Abstract
Reactive oxygen/nitrogen species suppress myocardial oxygen consumption. In this study, we determined that endogenous hydrogen peroxide through dismutation of superoxide enhances postischemic myocardial blood perfusion and oxygen consumption. Electron paramagnetic resonance oximetry was applied to monitor in vivo tissue Po2 in mouse heart subjected to regional ischemia reperfusion. Heart rate, arterial blood pressure, blood flow, infarction, and activities of mitochondrial NADH dehydrogenase and cytochrome c oxidase were measured in six groups of wild-type (WT) and endothelial nitric-oxide synthase knock-out (eNOS−/−) mice treated with phosphate-buffered saline (PBS), superoxide dismutase mimetic (SODm) M40403 [a manganese(II)-bis(cyclohexylpyridine)-substituted macrocyclic superoxide dismutase mimetic, C21H35Cl2MnN5], 10006329 EUK 134 [EUK134, manganese 3-methoxy N,N1-bis(salicyclidene)ethylenediamine chloride], and SODm plus glibenclamide to study the protective effect of hydrogen peroxide via dismutation of superoxide on the activation of sarcolemmal potassium channels. In the PBS group, there was an overshoot of tissue Po2 after reperfusion. Treatment with SODm, EUK134, and SODm + glibenclamide protected mitochondrial enzyme activities, reduced infarct size, and suppressed the post-ischemic hyperoxygenation. In particular, in the SODm-treated group, there was a transient peak of tissue Po2 at 9 min after reperfusion, which was dependent on endogenous hydrogen peroxide but not nitric oxide formation as it appeared in both WT and eNOS−/− mice. Blood flow and rate pressure product were higher in the SODm group than in other groups, which contributed to the transient oxygen peak. Thus, SOD mimetics protected mouse heart from superoxide-induced reperfusion injury. With treatment of different SOD mimetics, it is concluded that endogenous hydrogen peroxide via dismutation of superoxide at reperfusion enhances postischemic myocardial blood perfusion and mitochondrial oxygen consumption, possibly through activation of sarcolemmal ATP-sensitive potassium channels.
Myocardial ischemia and acute infarction arises secondary to atherosclerotic lesions followed by plaque rupture and thrombosis. Current treatments aim to terminate the ischemia by early re-establishment of blood flow. However, reperfusion may cause new damage to the region at risk (Rapapaport, 1989). The generation of reactive oxygen/nitrogen (ROS/ RNS) species after reperfusion has been realized as one of the critical insults that trigger many of the observed mechanisms of cell injury (Zweier et al., 1988, 1989; Kuppusamy and Zweier, 1994). Among these ROS/RNS, NO, superoxide, and peroxynitrite are the most prominent oxidants. During the burst, NO may react with superoxide to generate peroxynitrite and cause a nonselective and irreversible inhibition of many mitochondrial components (Brown and Borutaite, 2002).
Yet another superoxide-related oxygen species, hydrogen peroxide, has long been recognized as one of the possible endothelium-derived hyperpolarizing factors (Matoba et al., 2000). In normal aerobic mitochondrial metabolism, superoxide, as the byproduct of mitochondrial electron leakage (Wang et al., 2008), is dismutated by superoxide dismutases in the mitochondria (matrix manganese SOD as well as intermembrane space SOD) to hydrogen peroxide, which plays an important role in cell signaling (Freeman and Crapo, 1982). Hydrogen peroxide potentially dilates coronary arterial microvessels in both endothelium-dependent and independent manners (Thengchaisri and Kuo, 2003). At the beginning of reperfusion following ischemia, there is a burst formation of NO from shear stress on endothelial cells and superoxide, mainly from mitochondria after re-establishment of the blood flow. The burst superoxide reacts with the burst NO at a diffusion-controlled rate to form the very potent oxidant peroxynitrite, thereby degrading the bioavailability of NO. Therefore, dismutation of superoxide would be beneficial to the postischemic heart by increasing the bioavailability of NO and reducing the detrimental effect of peroxynitrite. It would be ideal to selectively dismutate superoxide and convert it to a potential beneficial product because hydrogen peroxide has a number of beneficial effects as mentioned above.
SOD mimetic M40403 (SODm) is a very selective superoxide dismutase (Masini et al., 2002). After intravenous injection, SODm distributes widely into the heart, lungs, brain, liver, and kidneys while retaining its intact chemical identity (Salvemini et al., 1999). It has been used as a selective superoxide dismutase in ischemia and reperfusion injury in rat heart to reduce the extent of myocardial damage, mast cell degranulation, and the incidence of ventricular arrhythmias. In our current study, SODm was given to mice to selectively dismutate superoxide generated at the beginning of reperfusion to reduce peroxynitrite production. We also used EUK134, which has both SOD and catalase activities, to study its effect on postischemic tissue oxygenation and to differentiate the effect of hydrogen peroxide (Izumi et al., 2002). If peroxynitrite contributes to the suppression of oxygen consumption, then by removing superoxide, the suppression of oxygen consumption should be reversed. In addition, we will test whether dismutation of superoxide and the subsequent formation of hydrogen peroxide will enhance the postischemic reperfusion and myocardial contractile function.
To determine the regulatory role of these ROS/RNS on mitochondrial respiration, oxygen consumption must be measured in the tissue in vivo. Tissue O2 consumption can be determined by measuring the change in tissue O2 supply and Po2. A significant overshoot of myocardial Po2 was reported previously after release of the aortic cross-clamp during coronary bypass surgery; however, the mechanism was not shown (Al-Obaidi et al., 2000). We demonstrated that the hyperoxygenation after reperfusion was due to the interaction of the burst NO and its derivative peroxynitrite on the mitochondrial respiration. In the prolonged high-level phase of Po2, peroxynitrite caused an irreversible change to the subcomponents of the mitochondrial respiratory chain, such as NADH dehydrogenase (NADH-DH) and cytochrome c oxidase (CcO) (Zhao et al., 2005).
