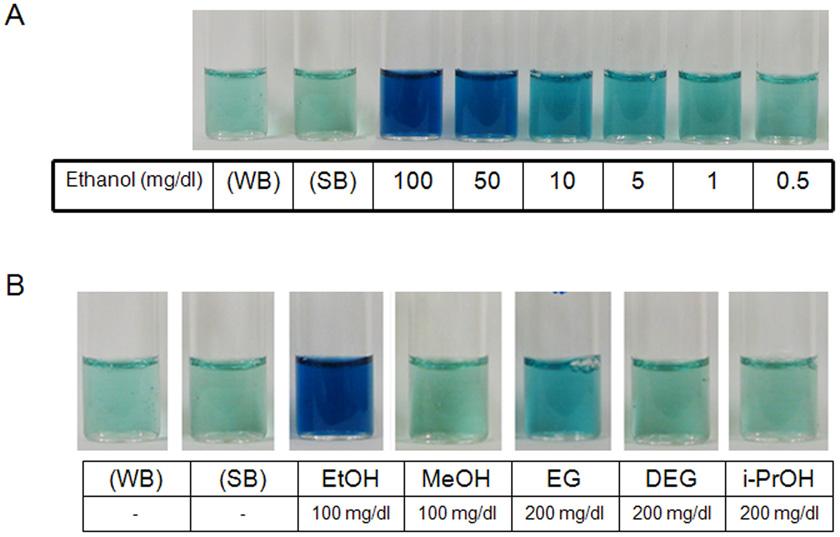
A common method of detecting ethanol is the alcohol dehydrogenase method. As depicted in panel A of figure 2, with this method, a blue hue is visibly perceived at a concentration of ~ 10 mg/dl and color intensity increased perceptively as its concentration rose to 50 mg/dl. A little difference of the color intensity could be detected on visual inspection between 50 and 100 mg/dl. By contrast, as shown in panel B, the presence in saliva of methanol at a concentration up to 100 mg/dl or isopropanol or diethylene glycol at a concentration of as much as 200 mg/dl produced no visually detectable color. When ethylene glycol was present at a very high concentration, ~ 200 mg/dl, there was some detectable increase in color intensity; however, this was considerably less than that observed at a four-fold lower ethanol concentration.
Figure 2. An example of typical experiments using the alcohol dehydrogenase method to detect the different alcohols in saliva.
Panel A) A light blue hue develops in the sample containing ethanol at a concentration of 1 mg/dl. The intensity of the color increases proportionately with the increase in ethanol concentration from 1 mg/dl to 100 mg/dl. WB, water negative control; SB, saliva negative control. Panel B) Comparison of the results with methanol (MeOH), ethanol (EtOH), isopropanol (i-PrOH), ethylene glycol (EG), and diethylene glycol (DEG) in saliva. The method was most sensitive for ethanol. Some slight increase in color intensity was detected with a ethylene glycol concentration of 200 mg/dl.
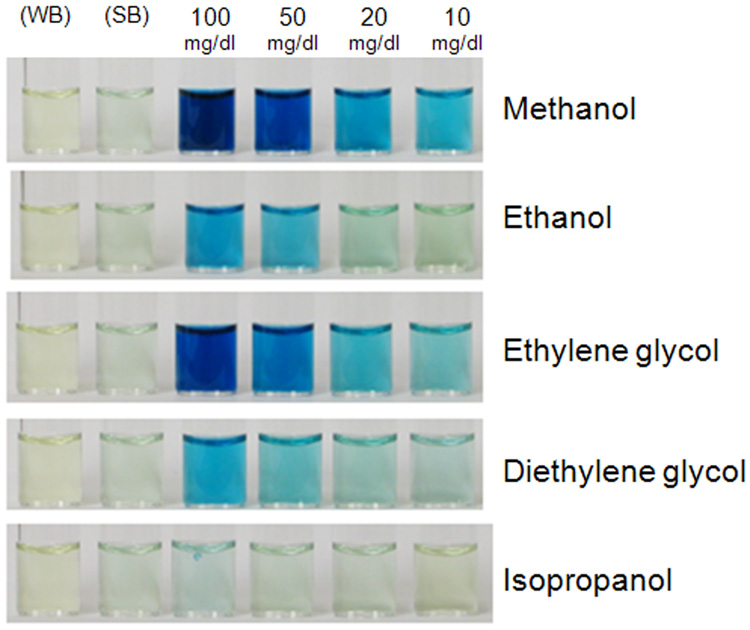
As depicted in Figure 3, ethanol, methanol, ethylene glycol, and diethylene glycol were all detected with the potassium permanganate method. However, this method was most sensitive in the detection of methanol with an order of sensitivity of methanol > ethylene glycol > diethylene glycol > ethanol. It is noteworthy to mention that at concentration ≥10 mg/dl, both methanol and ethylene glycol produced a detectable color; whereas, at this same concentration ethanol and diethylene glycol revealed little or no color. Isopropanol even at a concentration of 100 mg/dl was not detected by the permanganate method.
