Abstract
The ERN is a negative deflection in the event-related potential that peaks approximately 50 ms after the commission of an error. The ERN is thought to reflect early error-processing activity of the anterior cingulate cortex (ACC). First, we review current functional, neurobiological, and developmental data on the ERN. Next, the ERN is discussed in terms of three psychiatric disorders characterized by abnormal response monitoring: anxiety disorders, depression, and substance abuse. These data indicate that increased and decreased error-related brain activity is associated with the internalizing and externalizing dimensions of psychopathology, respectively. Recent data further suggest that abnormal error-processing indexed by the ERN indexes trait- but not state-related symptoms, especially related to anxiety. Overall, these data point to utility of ERN in studying risk for psychiatric disorders, and are discussed in terms of the endophenotype construct.
Research has begun to identify risk factors for psychiatric disorders. For example, having a parent with the disorder is a significant risk factor for anxiety disorders (Beidel & Turner, 1997), depression (Downey & Coyne, 1990), and substance abuse (Hartman, Lessem, Hopfer, Crowley, & Stallings, 2006). Personality factors are also a strong predictor for developing psychiatric disorders. For example, individuals reporting high levels of negative affect and the related personality trait of neuroticism report more anxiety and depressive symptoms (Jylha & Isometsa, 2006) and are commonly diagnosed with anxiety disorders (Hettema, Prescott, & Kendler, 2004), and major depressive disorder (Brown, Chorpita, & Barlow, 1998; Schmitz, Kugler, & Rollnik, 2003). In contrast, novelty seeking is a personality trait that is characterized by impulsivity and sensation seeking, and is a significant predictor for developing substance use disorders (Sher, Bartholow, & Wood, 2000).
High heritability rates of these disorders suggest that genetics play an important role in their etiology (Hicks, Krueger, Iacono, McGue, & Patrick, 2004; Kendler, Gardner, Gatz, & Pedersen, 2007; Middeldorp, Cath, Van Dyck, & Boomsma, 2005). However, isolating genetic causes of these potentially heterogeneous and complex diseases had proved to be difficult (Chakravarti & Little, 2003; Lewis, 2002). Recently, there is increasing focus on identifying neural and information-processing abnormalities that may place individuals at risk for developing psychopathology. One way to do this is by studying endophenotypes, which are unobservable characteristics that mediate the relationship between genes and a given behavioral phenotype (Gottesman & Gould, 2003). Insofar as endophenotypes are less complex and heterogeneous than the associated disorder, endophenotypes might be determined by fewer genes and be more amenable to study.
The present paper will focus on neural activity related to action monitoring that is measured with event-related potentials (ERPs)—in particular, brain activity that indexes the on-going monitoring of correct and incorrect behavior. This review will concentrate on error-related negativity (ERN), focusing on its neural correlates and functional significance. The ERN will then be discussed in relation to psychiatric disorders and related personality traits. These data suggest that increased action monitoring indexed by the ERN might serve as a putative risk factor for anxiety and depression, whereas reduced ERNs might relate more to substance abuse disorders. Finally, these data are discussed in terms of the endophenotype construct, and future directions for relevant research are discussed.
Response Monitoring
Response monitoring tasks
The present paper focuses on ERP data recorded during a number of different speeded response tasks, including the Erikson Flankers task, the Simon task, color Stroop task, and the Go/No-Go task. In the arrows version of the Erikson Flankers task, participants respond to the direction of a central arrowhead which is flanked by either compatible (e.g., < < < < <) or incompatible (e.g., < < > < <) arrowheads (Eriksen & Eriksen, 1974). Similarly, in the Simon task, participants respond to a red circle with their left index finger and to a blue circle with their right index finger (Simon, 1969). Stimuli are presented either to the right or to the left of the center fixation cross, leading to congruent (e.g., red circle on the left side) or incongruent (e.g., red circle on the right side) trials. In the color Stroop task, names of colors are presented on a screen and participants are asked to respond to the color of the word. On congruent trials, the color of the target word matches the word (i.e., the word “red” is presented in red font), on incongruent trials, the color of the target word does not match the word (i.e., the word “red” presented in green font); on neutral trials, the word does not map directly to either response (i.e., the word “blue” presented in red font). Finally, the Go/No-Go task requires participants to respond to a target (i.e., Go stimulus) and withhold their response to a non-target distracter (i.e., No-Go stimulus).
Participants typically perform between 500 – 1500 trials in these tasks in relatively rapid succession. In all of these tasks, errors are relatively rare and are characterized by fast reaction times compared to correct responses (Rabbitt, 1966). This suggests that errors are due to impulsive responding, prior to complete processing of the stimulus (Rabbitt & Vyas, 1981). Interestingly, reaction times on correct trials after errors are typically slower than the average reaction time on correct trials. It is thought that such post-error slowing occurs in order to increase the likelihood of a correct response on the subsequent trial (Hajcak, McDonald, & Simons, 2003b; Rabbitt, 1981).
Error-related negativity and related components
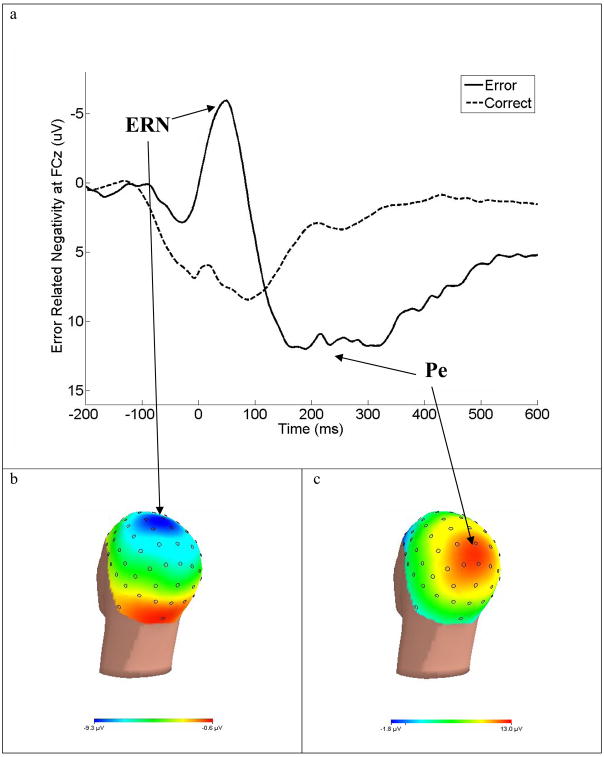
When participants make errors in these speeded response tasks, an ERP component, the error-related negativity (ERN)1, presents as a negative deflection approximately 50–100 ms following the erroneous response (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; W.J. Gehring, Goss, Coles, Meyer, & Donchin, 1993). Typical response-locked ERPs for error and correct trials are presented in Figure 1a. The ERN has been observed across tasks that employ a variety of stimulus and response modalities (Bernstein, Scheffers, & Coles, 1995; Holroyd, Dien, & Coles, 1998; Van’t Ent & Apkarian, 1999) and task difficulty (Band & Kok, 2000; Mathalon et al., 2003; Mathewson, Dywan, & Segalowitz, 2005; Moser, Hajcak, & Simons, 2005; Themanson, Hillman, & Curtin, 2006). The ERN is typically measured at midline frontal or central sites where the ERN is largest (i.e., FCz); Figure 1b presents the typical scalp distribution of the ERN. It is hypothesized that the anterior cingulate cortex (ACC) is the generator of the ERN as evidenced by studies using source localization (Dehaene, Posner, & Tucker, 1994; Holroyd et al., 1998; van Veen & Carter, 2002), magnetoencephalography (W. H. Miltner et al., 2003), and intracerebral recording (Brazdil, Roman, Daniel, & Rektor, 2005). Additional confirmation of the relationship between the ERN and the ACC stems from human lesions studies showing that patients with ACC lesions have diminished ERNs (Stemmer, Segalowitz, Witzke, & Schonle, 2004) and from single-unit studies that show increased error potentials in the anterior cingulate cortex in monkeys (Gemba, Sasaki, & Brooks, 1986; Ito, Stuphorn, Brown, & Schall, 2003).