To measure tissue Po2 in vivo, a reliable oximetry technique is required. Several techniques have been employed including fiber optic, lucigenin chemiluminescence, microelectrode, nuclear magnetic resonance, and recently developed electron paramagnetic resonance (EPR) oximetry techniques (Kreutzer and Jue, 1995; Xie et al., 1998; Clementi et al., 1999; He et al., 1999; Zhao et al., 1999; Dunn and Swartz, 2003; Pandian et al., 2003; Swartz and Dunn, 2003). Among different techniques, microelectrode and EPR oximetry with particulates are the two direct ways to measure tissue Po2 (Dunn and Swartz, 2003; Swartz and Dunn, 2003). However, with insertion of polarographic needles directly into tissue, microelectrode methods preclude repeated measurements. EPR oximetry, due to its noninvasive property, has emerged as a widely used method for measuring cellular, tissue, organ, and even whole-body tissue Po2 in different models (He et al., 1999; Pandian et al., 2003).
The current study will use EPR oximetry to measure tissue Po2 in the in vivo mouse heart subjected to 30 min of regional ischemia and 60 min of reperfusion to study the regulatory role of mitochondria-derived superoxide and its derivative peroxynitrite on myocardial tissue oxygen consumption. EPR oximetry will also be used to determine whether it is NO or hydrogen peroxide that plays a significant role in the enhancement of the postischemic reperfusion and cardiac function after treatment of SODm.
Materials and Methods
Chemicals
SODm is a gift from MetaPhore Pharmaceuticals (St. Louis, MO) (Masini et al., 2002). SODm, 10006329 EUK 134 (EUK134; Cayman Chemical, Ann Arbor, MI) (Izumi et al., 2002), and glibenclamide (Glib; Sigma-Aldrich, St. Louis, MO) were prepared into injection solutions in PBS with less than 0.1% DMSO as needed.
Animals and Myocardial Ischemia Reperfusion Model
Male wild-type (WT) C57BL/6 and eNOS knock-out (eNOS−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). WT mice were randomly divided into groups administered with SODm (4 mg/kg i.v.), EUK134 (10 mg/kg i.v.), and SODm (4 mg/kg i.v.) plus glibenclamide (2 mg/kg i.v.) bolus injections at the desired experimental time point. All procedures were performed with the approval of the Institutional Animal Care and Use Committee at The Ohio State University (Columbus, OH) and conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication 85–23, revised 1996).
The IR model and the surgical protocol were similar to the methods described previously (Zhao et al., 2005; Zhu et al., 2007). Mice were anesthetized with ketamine (50 mg/kg) plus xylazine (15 mg/kg). Atropine (0.05 mg s.c.) was administered to reduce airway secretions. Animals were orally intubated with PE-90 tubing and connected to a mouse mini-ventilator (model 845; Harvard Apparatus Inc., Holliston, MA). Core body temperature was maintained at 37°C with a thermo heating pad and monitored with the rectal thermometer.
A thoracotomy was performed. In brief, using the left armpit as the landmark, an oblique 8-mm incision was made 2 mm away from the left sternal border toward the left armpit. The fourth intercostal space was opened gently to spread the wound 8 to 10 mm in width. Afterward, the pericardium was picked up gently. Then the left anterior descending coronary artery (LAD) was visualized and completely ligated for 30 min in IR mice by tightening a 7-0 silk suture after passing it over a length of PE-10 tubing beneath the LAD at points 1 to 2 mm inferior to the left auricle. The 7-0 suture was similarly placed in the sham group but without LAD occlusion. The PE-10 tubing was ligated with the LAD to achieve easier ligature release and better reperfusion. Ischemia was confirmed visually by the appearance of pale and bulging myocardium in the area at risk (AAR). The ligature was removed, and reperfusion was visually confirmed after 30 min of LAD occlusion. Reperfusion was maintained for 60 min to obtain oximetry, blood flow, and hemodynamic measurements and 24 h for infarct size measurement.
EPR Oximetry
Approximately10 µg of lithium phthalocyanine (LiPc, the oxygen-sensitive probe) was loaded in a 27-gauge needle and implanted in the midmyocardium of the AAR after the surgery. The location of the LiPc was confirmed by histology as in the midmyocardium in the area at risk. The mouse then was transferred to the L-band EPR spectrometer, and EPR spectra were collected after 30-min equilibration of the probe with the surrounding tissue before, during, and after ischemia and reperfusion with the following parameters: frequency, 1.1 GHz; microwave power, 16 mW; and modulation filed, 0.045 G. The sensitivity of the probe to tissue Po2 is 5.8 mG/mm Hg.
Regional Blood Flow Measurement
An optical surface suction probe with a diameter of 2 mm (P10d; Moor Instruments, Devon, UK) was placed on the surface of the heart at the AAR to be able to move with the beating heart. The probe was connected to a laser Doppler tissue perfusion monitor (Moor Instruments) after thoracotomy and exposure of the heart. Blood flow was monitored during preischemia, 30-min ischemia, and 60-min reperfusion periods and the readings are expressed as a relative value without unit.
Hemodynamic Measurements
The right common carotid artery was cannulated with a PE-50 tubing tipped with 1-cm long PE-10 tubing. The cannula was attached to a transducer and monitor (PowerLab/400; ADInstruments Ltd., Chalgrove, Oxfordshire, UK) for analysis of mean arterial blood pressure (MABP) and heart rate (HR). The MABP was calculated as end diastolic arterial pressure +(systolic arterial pressure − end diastolic arterial pressure)/3. The rate pressure product (RPP) was determined from the following formula: RPP = MABP × HR (in mm Hg per minute).