Figure 3. An example of a typical experiment using the permanganate-PA method to detect the different alcohols in saliva.
The concentration of the individual alcohols was varied from 10 to 100 mg/dl. Methanol and ethylene glycol produced a blue hue at a concentration of 10 mg/dl which increased in intensity at higher concentrations. A blue hue appeared with ethanol and diethylene glycol at a concentration ~ 50 mg/dl. No color was detected with the water (WB) and saliva (SB) controls and isopropanol.
As depicted in panel A of Figure 4, with the sodium periodate method, the presence of ethylene glycol in saliva at a concentration as low as 1 mg/dl provided a blue hue, the intensity of which increased with increasing concentration. With this method, no color was detected in the presence of ethanol till its concentration was above 20 mg/dl. By contrast, as depicted in Panel B, the presence of methanol, isopropanol, or diethylene glycol could not be detected even at concentrations as great as 50 mg/dl In addition to visual inspection of color which can be observer dependent, a spectrophotometer can be used to get a semi-quantitative assessment of the concentration of the individual alcohol in saliva. A typical concentration- absorbance curve for ethylene glycol is depicted in panel C of Figure 4. As can be seen, there is a linear relationship between absorbance and the alcohol concentration when the color intensity was under absorbance 1. A similar relationship is anticipated for the other methods as long as the absorbance is maintained below 1. Maintaining the absorbance below 1 can be achieved by dilution of the samples.
Table 1 summarizes the relative sensitivity and specificity of the different methods for detection of the major alcohol intoxications. The alcohol oxidase was most sensitive in the detection of methanol (> 1mg/dl) followed by the potassium permanganate method; whereas, the alcohol dehydrogenase and sodium periodate were the least sensitive being negative at relatively high concentrations of 50 mg/dl. The alcohol dehydrogenase method was most sensitive for the detection of ethanol followed by the potassium permanganate, alcohol oxidase, and sodium periodate methods.
Table 1.
Relative Value of Methods for Detection of Toxic Alcohols
| Alcohol | Alcohol | Alcohol | Sodium | Potassium |
|---|---|---|---|---|
| Dehydrogenase | Oxidase | Periodate | Permanganate Method | |
| Method | Method | Method | ||
| Ethanol | ++++ | ++ | + | +++ |
| Ethylene glycol | + | − | ++++ | ++++ |
| Diethylene glycol | − | − | − | +++ |
| Isopropanol | − | − | − | − |
| Methanol | − | ++++ | − | ++++ |
The sodium periodate method was most sensitive in the detection of ethylene glycol followed by the potassium permanganate method. Both the alcohol dehydrogenase method and alcohol oxidase method were insensitive in the detection of this alcohol. Diethylene glycol could be detected by the potassium permanganate method, but it was not detected with any of the other methods even at a high concentration.
Isopropanol most commonly found in rubbing alcohol is often considered in the differential diagnosis of the toxic alcohol syndromes (1,2). Addition of isopropanol to saliva was not detected with all the tests even at a concentration of 100 mg/dl.
Discussion
Methanol, ethylene glycol, diethylene glycol are toxic alcohols which are important causes of poisoning of children and adults alike (1,2,11,12). The reported mortality in the United States arising from methanol and ethylene glycol intoxication is relatively low ~ 1% (12). However, the mortality rises strikingly if there is a significant delay in initiating proper treatment (13). The mortality of diethylene glycol is greater (as high as 70%) reflecting difficulty in making the diagnosis in a timely fashion (2,11).