Figure 1.
The response locked ERPs for error and correct trials at FCz, where the ERN was maximal (a). The response onset occurred at 0 msec and negative is plotted up. Scalp topography of error-related brain activity from 0 to 100 msec post-response (b). Scalp topography of error positivity from 200 to 400 msec post-response (c).
Interestingly, a small ERN-like component is sometimes evident on correct response trials (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Ford, 1999; Gehring & Knight, 2000; Scheffers & Coles, 2000; Vidal, Hasbroucq, Grapperon, & Bonnet, 2000). Because of its similarity to the ERN, this component has been referred to as the correct response negativity (CRN; Ford, 1999). Authors have suggested that CRN reflects a response comparison process (Falkenstein et al., 2000; Vidal et al., 2000), an emotional reaction (Luu, Collins, & Tucker, 2000), uncertainty of a correct response (Coles, Scheffers, & Holroyd, 2001; Pailing, Segalowitz, Dywan, & Davies, 2002), or coactivation of correct and incorrect responses (Luu et al., 2000; Scheffers, Coles, Bernstein, Gehring, & Donchin, 1996; Vidal et al., 2000).
Studies have consistently found that the ERN amplitude is larger when subjects make fewer errors (Amodio, Jost, Master, & Yee, 2007; Amodio, Master, Yee, & Taylor, 2007; Gehring et al., 1993; Hajcak et al., 2003b). Infrequent errors might make error commission especially significant, thus altering the amplitude of error signaling. Differences in subjects’ performance may thus pose a confound when comparing ERP across groups with different error rates and reaction times (Hajcak, Vidal, & Simons, 2004; Yeung, 2004). That is, if a group of participants has larger ERNs than a control group, and commit fewer errors than the control group, it is possible that the between-group behavioral differences might account for variation in the ERN. However it is possible to control subjects’ performance by providing feedback throughout the task (e.g., Hajcak & Foti, in press) or by only comparing subjects who are matched on performance (e.g., Tsai, Young, Hsieh, & Lee, 2005).
Trials with large ERN amplitudes have also been associated with increased post-error slowing (Debener et al., 2005; Gehring, Goss, Coles, Meyer, & Donchin, 1993). However, not all studies have replicated this pattern of results (Gehring & Fencsik, 2001; Scheffers, Humphrey, Stanny, Kramer, & Coles, 1999). Interestingly, studies show that it is possible for subjects to make errors that they are unaware of – and these unaware errors are associated with normal ERNs but reduced post-error slowing (Endrass, Reuter, & Kathmann, 2007; Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001). Therefore, post-error slowing appears to depend on error awareness, whereas the ERN does not.
Functional Significance of the ERN
Several theories and computational models have been developed regarding the functional significance of the ERN. Some suggest that the ERN reflects the error-detection process (e.g., Falkenstein et al., 1991; Falkenstein et al., 2000; Nieuwenhuis et al., 2001; Scheffers et al., 1996), an error signal at the remedial action system (e.g., Holroyd & Coles, 2002), the conflict-detection process (e.g., Yeung, Cohen, & Botvinick, 2004), or a more emotionally- or motivationally-relevant response to errors (Bush, Luu, & Posner, 2000; Gehring & Willoughby, 2002; Pailing et al., 2002).
Mismatch Theory
According to the early mismatch theory, the ERN represents the detection of a mismatch between the representations of the actual and intended response (Bernstein et al., 1995; Coles et al., 2001; Falkenstein et al., 1991). Thus, ERN amplitude should be directly related to the degree of mismatch between the correct and actual response. Indeed, studies confirm that subjects have larger ERNs when the error response and the correct response are more dissimilar (Bernstein et al., 1995; Falkenstein, Hohnsbein, & Hoormann, 1995; Scheffers et al., 1996). This mismatch should also be larger when subjects are more certain of their errors. In fact, studies have shown that the ERN amplitude is increased when subjects are sure that they made an error, regardless of whether or not they actually make a mistake (Scheffers & Coles, 2000).
Reinforcement and Learning-based Theories
One of the more prominent computational models, based in part on the mismatch theory, is the reinforcement learning theory of the ERN (RL-ERN; Holroyd & Coles, 2002). According to the RL-ERN, the basal ganglia monitor information from both the environment (external) and self-generated actions (internal), and evaluate on-going events based on learned expectations (Holroyd & Coles, 2002). The RL-ERN is rooted in non-human animal work indicating that the basal ganglia induce an increase or decrease in phasic midbrain dopamine (DA) activity, when events are better or worse than expected, respectively (for review see Barto, 1995; Houk, Adams, & Barto, 1995; Schultz, 2002). The RL-ERN theory proposes that the ERN is the result of disinhibition of the ACC by DA neurons signaling events as worse than anticipated. From this perspective, error signals are important for learning because they are used to predict future rewards and non-rewards and to modify ongoing behavior (Barto, 1995; Montague, Dayan, & Sejnowski, 1996; Schultz, 2002).
Consistent with the RL-ERN’s hypothesized role of DA in the generation of the ERN, administration of the DA agonist D-amphetamine leads to an increase in the ERN amplitude (de Bruijn, Hulstijn, Verkes, Ruigt, & Sabbe, 2004), whereas the administration of the DA antagonist haloperidol leads to a decrease in the ERN amplitude (de Bruijn, Sabbe, Hulstijn, Ruigt, & Verkes, 2006; Zirnheld et al., 2004). Demonstrating the specificity of DA neurotransmitter levels on the ERN, one study found that the selective serotonin reuptake inhibitor paroxetine had no effect on ERN amplitude (de Bruijn et al., 2006).
It is likely that changes in baseline DA levels are important for understanding the effects of dopaminergic agents on the ERN. This has been proposed in a new computational model, which builds upon the RL-ERN model, and states that basal DA levels can have a significant effect on reinforcement learning due to possible range restrictions on phasic DA bursts (Frank, 2005). For example, individuals with Parkinson’s disease who are being treated with DA agonists may have elevated tonic DA levels that prevent effective phasic dips, which could hinder negative reinforcement learning. Since these phasic dips characterize the ERN signal, the ERN amplitude would be diminished in Parkinson’s disease patients, which is consistent with the literature (Falkenstein, Hielscher et al., 2001; Ito & Kitagawa, 2006; Stemmer, Segalowitz, Dywan, Panisset, & Melmed, 2007).