Activities of NADH-DH and CcO
Heart tissue from the risk area was excised, washed, and frozen immediately at the end of 60-min reperfusion. Tissue samples then were homogenized in ice-cold HEPES buffer (3 mM, pH 7.2) containing sucrose (0.25 M), EGTA (0.5 mM), and protease-inhibitor cocktail (1:40; Roche Diagnostics, Indianapolis, IN). NADH-DH activity was measured in the presence of Tris-HCl buffer (20 mM, pH 8.0), NADH (150 M; Sigma-Aldrich), and coenzyme Q1 (100 M; Sigma-Aldrich). CcO activity was measured in the presence of phosphate buffer (50 mM, pH 7.4) and reduced cytochrome c (60 M; Sigma-Aldrich). The extinction coefficients, ε340 nm = 6.22 mM · cm for NADH and ε550 nm = 18.5 mM · cm for cytochrome c were used for activity calculation (Chen et al., 2004). Protein concentration of the tissue homogenate was measured by the Lowry method, and the activities were normalized to protein concentrations as micromoles per minute per milligram protein.
Infarct Size
Twenty-four hours after ligation and reperfusion, animals were sacrificed with pentobarbital (50 mg/kg i.p.), and hearts were excised and cannulated through the ascending aorta with a 23-gauge needle for perfusion with 3 to 4 ml of 1.0% triphenyl tetrazolium chloride (TTC) in phosphate buffer (pH 7.4, 37°C). The LAD was reoccluded by tightening the suture left in the myocardium after IR and TTC staining. The hearts were then perfused with 2 to 3 ml of 2.5% Evans Blue (Sigma-Aldrich) to delineate nonischemic myocardium. The hearts were weighed, frozen, and cut into five transverse slices, each with ~1-mm thickness. Each slice was photographed from both sides with a high-resolution digital camera on a dissecting microscope. The sections were photographed and contoured with a planimeter (MetaVue Version 6.2r6) to delineate the borders of the entire heart, the nonischemic area, and the infarct area. The sizes of the AAR [total left ventricle (LV) area – nonischemic area] and the infarct area were calculated as percentages of the total LV area and the AAR multiplied by the total weight of that slice.
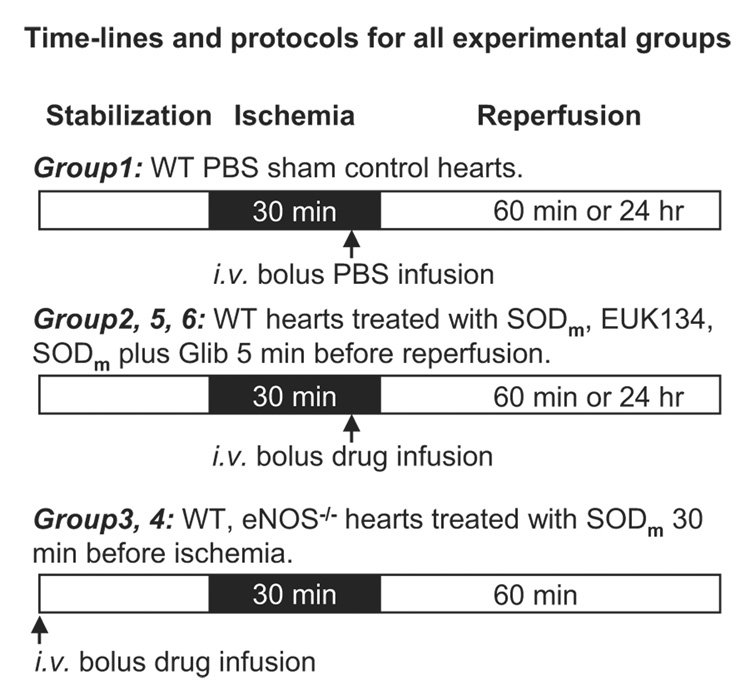
The time lines and protocols used for all of the experiments were indicated in Fig. 1. Wild-type and eNOS−/− mice were subjected to 30 min of LAD occlusion followed by 60 min or 24 h of reperfusion as indicated with the open and closed boxes. For group 1 (n = 7), intravenous bolus injection of PBS was given to wild-type mice 5 min before reperfusion as the sham control. In groups 2 (n = 7), 5 (n = 7), and 6 (n = 7), wild-type mice were treated with intravenous bolus injection of SODm, EUK134, SODm plus glibenclamide 5 min before reperfusion. In groups 3 (n = 7) and 4 (n = 7), wild-type and eNOS−/− mice were treated with intravenous bolus injection of SODm 30 min before ischemia.
Fig. 1.
Time lines and protocols for all experimental groups. After 30 min of stabilization, all mice underwent 30-min LAD occlusion followed by 60-min reperfusion represented with a sequence of white/black/white horizontal boxes. Group 1 (n = 7), WT mice subjected to 30-min LAD occlusion and 60-min reperfusion with intravenous injection of PBS 5 min before reperfusion. Groups 2 (n = 7), 5 (n = 7), and 6 (n = 7), WT mouse hearts subjected to 30-min ischemia and 60-min reperfusion with intravenous bolus infusion of SODm, EUK134, and SODm plus glibenclamide 5 min before reperfusion. Groups 3 (n = 7) and 4 (n = 7), eNOS−/− mouse hearts subjected to 30-min ischemia and 60-min reperfusion with intravenous bolus infusion of SODm 30 min before ischemia. Additional groups of hearts were subjected to 30-min ischemia and 24-h reperfusion for measurements of infarct size and area at risk.