In the absence of a history of ingestion of any of these alcohols, the diagnosis of these intoxications can be suspected in individuals with changes in mental status associated with visual disturbances as with methanol intoxication (2,4), or acute renal failure with ethylene glycol and diethylene glycol intoxication (2,5). The laboratory findings of an increased serum osmolality and high anion gap metabolic acidosis can also suggest the presence of these intoxications (1,2). However, the clinical findings described above are not specific for any of these disorders. Moreover, the presence and degree of hyperosmolality and metabolic acidosis may depend upon the time after ingestion blood is obtained for analysis (2,3). Early after ingestion, the serum osmolality may be elevated with little evidence of metabolic acidosis (3). Consequently, as the alcohol is metabolized to organic acids, serum osmolarilty can fall appreciably while the acidosis becomes more profound. The characteristic combination of hyperosmolality and metabolic acidosis may not be present in some cases (2,3). Moreover, measurement of serum osmolality is not done on a routine basis in clinical laboratories, even in patients with changes in mental status or suspected poisoning making the diagnosis of these alcohol intoxications problematic.
Fluorescein is commonly added to some brands of antifreeze containing ethylene glycol to make detection of leaks from cars more visible (14,15). Examination of the urine with a Woods lamp to detect the fluorescence has been suggested as another method of diagnosing this intoxication (14,15). However, false positives and negatives have been reported with this test lessening the value of the urine examination (14,15).
Actual confirmation of one of these intoxications is generally accomplished by measurement of the alcohol or its toxic metabolites in blood. A test for blood ethanol or saliva levels is inexpensive and easy to perform. However, measurement of methanol, ethylene glycol, and diethylene glycol or their organic acid metabolites is usually done with blood and requires sophisticated equipment such as gas chromatography (4,5). As a consequence, this test cannot be done in many clinical laboratories and blood has to be sent to a specialty laboratory. As reported by one of the authors, it can take 48 hours or longer to get the results (8). A long delay before recognition is important because treatment of the intoxications to be effective should be initiated within several hours or less after ingestion to prevent serious adverse consequences (2,4,5,13). Therefore, there is a need for a rapid screening test to diagnose these intoxications.
A simple saliva test for semi-quantitative determination of ethanol is available, the ALCO-Screen (6,7). The method is based on the alcohol oxidase method. The dipstick has one reactive area impregnated with alcohol oxidase and a dye that allows a colorimetric determination of ethanol concentration in both blood and saliva. can also detect in blood concentrations of methanol as low as ~ 5 mg/dl (7). However, it can only detect very high concentrations of ethylene glycol ~ 300 mg/dl (7), whereas toxicity is reported at blood concentration < 10 fold of this concentration. Also, based on our studies using the alcohol oxidase method, this would not detect diethylene glycol at concentrations that would require treatment. Finally, the AlCO-Screen test will be positive in the presence of either ethanol or methanol, and therefore would not be useful in cases of coingestion of both moities.
In the present study, we combined two enzyme-based methods (alcohol oxidase and alcohol dehydrogenase) and two that used oxidizing agents (sodium periodate and potassium permanganate). The inclusion of these methods allowed us to detect all three important alcohol intoxications: methanol, ethylene glycol, and diethylene glycol, and also ethanol and isopropanol. The latter two entities often need to be excluded in evaluating patients with possible toxic alcohol intoxications (2,8). Therefore, utilization of the separate methods substantially improves the specificity and sensitivity for detection of all the important toxic alcohol intoxications.
The methods utilize easily obtainable and relatively inexpensive reagents and no sophisticated equipment. All the studies can be completed within 40 minutes and thus can be performed either in a clinical facility or even outside the facility as the patient is being transported. Thus, these studies can be done at little expense in many areas without access to sophisticated equipment, including undeveloped countries. This is important as outbreaks of several of the intoxications are reported in third world countries (2).
In preliminary studies, blood was utilized. However, substances in blood interfered with some of the methods requiring special processing of the samples. Utilization of saliva eliminated this problem.