Conflict Monitoring Theory
Both the mismatch theory and the RL-ERN theory provide significant insight into the function of the ERN; however, in the context of these theories, it is unclear why an error occurs in the first place, especially if the correct representation is present at the time of the error (cf. Yeung et al., 2004). Another computational model, the conflict monitoring theory, addresses this concern by focusing on conflict detection rather than error detection, per se. The conflict monitoring theory is based on the idea that the role of the ACC is to monitor conflict during response selection and that errors are simply a specific example of response conflict that occurs between an erroneous and error-correcting response (Yeung et al., 2004). Specifically, both error and correct response representations are dynamically activated as a result of continued stimulus processing; on error trials, an error is therefore followed by an error-correcting response, which elicits conflict between the error and the error-correcting correct response (Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988; Rabbitt, 1981; Yeung et al., 2004). From this point of view, errors are simply trials on which response conflict crosses some threshold. The conflict monitoring theory identifies the ACC as the neural seat of conflict monitoring. In support of this, neuroimaging studies show that the ACC is activate not only following errors, but also on correct trials that are incongruent and elicited high response conflict (Bench et al., 1993; Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Carter et al., 1998; Carter et al., 2000; MacDonald, Cohen, Stenger, & Carter, 2000; Pardo, Pardo, Janer, & Raichle, 1990).
A number of investigators has also compared the ERN to a negative deflection in the stimulus-locked ERP: the N2 peaks between 200–400 msec after stimulus presentation, and thus generally occurs prior to the execution of a response. It is most pronounced on trials with high response conflict, and is evident in the incongruent minus congruent stimulus-locked difference waveform on correct trials. For example, in the Flankers task, high response conflict occurs when the flanking arrows are in the opposite direction as the center arrow (< < > < <). The N2 is thought to reflect the coactivation of the correct and incorrect response on correct trials (Falkenstein, Hoormann, & Hohnsbein, 1999; Yeung et al., 2004). Some source localization studies show that the N2 and the ERN have similar scalp topographies and a common neural source (caudal ACC), but they occur at different times relative to the response. That is, N2 occurs prior to correct responses and the ERN occurs following erroneous responses (Carter et al., 1998; van Veen & Carter, 2002; van Veen, Cohen, Botvinick, Stenger, & Carter, 2001; van Veen, Holroyd, Cohen, Stenger, & Carter, 2004; Yeung et al., 2004). Collectively, these results raise the possibility that ERN-like activity might reflect both error-detection and the more general detection of response conflict on correct trials.
The original version of the conflict monitoring theory does not account for the CRN. In the conflict monitoring theory, increased conflict should occur prior to the response on correct trials but after the response on error trials (Yeung et al., 2004). However, it has recently been suggested that conflict might occur at multiple levels. Van Veen and Carter (2005) studied both stimulus and response conflict while participants performed a Stroop task. They found that although the ACC was active during both forms of conflict, specific regions of the ACC differed, which suggests that different forms of conflict processing occur in parallel. Therefore, CRN might be related to conflict at the response level. In fact, work by Bartholow and colleagues indicates that incompatible trials yield a larger CRN amplitude than compatible trials (Bartholow et al., 2005). Thus, the relatively new suggestion that conflict may influence ERPs at multiple levels of stimulus and response processing appears to allow for the possibility that the ERN and CRN, as well as the stimulus-locked N2, reflect conflict monitoring.
Motivational Significance Theory
A major shortcoming of existing computational models is that they do not account for motivational and individual differences. Errors are motivationally salient events that prompt psychophysiological changes that include skin conductance and heart rate deceleration (Hajcak et al., 2003b; Hajcak, McDonald, & Simons, 2004). Although these peripheral responses are consistent with both a defensive and orienting response, a recent study from our lab found that the defensive startle reflex was larger following errors than correct responses (Hajcak & Foti, 2008). In fact, Hajcak and Foti found that the ERN predicted degree to which the startle reflex was potentiated after errors. Collectively, these data suggest that the ERN might reflect error-detection that is utilized for motivational ends. Thus, amplitude of the ERN might relate to the significance of an error. This possibility was first suggested by Gehring and colleagues (1993) who reported that the amplitude of the ERN was larger when an error was made when participants were told to be more accurate, whereas the ERN was smaller when participants were told to respond faster. Therefore, by emphasizing accuracy, the authors could have made errors more salient which in turn could have made them more important or negative (Hajcak, Moser, Yeung, & Simons, 2005).
Additional studies suggest a role for motivational significance in modulating amplitude of the ERN. One study used two different paradigms to assess motivational effects on ERN amplitude: a Flankers task in which subjects could either win or lose 5 or 100 points, and a Flankers task administered under conditions of performance evaluation (Hajcak et al., 2005). In both of these experiments, errors associated with a motivationally significant context (i.e., losing 100 points or being evaluated) coincided with a larger ERN amplitude compared to the other conditions. More recent studies replicate these results and indicate that more valuable or significant errors result in a larger ERN (Chiu & Deldin, 2007; Kim, Iwaki, Uno, & Fujita, 2005).
The motivational significance view of the ERN is often discussed in terms of affective processes as well. Vidal and colleagues (2000) suggested that the ERN may be influenced by an individual’s emotional reaction to errors. Interestingly, one study found that motivational significance was only influential in the magnitude of the ERN in individuals with certain personality traits (Pailing & Segalowitz, 2004). For example, individuals rating high on conscientiousness and low on neuroticism had smaller motivation-related effects on ERN amplitude.
Error-Monitoring and Individual Differences
Error monitoring and age
A number of studies has examined developmental changes in the ERN. In a large study that examined the ERN in children from age 7 to 18, Davies and colleagues did not find an observable ERN in children younger than 12 (Davies, Segalowitz, & Gavin, 2004a, 2004b). Contrary to this, other studies have reported ERNs in children ranging in age from 7 to 11 (Hajcak, Franklin, Foa, & Simons, 2007; Kim, Iwaki, Imashioya, Uno, & Fujita, 2007; Wiersema, van der Meere, & Roeyers, 2007). Preliminary data from our lab provides evidence that ERNs are even present in children as young as 5, provided simple tasks are employed (Torpey, Hajcak, & Klein, 2007). In regard to developmental trends in ERN amplitude, several researchers have reported smaller ERN amplitudes in children (ages 7–18) compared to adults (Kim et al., 2007; Wiersema et al., 2007). The ERN has also been correlated with age, suggesting that it increases with development (Hajcak et al., 2007).
One study showed that adolescents (ages 13 – 14) have comparable ERN amplitudes to adults (ages 23 – 24; Wiersema et al., 2007). On the other hand, Ladouceur and colleagues (2004) found that the ERN amplitude was larger in late adolescents (ages 14 – 17) compared to early adolescents (ages 9 – 14). Overall, these findings suggest that changes in ERN amplitude reflect developmental changes in the brain, possibly reflecting the continued maturation of the medial prefrontal cortex, with increasing ERN amplitude through childhood and adolescence that plateaus in late adolescents or early adulthood (Davies et al., 2004a, 2004b)
In older adults (ranging between ages 54 – 85), studies have consistently found decreased ERN amplitude when compared to young adults (ranging between ages 18–28; Band & Kok, 2000; Falkenstein, Hoormann, & Hohnsbein, 2001; Mathalon et al., 2003; Mathewson et al., 2005; Nieuwenhuis et al., 2002; Themanson et al., 2006). It is important to note, though, that some of these findings may be confounded by behavioral differences, such as decreased accuracy in older adult groups (Band & Kok, 2000; Mathewson et al., 2005; Nieuwenhuis et al., 2002).