Statistical Analysis
A two-way analysis of variance was used for analysis of Po2, mean arterial pressure, and RPP. A one-way analysis of variance was used for analysis of enzyme activities; these were followed by Newman-Keuls multiple-comparison test among the groups. A t test was used for analysis of AAR and infarct size. Data were represented as means ± S.E. A value of p < 0.05 was considered significant.
Results
EPR Oximetry
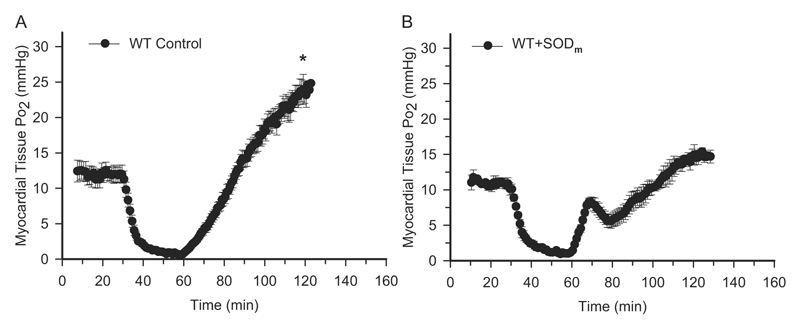
EPR oximetry was performed before, during, and after 30-min ischemia and 60-min reperfusion on all groups of mice. As shown in Fig. 2A on wild-type mice treated with PBS, the value of tissue Po2 before ischemia was measured as 12.0 ± 1.3 mm Hg. At the end of 30-min ischemia, the value of tissue Po2 reached a level of 0.7 ± 0.1 mm Hg. After reperfusion, tissue Po2 increased gradually and overshot the preischemic level. Previously, we have demonstrated that the overshoot of tissue Po2 after reperfusion in the wild-type control mice was due to the suppression of mitochondrial oxygen consumption through endothelium-derived NO and its derivative peroxynitrite (Zhao et al., 2005). When the mice were treated with SODm 5 min before reperfusion, the hyperoxygenation status after reperfusion was suppressed (Fig. 2B). It is interesting that the Po2 value went up initially and peaked at a value of 8.2 ± 0.6 mm Hg at 9 min after reperfusion. After the transient peak, tissue Po2 decreased to a level of 5.8 ± 1.0 mm Hg at 20 min after reperfusion and then increased gradually. From the balance of supply and demand point of view, this transient peak could be due to a 2-fold reason; the increased blood flow at the beginning of reperfusion and the enhanced cardiac function after reperfusion therefore increased oxygen consumption afterward.
Fig. 2.
EPR oximetry on WT control and SODm-treated mouse hearts. After thoracotomy and exposure of the heart, ~10 µg of LiPc was implanted into the midmyocardium in the risk area, and EPR spectra were recorded. Time-dependent EPR line width was measured, and tissue Po2 was calculated. A, myocardial tissue Po2 before and during ischemia and after reperfusion in WT control mouse hearts. B, myocardial tissue Po2 before and during ischemia and after reperfusion in SODm-treated mouse hearts (bolus intravenous injection at a dose of 4 mg/kg 5 min before reperfusion). These measurements demonstrated that there is an overshoot of tissue Po2 after reperfusion, and this hyperoxygenation is attenuated after treatment with SODm. In addition, there is a transient peak of tissue Po2 at 9 min of reperfusion in SODm-treated mouse hearts. *, p < 0.01, WT Control versus WT + SODm at 60-min reperfusion, n = 7.
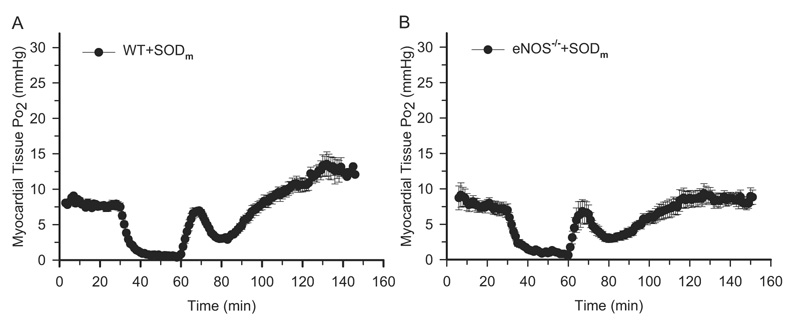
When the burst superoxide was dismutated with the treatment of SODm, NO bioavailability should have increased. It has been reported that NO can reversibly inhibit mitochondrial oxygen consumption on the site of cytochrome c oxidase (Lizasoain et al., 1996; Loke et al., 1999). To determine whether NO contributes to this transient peak of tissue Po2 after treatment with SODm, we performed EPR oximetry on eNOS−/− mice in which there is no production of NO after reperfusion. In addition, to clear out any potential transient impact of the delivery of the drug to the hemodynamics, we performed EPR oximetry on the mice treated with SODm 30 min before ischemia, so the injection time point was far away from the reperfusion time that any unequilibrated drug delivery process would be ruled out. As shown in Fig. 3, in the wild-type and eNOS−/− mice, SODm treatment suppressed the hyperoxygenation status after reperfusion. Interestingly, there are similar transient peaks of tissue Po2 after reperfusion in both the eNOS−/− as well as wild-type mice peaking at 9 min after reperfusion, which is obviously not dependent on the endothelium-derived NO.
Fig. 3.