Although we did not examine all possible interfering substances that might be present in saliva, other reports have shown high glucose concentrations interfere with the sodium periodate method. Therefore, in patients with significant hyperglycemia, a positive test with this method would have to be confirmed by other methods.
For the purposes of these studies, each of the alcohol was added to saliva in known concentrations rather than obtaining saliva from animals or patients with known toxic alcohol intoxications. Based on studies of the relationship between salivary and blood concentrations of ethanol and methanol, salivary concentrations of these alcohols have been found to be close to or slightly less than that of blood (6,10). The other toxic alcohols, ethylene glycol and diethylene glycol are also lipid soluble, not protein-bound, and non-ionized. Movement into saliva from plasma will therefore occur by simple diffusion. The only factor that will affect the rate of diffusion and therefore the relative concentrations of substances in blood and saliva will be their molecular weight. Moreover, ther molecular weight of ethylene glycol 62 daltons and diethylene glycol 106 daltons are sufficiently low so that transfer from blood into saliva should be rapid and their concentrations should not be much dissimilar from that of blood. (10, 17).. However, the precise relationship between blood and saliva concentrations for ethylene glycol and diethylene glycol will have to be established.
Importantly, very small quantity of saliva was required for these studies, namely 0.1 ml. This quantity of saliva should be easy to obtain even from an unconscious or impaired patient, by swabbing the mouth with one of the saliva collection systems (6). Secondly, each of the tests has different sensitivity and or specificity for the detection of the various alcohols. Therefore, by combining the results of the 4 methods, the clinician should come up with a reasonable determination of which alcohol is present.
In a substantial number of patients, ethanol and one of the other toxic alcohols might be present in body fluids if the intoxication results from ingestion of adulterated ethanol (2,4,5). In these cases, examining the results of the tests should enable the clinician to document both the presence of the other alcohols and ethanol.. Except for ethanol, toxic alcohol intoxications are often treated with dialysis to remove the offending alcohol and/or addition of fomepizole a specific inhibitor of the alcohol dehyrogenase to prevent generation of the toxic organic acids (2,4,5). Treatment is recommended when blood concentrations are ~ 20 mg/dl and dialysis has been recommended with values of 50 mg/dl or greater (2,4,5). Based on the sensitivity of the methods, these levels should easily be detected with the methods described.
In some instances, the parent alcohol is completely metabolized to its organic acids. Under these circumstances, methods described here will not be able to diagnose any of the intoxications. Of course, the same conclusion holds for most of the methods using blood to detect the parent alcohol. Examination of the literature suggests this is a relatively rare event, but is a limitation of the present methodology (2,3,4)..
As noted, each of the methods has a different sensitivity and specificity for the various alcohols. Therefore examination of the results of all the tests should allow the clinician to suspect which of the intoxications is present. Quantitation of body fluid concentrations then could be accomplished with the more sophisticated techniques. Appropriate treatment could then be initiated.
In summary, we describe 4 liquid-based methods to detect the major alcohol intoxications that use extremely minute quantities of saliva, can be performed with easily obtainable and inexpensive reagents, and can be completed within 30– 40 minutes. Since a colorometric method is utilized, documentation of the presence of the alcohol in saliva can be established by visual inspection alone. Subsequently, estimates of actual concentrations of the alcohols could be obtained using a spectrophotometer sufficient to initiate therapy, since intensity of the color formed is proportional to the concentration of the alcohol. Once the screening test suggests the presence of one of the alcohols,, more precise measurements of the alcohol concentration can be obtained.