One of the initial studies suggested that decreased ERN amplitude in older adults may result from a decreased ability to detect errors (Band & Kok, 2000). Although this may be true in more difficult tasks, it is unclear why the ERN would be reduced when subjects perform relatively simple tasks. Another suggestion is that decreased ERN amplitude is due to diminished dopaminergic function that occurs with normal aging (Nieuwenhuis et al., 2002). Likewise, decreased ERN amplitude in children may be due to neurodevelopment of medial prefrontal cortex (Stuss, 1992) and dopaminergic neurons that continues until early adulthood (Levitt, 2003; Segawa, 2000). In support of this view, the scalp distribution of the ERN in 5 – 6 year olds appears to be more posterior compared to adults (Torpey et al., 2007), suggesting some anteriorization over the course of development. Therefore, it is possible that developmental changes in DA and the prefrontal cortex across the life span are at the root of developmental changes in ERN amplitude.
Error-monitoring and psychopathology
ERN and anxiety
Consistent with the notion that obsessive-compulsive disorder might be characterized by abnormal action monitoring (Pitman, 1987), Gehring and colleagues first reported that patients with OCD had increased ERNs compared to age-matched controls (Gehring, Himle, & Nisenson, 2000). This pattern of results has now been replicated (Johannes et al., 2001; Ruchsow, Gron et al., 2005), and consistent results have also been reported in children with both generalized anxiety disorder (GAD) and OCD (Hajcak et al., 2007; Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006). Additionally, several fMRI studies have confirmed the findings of increased error-related brain activity in OCD patients (Fitzgerald et al., 2005; Maltby, Tolin, Worhunsky, O’Keefe, & Kiehl, 2005; Ursu, Stenger, Shear, Jones, & Carter, 2003). One study found increased ACC activity in both error and correct trials in OCD patients, signifying an overall hyperactivity in the ACC during response monitoring (Ursu et al., 2003); similar results were reported in an ERP study that showed both an increased ERN and CRN in high-OC subjects (Hajcak & Simons, 2002).
Hajcak and colleagues (2007) demonstrated that ERN amplitude does not change after successful treatment in pediatric OCD. These findings have since been replicated in an additional report (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2007). Another study that examined state-related changes in anxiety with spider phobic individuals found that the ERN amplitude did not change during symptom provocation with the presence of a tarantula (Moser et al., 2005). Combined, these studies suggest that the ERN is not affected by state-related changes in anxious symptoms.
In support of this possibility, several studies have examined the ERN with respect to personality traits that are closely related to pathological anxiety. Two studies have found that subjects scoring high on negative affect have significantly larger ERN amplitudes compared to subjects scoring low on negative affect (Hajcak, McDonald et al., 2004; Luu et al., 2000). Additionally, pathological worry has been associated with increased ERN amplitude (Hajcak, McDonald, & Simons, 2003a). An fMRI study similarly found increased ACC activity on error trials in individuals with high trait anxiety (Paulus, Feinstein, Simmons, & Stein, 2004).
Other evidence suggests that negative affect is strongly related to individual differences in punishment sensitivity (Carver & White, 1994; Watson, Wiese, Validya, & Tellegen, 1999). Two studies have examined behavioral inhibition system (BIS) and behavioral activation system (BAS) scores relative to ERN amplitude (Amodio, Master et al., 2007; Boksem, Tops, Wester, Meijman, & Lorist, 2006). BIS and BAS scores are thought to represent punishment sensitivity and reward seeking, respectively (Carver & White, 1994; Gray, 1972, 1981). Consistent with the notion that errors are aversive, both Boksem and colleagues (2006) and Amodio and colleagues (2007) found that subjects with high BIS scores also had larger ERNs. BIS and BAS traits relate to biases towards reactive and proactive control respectively (Boksem et al., 2006; Braver, Gray, & Burgess, 2007; Gray & Braver, 2002), and may also relate to learning style. For example, another study found that participants who learned by avoiding negative events (i.e., reactive control) had larger ERNs than individuals who learned from positive events (i.e., proactive control; Frank, Woroch, & Curran, 2005).
ERN and depression
Depressed individuals exhibit increased sensitivity to mistakes and negative feedback (Elliott, Sahakian, Michael, Paykel, & Dolan, 1998; Steffens, Wagner, Levy, Horn, & Krishnan, 2001). Individuals endorsing depressive symptoms were shown to have decreased accuracy after incorrect compared to correct trials, which is evidence of poor performance adjustments following errors (Holmes & Pizzagalli, 2007; Pizzagalli, Peccoralo, Davidson, & Cohen, 2006). A negatively biased view of the environment is also thought to be a strong factor in the development of depression (Beck, 1967; Leppanen, 2006). Depressed individuals accurately judged the number of error responses they made, but underestimated the number of correct responses in a working memory task (Dunn, Dalgleish, Lawrence, & Ogilvie, 2007).
In line with these negative processing biases, depressed individuals also exhibit abnormal error-related activity. For example, while performing a Stroop task, depressed subjects had greater ERN amplitude compared to controls (Holmes & Pizzagalli, 2008). Additionally, while performing a Flanker’s task, depressed subjects had greater ERN amplitude in neutral and punishment conditions compared to controls, but no difference in ERN amplitude during a reward condition which supports the notion that depressed individuals are especially sensitive to punishment (Chiu & Deldin, 2007). An fMRI study likewise found increased rostral ACC activity in depressed patients compared to controls during error trials (Steele, Meyer, & Ebmeier, 2004).
Conceptually, both anxiety and depression appear to be characterized by an increased sensitivity to committing errors – consistent with studies that report an increased ERN in relation to anxiety and depression. In fact, Hajcak and colleagues (2004) argued that abnormal ERN amplitude may not be specific to pathological conditions of depression or pathological anxiety, but rather reflect an underlying characteristic that is central to both of these disorders – negative affect (cf., Luu et al., 2000). However, an alternative possibility is that the ERN is increased in all forms of psychopathology. Next, we review evidence that the ERN shows an opposite pattern of effects in disorders characterized by insensitivity to errors and other forms of punishment - namely substance abuse.
ERN and substance abuse
In the only study to date that has examined the ERN in relation to substance abuse, Franken and colleagues found that cocaine dependent patients had decreased ERN amplitudes compared to a control group (Franken, van Strien, Franzek, & van de Wetering, 2007). The relation between substances of abuse and error monitoring has also has also been studied by acutely administering alcohol to healthy volunteers. Both low and moderate doses of alcohol decrease ERN amplitude in healthy individuals (Easdon, Izenberg, Armilio, Yu, & Alain, 2005; Ridderinkhof et al., 2002). However, no studies to date have looked at state-related ERN changes in substance abusers.
Imaging studies, however, support the notion that substance abusers, regardless of what drugs they abuse, exhibit reduced error-related ACC activity. For example studies have found decreased error-related ACC activity in individuals who use marijuana (Gruber & Yurgelun-Todd, 2005), opiates (Forman et al., 2004), cocaine (Goldstein et al., 2007; Kaufman, Ross, Stein, & Garavan, 2003) and methamphetamine (London et al., 2005). Overall, these results suggest that individuals with substance abuse may have a decrement in error-processing, although more ERP studies are required to examine this with specific regard to the ERN.
A hallmark personality characteristic in substance abuse is impulsivity (Moeller et al., 2001) which is often characterized by enhanced sensitivity to reward and reduced sensitivity to punishment (Corr, 2002; Monterosso & Ainslie, 1999). Similarly, those at risk for developing substance abuse disorders are more sensitive to rewards than punishment (Finn, Kessler, & Hussong, 1994) and are particularly insensitive to long-term negative consequences which are usually dismissed in favor of short-term rewards (Grant, Contoreggi, & London, 2000; Petry, Bickel, & Arnett, 1998).