EPR oximetry on WT SODm-treated and eNOS−/− SODm-treated mouse hearts. Surgical procedures and EPR measurements were the same as described in Fig. 1. SODm was given as a bolus (4 mg/kg) at 30 min before ischemia. A, myocardial tissue Po2 before and during ischemia and after reperfusion on WT SODm-treated mouse hearts. There is a transient peak of tissue Po2 at 9 min after reperfusion, even when SODm was given 30 min before ischemia. B, myocardial tissue Po2 before and during ischemia and after reperfusion on eNOS−/− SODm-treated mouse hearts. The transient peak of Po2 exists even in the eNOS−/− mouse hearts. n = 7 for each group. These measurements demonstrated that the transient peak of tissue Po2 after treatment with SODm is independent of endothelium-derived NO.
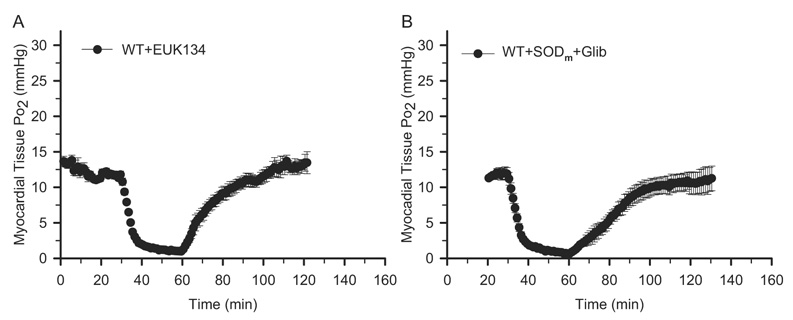
The SODm we used in the current study has been reported as a selective superoxide dismutase mimetic (Masini et al., 2002). This means that the burst formation of superoxide after reperfusion (Zweier, 1988) is dismutated to hydrogen peroxide selectively by SODm. It has also been reported that hydrogen peroxide can dilate vasculature in the heart and cause an increase of tissue blood perfusion (Burgoyne et al., 2007; Kokusho et al., 2007; Yada et al., 2008). To test whether hydrogen peroxide contributes to this transient peak of tissue Po2 after the treatment of SODm, we performed EPR oximetry on the wild-type mice treated with EUK134, which is a superoxide dismutase mimetic with catalase activity (Izumi et al., 2002). As shown in Fig. 4A, after treatment with EUK134 in the wild-type mice 5 min before reperfusion, there is no overshoot of tissue Po2, which indicates that the hyperoxygenation status was attenuated due to the dismutation of the burst formation of superoxide by EUK134 after reperfusion. Interestingly, the transient peak of tissue Po2 at 9 min of reperfusion as shown in the control mice has also been suppressed. These data strongly suggested that the transient peak of tissue Po2 after reperfusion with treatment of SODm in the control group was due to the selective dismutation of the burst formation of superoxide and the subsequent formation of hydrogen peroxide.
Fig. 4.
EPR oximetry on EUK134- and SODm + Glib-treated mouse hearts. All experimental procedures are the same as described in Fig. 1. EUK134 (10 mg/kg), SODm (4 mg/kg), and glibenclamide (Glib, 2 mg/kg) were all given as a bolus at 5 min before reperfusion. A, myocardial tissue Po2 before and during ischemia and after reperfusion on WT EUK134-treated mouse hearts. B, myocardial tissue Po2 before and during ischemia and after reperfusion on WT SODm + Glib-treated mouse hearts. n = 7 for each group. These measurements demonstrated that EUK134 treatment suppressed the hyperoxygenation in the WT control mouse hearts without the occurrence of the transient peak. In addition, glibenclamide treatment also suppressed the transient peak of Po2 in the SODm-treated mouse hearts.
To test that the endogenous hydrogen peroxide activates the sarcolemmal ATP-dependent potassium (sKATP) channels and protects the heart, we performed EPR oximetry on the wild-type mice treated with SODm plus glibenclamide, which is an inhibitor of the sKATP channels. As shown in Fig. 4B, the transient peak of tissue Po2 after treatment with SODm (Fig. 2B) was suppressed after the addition of glibenclamide 5 min before reperfusion. These data suggested that hydrogen peroxide and the sKATP channels are involved in the appearance of the transient peak of tissue Po2 after treatment with SODm.
Regional Blood Flow Measurement
To test our hypothesis that the selectively dismutated superoxide and subsequently formed hydrogen peroxide increases blood flow after reperfusion, blood perfusion in the area of risk was measured using a surface Doppler blood perfusion probe as described under Materials and Methods. As shown in Fig. 5, A and B, the blood perfusion before ischemia was measured as 6.1 ± 0.1, 6.0 ± 0.1, 5.6 ± 0.2, and 6.0 ± 0.2 in PBS, SODm, EUK134, and SODm plus glibenclamide groups. After 30 min of regional ischemia, values of blood perfusion reached a similar level of 1.8 ± 0.1, 1.8 ± 0.2, 1.9 ± 0.2, and 1.7 ± 0.1 in PBS, SODm, EUK134, and SODm plus glibenclamide groups. After reperfusion, the value of blood perfusion reached a level of 4.9 ± 0.3 in the SODm group, which is significantly higher than that of 4.4 ± 0.1, 4.5 ± 0.4, and 4.2 ± 0.2 in PBS, EUK134, and SODm plus glibenclamide groups. The higher blood perfusion after treatment with SODm was suppressed by additional treatment with glibenclamide, which is an inhibitor of the sKATP channel. These data suggested that the dismutation of superoxide and subsequent formation of hydrogen peroxide, as well as the sKATP channels, are involved in the enhanced blood perfusion after reperfusion with treatment of SODm.