Acknowledgements
These studies were supported by funds from Veterans Administration. Dr Shin was supported in part by NIH grants DK058333 and DK053642.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abramson S, Singh AK. Treatment of the alcohol intoxications: ethylene glycol, methanol and isopropanol. Curr Opin Nephrol Hypertens. 2000;9:695–701. doi: 10.1097/00041552-200011000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Kraut JA, Kurtz I. Toxic alcohol ingestions: clinical features, diagnosis, and management. CJASN. 2008;3:208–225. doi: 10.2215/CJN.03220807. [DOI] [PubMed] [Google Scholar]
- 3.Hovda KE, Hunderi OH, Rudberg N, Froyshov S, Jacobsen D. Anion and osmolal gaps in the diagnosis of methanol poisoning: clinical study in 28 patients. Intensive Care Med. 2004;30:1842–1846. doi: 10.1007/s00134-004-2373-7. [DOI] [PubMed] [Google Scholar]
- 4.Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol. 2002;40:415–446. doi: 10.1081/clt-120006745. [DOI] [PubMed] [Google Scholar]
- 5.Barceloux DG, Krenzelok EP, Olson K, Watson W. American academy of clinical toxicology practice guidelines on the treatment of ethylene glycol poisoning. Jf Toxicol Clin Toxicol. 1999;37:537–560. doi: 10.1081/clt-100102445. [DOI] [PubMed] [Google Scholar]
- 6.Hack JB, Chiang WK, Howland MA, Patel H, Goldfrank LR. The utility of an alcohol oxidase reaction test to expedite the detection of toxic alcohol exposures. Acad Emerg Med. 2000;7:294–297. doi: 10.1111/j.1553-2712.2000.tb01082.x. [DOI] [PubMed] [Google Scholar]
- 7.Hack JB, Early J, Brewer KL. An alcohol oxidase dipstick rapidly detects methanol in the serum of mice. Acad Emerg Med. 2007;14:1130–1134. doi: 10.1197/j.aem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Rastogi A, Tagaki B, Bajwa M, Kraut JA. Spurious Elevation in Serum Creatinine Caused by Ingestion of Nitromethane: Implication for the Diagnosis and Treatment of Methanol Intoxication. Am J Kidney Dis. 2008 doi: 10.1053/j.ajkd.2007.12.010. (In Press) [DOI] [PubMed] [Google Scholar]
- 9.Liu JJ, Daya MR, Carrasquillo O, Kales SN. Prognostic factors in patients with methanol poisoning. J Toxicol Clin Toxicol. 1999;36:175–180. doi: 10.3109/15563659809028937. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman E, Lamster IB. The diagnostic applications of saliva - A review. Crit Rev in Oral Biol & Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien KL, Selanikio JD, Hecdivert C, Placide MF, Louis M, Barr DB, et al. Epidemic of pediatric deaths from acute renal failure caused by diethylene glycol poisoning. JAMA. 1998;279:1175–1180. doi: 10.1001/jama.279.15.1175. [DOI] [PubMed] [Google Scholar]
- 12.Litovitz TL, Klein-Schwartz W, White S, Cobaugh DJ, Youniss J, Drab A, Benson BE. 1999 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2000;18:517–574. doi: 10.1053/ajem.2000.9261. [DOI] [PubMed] [Google Scholar]
- 13.Hovda KE, Hunderi OH, Tafjord AB, Dunlop O, Rudberg N, Jacobsen D. Methanol outbreak in Norway 2002–2004: epidemiology, clinical features and prognostic signs. J Intern Med. 2005;258:181–190. doi: 10.1111/j.1365-2796.2005.01521.x. [DOI] [PubMed] [Google Scholar]
- 14.Winter ML, Ellis MD, Snodgrass WR. Urine fluorescence using a Wood's lamp to detect the antifreeze additive sodium fluorescein:a qualitative adjunctive test in suspected ethylene glycol ingestions. Ann Emerg Med. 1990;19:663–667. doi: 10.1016/s0196-0644(05)82472-2. [DOI] [PubMed] [Google Scholar]
- 15.Parsa T, Cunningham SJ, Wall SP, Almo SC, Crain EF. The usefulness of urine fluorescence for suspected antifreeze ingestion in children. Am J Emerg Med. 2005;23:787–792. doi: 10.1016/j.ajem.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Megarbane B, Borron SW, Baud FJ. Current recommendations for treatment of severe toxic alcohol poisonings. Intensive Care Med. 2005;31:189–195. doi: 10.1007/s00134-004-2521-0. [DOI] [PubMed] [Google Scholar]
- 17.Tu G, Kapur B, Israel Y. Characteristics of a new urine, serum, and seliva alcohol reagent strip. Alcohol Clin Exp Res. 1992;16:222–227. doi: 10.1111/j.1530-0277.1992.tb01367.x. [DOI] [PubMed] [Google Scholar]