Consistent with the suggestion that impulsivity is related to increased sensitivity to reward and decreased sensitivity to punishment (Corr, 2002; Monterosso & Ainslie, 1999), studies show that individuals who score high on impulsivity scales have decreased ERN amplitudes in response to errors (Potts, George, Martin, & Barratt, 2006; Ruchsow, Spitzer, Gron, Grothe, & Kiefer, 2005). Other studies have found similar results using more indirect measures of impulsivity. For example, individuals characterized as externalizing often have impulsive control problems (Krueger, Hicks, Patrick, & Carlson, 2002; Krueger, Markon, Patrick, & Iacono, 2005). As expected, high externalizing individuals also had smaller ERN amplitudes (Hall, Bernat, & Patrick, 2007).
Overall then, increased ERNs appear specific to disorders such as anxiety and depression, whereas decreased ERNs have been reported in individuals with substance abuse and impulsive personality characteristics. This pattern of increased and decreased ERNs fit well within contemporary models of psychopathology that posit two higher-order factors of psychopathology (Krueger, 1999): specifically, internalizing and externalizing disorders might be characterized by hyperactive and hypoactive error-processing, respectively, as indexed by the ERN.
Summary
ERN is a reliable index of error-processing, evident just 50 ms after individuals make mistakes; the available evidence indicates that the ERN is generated in the ACC, and likely reflects dopaminergic activity related to the on-going evaluation of errors and response conflict (Holroyd & Coles, 2002; Yeung et al., 2004). There is increasing evidence that ERN also relates to motivational and affective variables, and might be tied to neural systems that support defensive behaviors and avoidance learning (Frank et al., 2005; Hajcak & Foti, 2008).
Individuals with internalizing disorders, characterized by increased sensitivity to errors (i.e., anxiety and depression), are characterized by increased ERNs. Similar results are found in personality traits closely related to internalizing forms of psychopathology: individuals that score high in negative affect, anxiety, worry, and behavioral inhibition all have increased ERN amplitudes (e.g., Amodio, Master et al., 2007; Boksem et al., 2006; Hajcak et al., 2003a; Hajcak, McDonald et al., 2004). Moreover, it appears that state-related changes in anxiety do not have a corresponding influence on the ERN (Hajcak et al., 2007; Moser et al., 2005). Collectively, these results support the notion that abnormalities of the ERN are related to stable characteristics related to internalizing disorders.
Externalizing disorders, on the other hand, are characterized by impulsivity and behaviors that go against societal norms (Krueger, Caspi, Moffitt, & Silva, 1998; Krueger, Markon, Patrick, Benning, & Kramer, 2007). Individuals with substance abuse problems are often typified by impulsive behaviors (Moeller et al., 2001) and often making decisions that result in short-term rewards at the expense of long-term negative consequences (Grant et al., 2000; Petry et al., 1998). Psychophysiological and imaging studies confirm that individuals with substance abuse disorders have decreased ERN amplitude (Franken et al., 2007) and error-related brain activation (i.e., Goldstein et al., 2007), indicating that these individuals are less sensitive to errors.
Personality traits, such as impulsivity, that are associated with externalizing are characterized by a similar reduction in the ERN (e.g., Potts et al., 2006); along similar lines, individuals who are characterized by insensitivity to punishment have reduced ERNs in the context of punishment but not reward (Dikman & Allen, 2000). These results support the notion that a reduced ERN appears related to the externalizing dimension of personality and psychopathology.
Conclusions and Future Directions
We have previously proposed that the ERN might be a useful endophenotype for internalizing disorders (Hajcak, Franklin, Foa, & Simons, 2007). In particular, the ERN might reflect information-processing abnormalities that mediate the pathway between genetic predisposition and disease states (Gottesmann & Gould 2003). Gottesmann and Gould (2003) highlighted several characteristics of endophenotypes. First, an endophenotype should be associated with a disease. Several studies have shown that increased error-related brain activity characterizes patients with anxious (Gehring, Himle, & Nisenson, 2000; Hajcak et al., 2003a; Hajcak, McDonald et al., 2004; Hajcak & Simons, 2002; Ruchsow, Spitzer et al., 2005) and depressive symptoms (Chiu & Deldin, 2007). Additionally, an endophenotype should be state-independent. This has been verified in anxiety disorders, with studies showing no change in ERN amplitude during symptom provocation (Moser et al., 2005) or after successful treatment (Hajcak et al., 2007; Ladouceur et al., 2007). To our knowledge, though, there have been no research studies to date that have looked at state changes in symptoms of depressed patients. Endophenotypes must also be heritable. Preliminary studies reported by Anohkin, Golosheykin, and Myers (2008) indicate that error-related brain activity is heritable, with estimates in the range of .30 to .50. Also, endophenotypes should be more evident in unaffected first-degree family members of patients compared to first-degree family members of non-patients. This has not been assessed, as of yet, and is certainly an important topic for future study.
Of course, the present review highlights the possibility that a reduced ERN might also relate to the externalizing dimension of personality and psychopathology; however, further research needs to be done to confirm and extend these findings. Future studies might clarify other remaining issues. For example, more studies are needed to assess changes in ERN amplitude after successful treatment in order to tease apart state and trait influences on the ERN. Some studies have already suggested that trait-related differences in anxiety relate to an increased ERN, whereas no studies have similarly examined the impact of state-related depressive changes on the ERN. Finally, longitudinal studies beginning in childhood are necessary to examine the ERN in children prior to the development of psychiatric disorders to establish a time-line for when differences in ERN amplitude begin to signify risk.
Although many psychiatric disorders can be categorized into the internalizing-externalizing dimension, psychotic disorders such as schizophrenia do not fit well within this model – and questions remains as to whether or not psychotic disorders represent an entirely unique dimension (see Krueger & Tackett, 2003). Interestingly, the literature suggests that individuals with schizophrenia represent a unique dimension: in contrast with the internalizing and externalizing dimensions characterized by increased and decreased error-sensitivity, respectively, schizophrenia is characterized by a general inability to self-monitor (Malenka, Angel, Hampton, & Berger, 1982; Stirling, Hellewell, & Quraishi, 1998).
Although most studies indicate that individuals with schizophrenia have smaller ERN amplitudes (Alain, McNeely, He, Christensen, & West, 2002; Bates, Kiehl, Laurens, & Liddle, 2002; Bates, Liddle, Kiehl, & Ngan, 2004; Kim et al., 2006; Morris, Heerey, Gold, & Holroyd, 2007; Morris, Yee, & Nuechterlein, 2006), some evidence suggests that individuals with schizophrenia also have larger CRN amplitudes that are comparable in magnitude to their ERNs (Mathalon et al., 2002; Morris et al., 2006). These findings are similar to what is seen in individuals with prefrontal cortex lesions who have no difference between their ERN and CRN amplitudes (Gehring & Knight, 2000). The similarities between schizophrenia and prefrontal cortex lesion patients are not surprising as deficits in the prefrontal cortex are thought to contribute to the etiology of schizophrenia (Galderisi et al., 2007; Zhou et al., 2007). One difference between these two groups is that PFC lesion patients have normal ERN amplitudes, whereas schizophrenia patients have smaller ERN amplitudes. During errors of commission, schizophrenic patients had a decreased hemodynamic response in the ACC (Carter, MacDonald, Ross, & Stenger, 2001) and more specifically in the rostral ACC (Laurens, Ngan, Bates, Kiehl, & Liddle, 2003). Additionally, studies suggest that schizophrenia is also characterized by decreased structure and function of the ACC (Zetzsche et al., 2007). Future studies should investigate both the ERN and the CRN in schizophrenia to determine whether changes in both of these components represent a general inability of individuals with schizophrenia to self-monitor. It is of interest to note that there is an abundant literature on the P300 component in schizophrenia which suggests that deficits in attention may impact task performance and related psychophysiological measures (see Ford, 1999 for a review). Thus, ERN findings may result from a more general deficit found in schizophrenia rather than being specific to response monitoring.