Fig. 5.
Regional blood flow measurement in mouse hearts during ischemia and reperfusion. Blood flow in the area at risk was measured before ischemia and during 30 min of ischemia and 60 min of reperfusion in PBS control, SODm (4 mg/kg), EUK134 (10 mg/kg), and SODm plus glibenclamide (2 mg/kg) (SODm+Glib)-treated mouse hearts. A, regional blood flow measured in PBS and SODm-treated mouse hearts. B, regional blood flow measured in EUK134 and SODm + Glib-treated mouse hearts. After SODm treatment, myocardial blood flow after reperfusion is significantly higher than that of PBS, EUK134, and SODm + Glib-treated hearts. *, p < 0.05, SODm versus PBS, EUK134, and SODm + Glib after 10 min of reperfusion, n = 7.
Hemodynamic Measurements
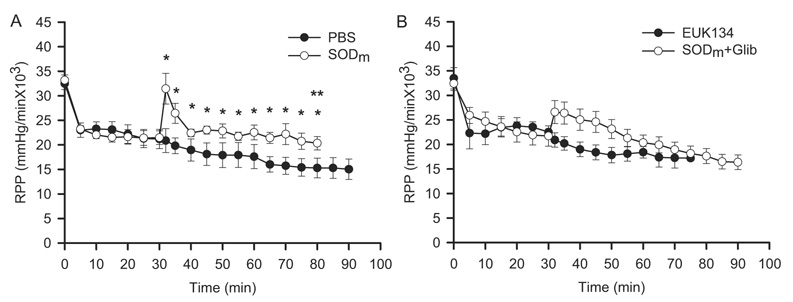
It has been demonstrated that openers of the sKATP channels improved the cardiac reperfusion contractile function (Grover et al., 1991; Venkatesh et al., 1992), whereas inhibitors of the channels suppressed the cardiac function (Grover et al., 1993). To determine that dismutation of superoxide and subsequent formation of hydrogen peroxide enhances the cardiac reperfusion contractile function through activation of the sKATP channels, we measured the HR and MABP and calculated the RPP, which is a hemodynamic index for cardiac contractile function and tissue oxygen consumption. As shown in Fig. 6, A and B, the RPP values before ischemia were measured as 33195 ± 1068, 32,471 ± 1204, 33,484 ± 2175, and 32,410 ± 1411 mm Hg/min in PBS, SODm, EUK134, and SODm plus glibenclamide-treated mice. There was no significant difference among the groups. After 30 min of ischemia, the values of RPP reached similar levels of 21,197 ± 1980, 21,478 ± 1394, 22,524 ± 1250, and 21,719 ± 2064 mm Hg/min in these four groups, respectively. After reperfusion, the value of RPP showed a transient peak and reached a significantly higher level of 31,452 ± 3126 mm Hg/min 2 min after reperfusion in the group treated with SODm compared with that of the PBS (20,860 ± 2440 mm Hg/min) and EUK134 (20,899 ± 1462 mm Hg/min) groups. After the initial transient peak in the SODm group, RPP values stayed significantly higher for the whole reperfusion time period (*, p < 0.05 versus PBS and EUK134 groups). However, in the SODm plus glibenclamide-treated group, the addition of glibenclamide attenuated the peak to a lesser extent of increase (26,600 ± 2343 mm Hg/min) compared with that of the SODm group. It is interesting that the RPP values gradually decreased after the initial increase with the addition of glibenclamide reaching a level of 17,625 ± 1525 mm Hg/min at 50-min reperfusion, which is significantly lower than that of the SODm group (20,344 ± 1358 mm Hg/min) at the corresponding time point. It is also noteworthy that there was no significant difference in the HR among all of the groups (data not shown). These data suggested that treatment with SODm enhanced the reperfusion contractile function, and glibenclamide attenuated this enhancement. Therefore, dismutation of superoxide and subsequent formation of hydrogen peroxide as well as the sKATP channels are involved in the enhancement of the reperfusion contractile function.
Fig. 6.
Measurement of the RPP of HR and MABP. MABP was calculated as: MABP = end diastolic ABP + (systolic ABP – end diastolic ABP)/3, where ABP is arterial blood pressure. RPP was calculated as: RPP = HR × MABP. A, RPP on PBS and SODm-treated mouse hearts before, during, and after ischemia and reperfusion. B, RPP on EUK134 and SODm + Glib-treated mouse hearts before, during, and after ischemia and reperfusion. These measurements demonstrated that with SODm treatment, the value of RPP was significant higher compared with PBS- and EUK134-treated hearts, including an initial peak after 2 min of reperfusion. After treatment with SODm plus glibenclamide, the value of RPP increased initially and then decreased after 25 min of reperfusion. At 50-min reperfusion, the value of RPP in the SODm + Glib hearts was significantly lower than that in the SODm-treated hearts. *, p < 0.05, SODm versus PBS and EUK134 after reperfusion; **, p < 0.05, SODm versus SODm + Glib hearts at 50-min reperfusion, n = 7.