Footnotes
The focus of this review is the response related ERN, however, a similar component is observed following negative feedback (feedback ERN; W.H. Miltner, Braun, & Coles, 1997). Although this feedback-related component is similar to what is observed after a response error, there are significant differences between the two which have led some researchers to suggest that they are not the same component (W. J. Gehring & Willoughby, 2004; Hajcak, Moser, Holroyd, & Simons, 2006). Thus, this review will focus the psychophysiological response to errors or slips in fast reaction time tasks.
Following the ERN, another ERP component occurs on error trials: the error positivity (Pe). The Pe follows the ERN as a positive deflection 200–400 msec after the commission of an error (Figure 1a; Falkenstein, Hoormann, Hohnsbein, & Kleinsorge, 2003; Nieuwenhuis et al., 2001; Overbeek, Nieuwenhuis, & Ridderinkhof, 2005) and has a more posterior midline scalp distribution than the ERN (Figure 1c; Falkenstein et al., 2000). There are several theories as to what the Pe represents, but studies have not systematically evaluated the Pe, so it is difficult to identify its role in error processing (Overbeek et al., 2005). Therefore, this review will only focus on the ERN and will not discuss Pe findings.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alain C, McNeely HE, He Y, Christensen BK, West R. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cerebral Cortex. 2002;12(8):840–846. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Jost JT, Master SL, Yee CM. Neurocognitive correlates of liberalism and conservatism. Nature Neuroscience. 2007;10(10):1246–1247. doi: 10.1038/nn1979. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology. 2007 doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008 doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Band GP, Kok A. Age effects on response monitoring in a mental-rotation task. Biological Psychology. 2000;51(2–3):201–221. doi: 10.1016/s0301-0511(99)00038-1. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Pearson MA, Dickter CL, Sher KJ, Fabiani M, Gratton G. Strategic control and medial frontal negativity: beyond errors and response conflict. Psychophysiology. 2005;42(1):33–42. doi: 10.1111/j.1469-8986.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- Barto AG. Adaptive critics and the basal ganglia. In: Houk J, Davis J, Beiser D, editors. Models of information processing in the basal ganglia. Cambridge, MA: MIT Press; 1995. pp. 215–232. [Google Scholar]
- Bates AT, Kiehl KA, Laurens KR, Liddle PF. Error-related negativity and correct response negativity in schizophrenia. Clinical Neurophysiology. 2002;113(9):1454–1463. doi: 10.1016/s1388-2457(02)00154-2. [DOI] [PubMed] [Google Scholar]
- Bates AT, Liddle PF, Kiehl KA, Ngan ET. State dependent changes in error monitoring in schizophrenia. Journal of Psychiatric Research. 2004;38(3):347–356. doi: 10.1016/j.jpsychires.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Causes and treatment. Philadelphia, PA: University of Pennsylvania Press; 1967. [Google Scholar]
- Beidel DC, Turner SM. At risk for anxiety: I. Psychopathology in the offspring of anxious parents. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):918–924. doi: 10.1097/00004583-199707000-00013. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RS, et al. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31(9):907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- Bernstein PS, Scheffers MK, Coles MGH. Where did I go wrong?” A psychophysiological analysis of error detection. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:1312–1322. doi: 10.1037//0096-1523.21.6.1312. [DOI] [PubMed] [Google Scholar]
- Boksem MA, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Res. 2006;1101(1):92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane M, Miyake A, Towse JN, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Brazdil M, Roman R, Daniel P, Rektor I. Intracerebral error-related negativity in a simply go/nogo task. Journal of Psychophysiology. 2005;19:244–255. [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology. 1998;107(2):179–192. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences. 2000;97(4):1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. American Journal of Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67(2):319–333. [Google Scholar]
- Chakravarti A, Little P. Nature, nurture and human disease. Nature. 2003;421(6921):412–414. doi: 10.1038/nature01401. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry. 2007;164(4):608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Coles MG, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biological Psychology. 2001;56(3):173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Corr PJ. J. A. Gray’s reinforcement sensitivity theory: tests of the joint substystems hypothesis of anxiety and impulsivity. Personality and Individual Differences. 2002;33:511–532. [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of error-monitoring event-related potentials in adolescents. Annals of the New York Academy of Sciences. 2004a;1021:324–328. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004b;25(3):355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- de Bruijn ER, Hulstijn W, Verkes RJ, Ruigt GS, Sabbe BG. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology (Berl) 2004;177(1–2):151–160. doi: 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- de Bruijn ER, Sabbe BG, Hulstijn W, Ruigt GS, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Research. 2006;1105(1):122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Dikman ZV, Allen JJ. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37(1):43–54. [PubMed] [Google Scholar]
- Downey G, Coyne JC. Children of depressed parents: an integrative review. Psychological Bulletin. 1990;108(1):50–76. doi: 10.1037/0033-2909.108.1.50. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD, Ogilvie AD. The accuracy of self-monitoring and its relationship to self-focused attention in dysphoria and clinical depression. Journal of Abnormal Psychology. 2007;116(1):1–15. doi: 10.1037/0021-843X.116.1.1. [DOI] [PubMed] [Google Scholar]
- Easdon C, Izenberg A, Armilio ML, Yu H, Alain C. Alcohol consumption impairs stimulus- and error-related processing during a Go/No-Go Task. Cognitive Brain Research. 2005;25(3):873–883. doi: 10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychological Medicine. 1998;28(3):559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task. European Journal of Neuroscience. 2007;26(6):1714–1720. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters on the identification of target letters in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hielscher H, Dziobek I, Schwarzenau P, Hoormann J, Sunderman B, et al. Action monitoring, error detection, and the basal ganglia: an ERP study. Neuroreport. 2001;12(1):157–161. doi: 10.1097/00001756-200101220-00039. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Event-related potential correlates of errors in reaction tasks. Electroencephalography and Clinical Neurophysiology Supplement. 1995;44:287–296. [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51(2–3):87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica (Amst) 1999;101(2–3):267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Changes of error-related ERPs with age. Experimental Brain Research. 2001;138(2):258–262. doi: 10.1007/s002210100712. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J, Kleinsorge T. Short-term mobilization of processing resources is revealed in the event-related potential. Psychophysiology. 2003;40(6):914–923. doi: 10.1111/1469-8986.00109. [DOI] [PubMed] [Google Scholar]
- Finn PR, Kessler DN, Hussong AM. Risk for alcoholism and classical conditioning to signals for punishment: evidence for a weak behavioral inhibition system? Journal of Abnormal Psychology. 1994;103(2):293–301. doi: 10.1037//0021-843x.103.2.293. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological Psychiatry. 2005;57(3):287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(6):667–682. [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, et al. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biological Psychiatry. 2004;55(5):531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. Journal of Cognitive Neuroscience. 2005;17(1):51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Woroch BS, Curran T. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 2005;47(4):495–501. doi: 10.1016/j.neuron.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biological Psychology. 2007;75(1):45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Quarantelli M, Volpe U, Mucci A, Cassano GB, Invernizzi G, et al. Patterns of Structural MRI Abnormalities in Deficit and Nondeficit Schizophrenia. Schizophrenia Bulletin. 2007 doi: 10.1093/schbul/sbm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11(1):1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3(5):516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Are all medial frontal negativities created equal? Toward a richer epirical basis for theories of action monitoring. In: Ullsperger M, Falkenstein M, editors. Errors, conflicts, and the brain: Current opinions on response monitoring. Leipzig: MPI of Cognitive Neuroscience; 2004. pp. 14–20. [Google Scholar]