Activities of NADH-DH and CcO
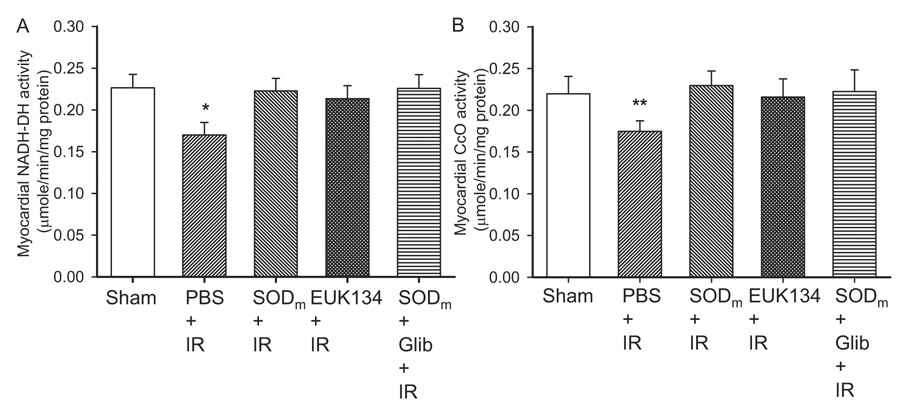
The tissue blood perfusion and EPR oximetry data demonstrated that oxygen consumption in the PBS groups was greatly attenuated after reperfusion. Therefore, the restored tissue oxygen consumption and the preserved reperfusion contractile function in SODm, EUK134, and SODm plus glibenclamide groups required conserved oxygen consumption from the mitochondria. To test whether mitochondrial oxygen consumption and enzyme activities are improved after the treatment, we measured NADH-DH and CcO activities in the postischemic myocardium. As shown in Fig. 7, A and B, the activities of NADH-DH and CcO were measured as 0.23 ± 0.02 and 0.22 ± 0.02, 0.17 ± 0.02 and 0.17 ± 0.01, 0.22 ± 0.01 and 0.23 ± 0.02, 0.21 ± 0.02 and 0.22 ± 0.02, and 0.23 ± 0.02 and 0.22 ± 0.03 µmol/min/mg protein in the Sham, PBS, SODm, EUK134, and SODm plus glibenclamide groups, respectively. These data demonstrated that, after ischemia reperfusion, the activities of NADH-DH and CcO were significantly reduced. With treatment of SODm, EUK134, and SODm plus glibenclamide, the activities of these two enzymes were restored to levels comparable to that before ischemia. Protein levels of these two enzymes were also measured (data not shown), and no difference was observed among the groups indicating that the modifications to the enzymes were post-translational. These data are consistent with the attenuation of the hyperoxygenation status after reperfusion in all of the treated groups.
Fig. 7.
Activities of mitochondrial enzymes NADH-DH and CcO after 30 min of ischemia and 60 min of reperfusion. A, NADH-DH activities of sham control and PBS, SODm, EUK134, and SODm + Glib-treated mouse hearts. B, CcO activities of sham control and PBS, SODm, EUK134, and SODm + Glib-treated mouse hearts. The activities of NADH-DH and CcO were significantly lower in PBS + IR hearts than those in the rest of the groups. SODm, EUK134, and SODm plus glibenclamide restored the activities to levels comparable to that of sham control hearts. n = 7 for each group.
Infarct Size
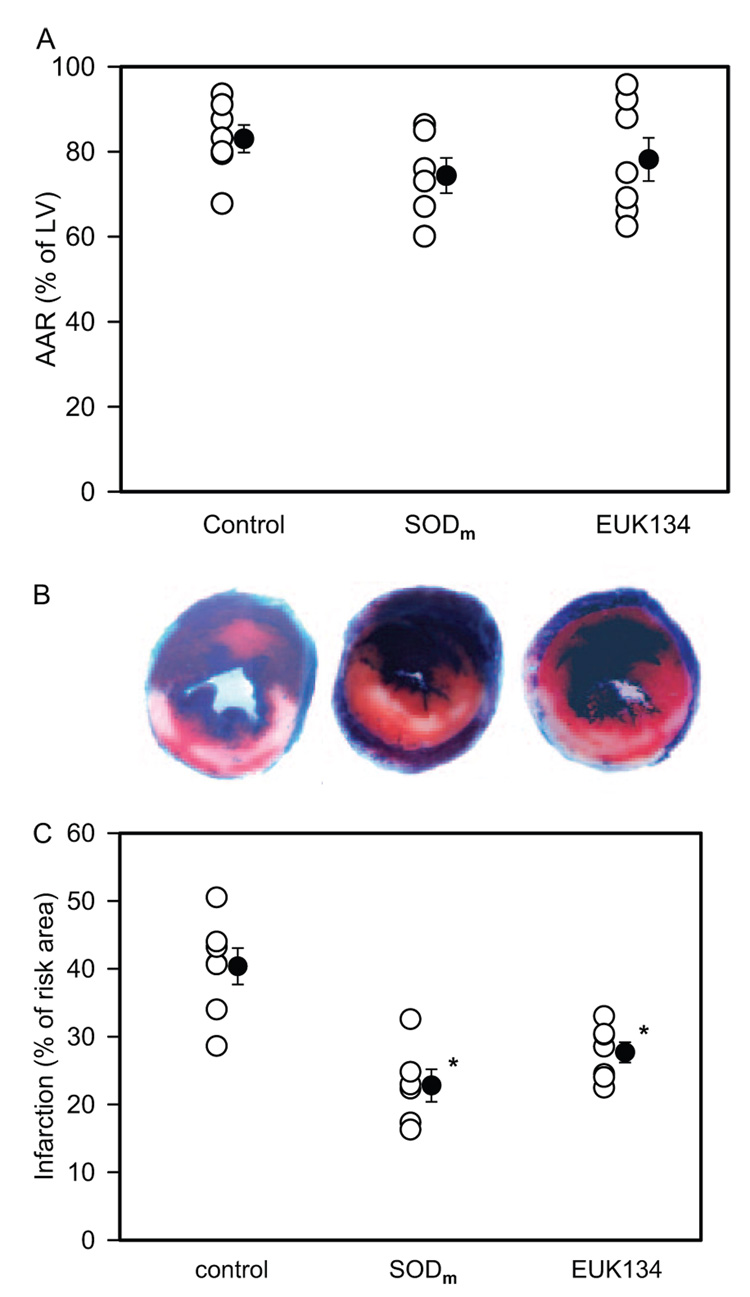
The ratio of AAR over the area of LV and infarct area over AAR was measured in the PBS, SODm, and EUK134 groups. It was shown (Fig. 8) that there were no differences in the ratio of AAR among all of the groups. Infarct size was significantly lower in SODm and EUK134 groups than that of the PBS group (22.7 ± 2.4 and 27.6 ± 1.5% versus 40.2 ± 2.7%; *, p < 0.05). These data confirmed the protection of SODm and EUK134 on the post-ischemic myocardium.