- Gemba H, Sasaki K, Brooks VB. ‘Error’ potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning. Neuroscience Letters. 1986;70(2):223–227. doi: 10.1016/0304-3940(86)90467-2. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144(4):1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Sirevaag EJ, Eriksen CW, Donchin E. Pre- and poststimulus activation of response channels: a psychophysiological analysis. Journal of Experimental Psychology: Human Perception Performance. 1988;14(3):331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion: A modification of Eysenck’s theory. In: Nebylitsyn VD, Gray JA, editors. The biological bases of individual behaviour. San Diego, CA: Academic Press; 1972. pp. 182–205. [Google Scholar]
- Gray JA. A critique of Eysenck’s theory of personality. In: Eysenck HJ, editor. A model for personality. Berlin: Springer-Verlag; 1981. pp. 246–276. [Google Scholar]
- Gray JR, Braver TS. Personality predicts working-memory-related activation in the caudal anterior cingulate cortex. Cognitive, Affective, & Behavioral Neuroscience. 2002;2(1):64–75. doi: 10.3758/cabn.2.1.64. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Cognitive Brain Research. 2005;23(1):107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Errors are aversive: defensive motivation and the error-related negativity. Psychol Sci. 2008;19(2):103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Errors are aversive: defensive motivation and the error-related negativity. Psychological Science. doi: 10.1111/j.1467-9280.2008.02053.x. in press. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased Error-Related Brain Activity in Pediatric Obsessive-Compulsive Disorder Before and After Treatment. American Journal of Psychiatry. 2007 doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003a;64(1–2):77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003b;40(6):895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain and Cognition. 2004;56(2):189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42(2):151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Research. 2002;110(1):63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Vidal F, Simons RF. Difficulties with Easy Tasks: ERN/Ne and stimulus component overlap. In: Ullsperger M, Falkenstein M, editors. Errors, conflicts, and the brain: Current opinions on response monitoring. Leipzig: MPI of Cognitive Neuroscience; 2004. pp. 204–211. [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18(4):326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CA, Lessem JM, Hopfer CJ, Crowley TJ, Stallings MC. The family transmission of adolescent alcohol abuse and dependence. Journal of Studies on Alcohol. 2006;67(5):657–664. doi: 10.15288/jsa.2006.67.657. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Kendler KS. Genetic and environmental sources of covariation between generalized anxiety disorder and neuroticism. American Journal of Psychiatry. 2004;161(9):1581–1587. doi: 10.1176/appi.ajp.161.9.1581. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Archives of General Psychiatry. 2004;61(9):922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Task feedback effects on conflict monitoring and executive control: relationship to subclinical measures of depression. Emotion. 2007;7(1):68–76. doi: 10.1037/1528-3542.7.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008;65(2):179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MG. Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neuroscience Letters. 1998;242(2):65–68. doi: 10.1016/s0304-3940(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Houk JC, Adams JL, Barto AG. A model of how the basal ganglia generate and use neural signals that predict reinforcement. In: Houk J, Davis J, Beiser D, editors. Models of information processing in the basal ganglia. Cambridge, MA: MIT Press; 1995. pp. 249–270. [Google Scholar]
- Ito J, Kitagawa J. Performance monitoring and error processing during a lexical decision task in patients with Parkinson’s disease. Journal of Geriatric Psychiatry and Neurology. 2006;19(1):46–54. doi: 10.1177/0891988705284716. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302(5642):120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, et al. Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Res. 2001;108(2):101–110. doi: 10.1016/s0925-4927(01)00117-2. [DOI] [PubMed] [Google Scholar]
- Jylha P, Isometsa E. The relationship of neuroticism and extraversion to symptoms of anxiety and depression in the general population. Depression and Anxiety. 2006;23(5):281–289. doi: 10.1002/da.20167. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2003;23(21):7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Gatz M, Pedersen NL. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychological Medicine. 2007;37(3):453–462. doi: 10.1017/S0033291706009135. [DOI] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Imashioya H, Uno H, Fujita T. Error-related negativity in a visual go/no-go task: children vs. adults. Developmental Neuropsychology. 2007;31(2):181–191. doi: 10.1080/87565640701190775. [DOI] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Uno H, Fujita T. Error-related negativity in children: effect of an observer. Dev Neuropsychol. 2005;28(3):871–883. doi: 10.1207/s15326942dn2803_7. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kang SS, Shin KS, Yoo SY, Kim YY, Kwon JS. Neuropsychological correlates of error negativity and positivity in schizophrenia patients. Psychiatry and Clinical Neurosciences. 2006;60(3):303–311. doi: 10.1111/j.1440-1819.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56(10):921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, Silva PA. The structure and stability of common mental disorders (DSM-III-R): a longitudinal-epidemiological study. Journal of Abnormal Psychology. 1998;107(2):216–227. doi: 10.1037//0021-843x.107.2.216. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR. Etiologic connections among substance dependence, antisocial behavior and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111(3):411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116(4):645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: a dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology. 2005;114(4):537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Tackett JL. Personality and psychopathology: working toward the bigger picture. Journal of Personality Disorders. 2003;17(2):109–128. doi: 10.1521/pedi.17.2.109.23986. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Decreased Pe, but not ERN, amplitude following treatment of children diagnosed with an anxiety disorder: preliminary results. Psychophysiology. 2007;44:s99. [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. ERP correlates of action monitoring in adolescence. Annals of the New York Academy of Sciences. 2004;1021:329–336. doi: 10.1196/annals.1308.040. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126(Pt 3):610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Current Opinion in Psychiatry. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. Journal of Pediatrics. 2003;143(4 Suppl):S35–45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Lewis DA. In pursuit of the pathogenesis and pathophysiology of schizophrenia: where do we stand? American Journal of Psychiatry. 2002;159(9):1467–1469. doi: 10.1176/appi.ajp.159.9.1467. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biological Psychiatry. 2005;58(10):770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129(1):43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Angel RW, Hampton B, Berger PA. Impaired central error-correcting behavior in schizophrenia. Archives of General Psychiatry. 1982;39(1):101–107. doi: 10.1001/archpsyc.1982.04290010073013. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24(2):495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Bennett A, Askari N, Gray EM, Rosenbloom MJ, Ford JM. Response-monitoring dysfunction in aging and Alzheimer’s disease: an event-related potential study. Neurobiology of Aging. 2003;24(5):675–685. doi: 10.1016/s0197-4580(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. Journal of Abnormal Psychology. 2002;111(1):22–41. [PubMed] [Google Scholar]
- Mathewson KJ, Dywan J, Segalowitz SJ. Brain bases of error-related ERPs as influenced by age and task. Biological Psychology. 2005;70(2):88–104. doi: 10.1016/j.biopsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI. The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychological Medicine. 2005;35(5):611–624. doi: 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- Miltner WH, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Miltner WH, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MG. Implementation of error-processing in the human anterior cingulate cortex: a source analysis of the magnetic equivalent of the error-related negativity. Biological Psychology. 2003;64(1–2):157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Abuse Treatment. 2001;21(4):193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. Journal of Neuroscience. 1996;16(5):1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. Beyond discounting: possible experimental models of impulse control. Psychopharmacology (Berl) 1999;146(4):339–347. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- Morris SE, Heerey EA, Gold JM, Holroyd CB. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophrenia Research. 2007 doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Yee CM, Nuechterlein KH. Electrophysiological analysis of error monitoring in schizophrenia. Journal of Abnormal Psychology. 2006;115(2):239–250. doi: 10.1037/0021-843X.115.2.239. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Simons RF. The effects of fear on performance monitoring and attentional allocation. Psychophysiology. 2005;42(3):261–268. doi: 10.1111/j.1469-8986.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38(5):752–760. [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Talsma D, Coles MG, Holroyd CB, Kok A, et al. A computational account of altered error processing in older age: dopamine and the error-related negativity. Cognitive, Affective, & Behavioral Neuroscience. 2002;2(1):19–36. doi: 10.3758/cabn.2.1.19. [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: on the functional significance of the Pe Vis-a-vis the ERN/Ne. Journal of Psychophysiology. 2005;19:319–329. [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41(1):84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology. 2002;39(2):198–206. doi: 10.1017/S0048577202010247. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Sciences. 1990;87(1):256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Simmons A, Stein MB. Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biological Psychiatry. 2004;55(12):1179–1187. doi: 10.1016/j.biopsych.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93(5):729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Pitman RK. A cybernetic model of obsessive-compulsive psychopathology. Comprehensive Psychiatry. 1987;28(4):334–343. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: a 128-channel EEG study. Human Brain Mapping. 2006;27(3):185–201. doi: 10.1002/hbm.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts GF, George MR, Martin LE, Barratt ES. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neuroscience Letters. 2006;397(1–2):130–134. doi: 10.1016/j.neulet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Errors and error correction in choice-response tasks. Journal of Experimental Psychology. 1966;71(2):264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Sequential reactions. In: Holding D, editor. Human skills. New York: Wiley; 1981. pp. 153–175. [Google Scholar]
- Rabbitt P, Vyas S. Processing a display even after you make a response to it. How perceptual errors can be corrected. Quarterly Journal of Experimental Psychology. 1981;33A:223–239. [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, et al. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298(5601):2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Gron G, Reuter K, Spitzer M, Hermle L, Kiefer M. Error-related brain activity in patients with obsessive-compulsive disorder and in healthy controls. Journal of Psychophysiology. 2005;19(4):298–304. [Google Scholar]
- Ruchsow M, Spitzer M, Gron G, Grothe J, Kiefer M. Error processing and impulsiveness in normals: evidence from event-related potentials. Cognitive Brain Research. 2005;24(2):317–325. doi: 10.1016/j.cogbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology: Human Perception and Performance. 2000;26(1):141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG, Bernstein P, Gehring WJ, Donchin E. Event-related brain potentials and error-related processing: an analysis of incorrect responses to go and no-go stimuli. Psychophysiology. 1996;33(1):42–53. doi: 10.1111/j.1469-8986.1996.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Kugler J, Rollnik J. On the relation between neuroticism, self-esteem, and depression: results from the National Comorbidity Survey. Comprehensive Psychiatry. 2003;44(3):169–176. doi: 10.1016/S0010-440X(03)00008-7. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Segawa M. Development of the nigrostriatal dopamine neuron and the pathways in the basal ganglia. Brain Development. 2000;22(Suppl 1):S1–4. doi: 10.1016/s0387-7604(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. Journal of Consulting and Clinical Psychology. 2000;68(5):818–829. [PubMed] [Google Scholar]
- Simon JR. Reactions toward the source of stimulation. Journal of Experimental Psychology. 1969;81(1):174–176. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- Steele JD, Meyer M, Ebmeier KP. Neural predictive error signal correlates with depressive illness severity in a game paradigm. Neuroimage. 2004;23(1):269–280. doi: 10.1016/j.neuroimage.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Wagner HR, Levy RM, Horn KA, Krishnan KR. Performance feedback deficit in geriatric depression. Biological Psychiatry. 2001;50(5):358–363. doi: 10.1016/s0006-3223(01)01165-9. [DOI] [PubMed] [Google Scholar]
- Stemmer B, Segalowitz SJ, Dywan J, Panisset M, Melmed C. The error negativity in nonmedicated and medicated patients with Parkinson’s disease. Clinical Neurophysiology. 2007;118(6):1223–1229. doi: 10.1016/j.clinph.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Stemmer B, Segalowitz SJ, Witzke W, Schonle PW. Error detection in patients with lesions to the medial prefrontal cortex: an ERP study. Neuropsychologia. 2004;42(1):118–130. doi: 10.1016/s0028-3932(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Stirling JD, Hellewell JS, Quraishi N. Self-monitoring dysfunction and the schizophrenic symptoms of alien control. Psychological Medicine. 1998;28(3):675–683. doi: 10.1017/s0033291798006679. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Biological and psychological development of executive functions. Brain and Cognition. 1992;20(1):8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Hillman CH, Curtin JJ. Age and physical activity influences on action monitoring during task switching. Neurobiology of Aging. 2006;27(9):1335–1345. doi: 10.1016/j.neurobiolaging.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Klein DN. The impact of motivational influences on error-related brain activity in young children. Psychophysiology. 2007;44:s25. [Google Scholar]
- Tsai LL, Young HY, Hsieh S, Lee CS. Impairment of error monitoring following sleep deprivation. Sleep. 2005;28(6):707–713. doi: 10.1093/sleep/28.6.707. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychological Science. 2003;14(4):347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Van’t Ent D, Apkarian P. Motoric response inhibition in finger movement and saccadic eye movement: A comparative study. Clinical Neurophysiology. 1999;110:1058–1072. doi: 10.1016/s1388-2457(98)00036-4. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Separating semantic conflict and response conflict in the Stroop task: a functional MRI study. Neuroimage. 2005;27(3):497–504. doi: 10.1016/j.neuroimage.2005.04.042. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14(6):1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- van Veen V, Holroyd CB, Cohen JD, Stenger VA, Carter CS. Errors without conflict: implications for performance monitoring theories of anterior cingulate cortex. Brain and Cognition. 2004;56(2):267–276. doi: 10.1016/j.bandc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the ‘error negativity’ specific to errors? Biological Psychology. 2000;51(2–3):109–128. doi: 10.1016/s0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Validya J, Tellegen A. The two general activation systems of affect: structural fundings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76:820–838. [Google Scholar]
- Wiersema JR, van der Meere JJ, Roeyers H. Developmental changes in error monitoring: an event-related potential study. Neuropsychologia. 2007;45(8):1649–1657. doi: 10.1016/j.neuropsychologia.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Yeung N. Relating cognitive and affective theories of the error-related negativity, Errors, Conflicts, and the Brain. In: Ullsperger M, Falkenstein M, editors. Current Opinions on Performance Monitoring. Leipzig: Max Planck Institute of Cognitive Neuroscience; 2004. pp. 63–70. [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review. 2004;111(4):931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Preuss U, Frodl T, Watz D, Schmitt G, Koutsouleris N, et al. In-vivo topography of structural alterations of the anterior cingulate in patients with schizophrenia: New findings and comparison with the literature. Schizophrenia Research. 2007;96(1–3):34–45. doi: 10.1016/j.schres.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neuroscience Letters. 2007;417(3):297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll CA, Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. Journal of Cognitive Neuroscience. 2004;16(6):1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]