Fig 8.
Evans Blue and TTC staining of the AAR and infarct size after 30 min of ischemia and 24 h of reperfusion expressed as percentage of LV mass and AAR mass, respectively. A, AAR in control (PBS+IR) (83.3 ± 3.3%), SODm + IR (74.6 ± 4.2%), and EUK134 + IR (78.4 ± 5.1%) groups. B, representative sections from control, SODm, and EUK134-treated groups. C, infarct size in control (40.2 ± 2.7%), SODm + IR (22.7 ± 2.4%), and EUK134 + IR (27.6 ± 1.5%) groups. There was no significant difference of AAR values among all of the groups. However, the infarct size was significantly reduced after treatment with SODm or EUK134. *, p < 0.05, SODm and EUK134 versus control groups, n = 7.
Discussion
It has been demonstrated that there was a burst formation of ROS/RNS, including NO (mainly from endothelial cells) and superoxide (mainly from mitochondria), at the very 1st min of reperfusion (Zweier, 1988; Wang and Zweier, 1996; Ferdinandy and Schulz, 2003). The formation of peroxynitrite from NO and superoxide has been reported to suppress the mitochondrial oxygen consumption and attenuate the postischemic myocardial function (Zhao et al., 2005; Zhu et al., 2007). Therefore, prevention of the formation of peroxynitrite and protection of the postischemic myocardial oxygen consumption may lead to salvage of the myocardium at risk. We have previously reported that inhibition of NO production protected the heart from ischemia reperfusion injury with the attenuation of the postischemic hyperoxygenation (Zhao et al., 2005). However, NO has a number of protective effects, such as vasodilation, inhibition of platelet aggregation, and antiapoptotic effects, all leading to cardiac tissue protection. Therefore, antioxidant therapy targeting on superoxide formation would be more preferable. It has also been reported that hydrogen peroxide can vasodilate through activation of different surface potassium channels (Burgoyne et al., 2007; Kokusho et al., 2007). Furthermore, the activation of the sKATP channels has been reported to enhance the reperfusion cardiac contractile function (Grover et al., 1991). Therefore, by using a selective SODm (Masini et al., 2002), dismutation of superoxide and subsequent formation of hydrogen peroxide may further improve the postischemic myocardial function.
In the current study, we have used different SOD mimetics to dismutate the burst formation of superoxide after ischemia reperfusion. EPR oximetry was applied to measure the in vivo tissue Po2 after treatment with SODm, EUK134, and SODm plus glibenclamide. It was observed that the hyperoxygenation status after ischemia reperfusion was attenuated after all treatments. It is interesting that, with the treatment of SODm, there was a transient peak of tissue Po2, which was independent of NO formation. Recently, it has been reported that hydrogen peroxide can activate protein kinase G and, therefore, activate sarcolemmal potassium channel and exert its hyperpolarization and vasorelaxation effect (Burgoyne et al., 2007). Based on our EPR oximetry data, we hypothesize that selective dismutation of the burst formation of superoxide and subsequent formation of hydrogen peroxide at the beginning of reperfusion activates the sKATP channels and, therefore, vasodilates and increases blood perfusion after reperfusion. With comparison to the treatment of EUK134, we hypothesized that the transient peak of tissue Po2 was due to the formation of hydrogen peroxide via the dismutation of superoxide with the selective SODm. The blood flow and hemodynamic measurements confirmed our hypothesis that the subsequent formation of hydrogen peroxide enhanced blood perfusion and cardiac contractile function after reperfusion. The data from the treatment of SODm plus glibenclamide suggested that the transient peak of tissue Po2 was possibly due to the opening of the sKATP channels by hydrogen peroxide. As reported, the opening of the mitochondrial ATP-dependent potassium (mitoKATP) channels also protects the heart from ischemia reperfusion injury (Mozaffari and Schaffer, 2008). The protective mechanism is due to the generation of reactive oxygen species to precondition the heart and the reduction of calcium overload in the mitochondria (Pasdois et al., 2007). Glibenclamide can also inhibit the opening of the mitoKATP channels, therefore, abrogating the protection. In our study, we coadministered glibenclamide with the SODm. Our data showed that glibenclamide prevented the transient peak of tissue Po2 due to its inhibitory effect on the increase of blood flow and enhancement of cardiac function after the treatment of the SODm. These results suggested that the effect of glibenclamide in this study is more likely through the sKATP channels, possibly in the endothelial and smooth muscle cells rather than the mitoKATP channels in the myocyte mitochondria.
It has been reported that NO reversibly inhibited cytochrome c oxidase (Lizasoain et al., 1996). Therefore, the interesting phenomenon of the transient peak of tissue Po2 after treatment of SODm in our oximetry studies could be due to the dismutation of superoxide; thus, more NO is available in the reperfused tissue. To determine whether NO is responsible for this transient oxygen peak, we have also performed the EPR oximetry on eNOS−/− mice. It was observed that the transient peak was not dependent on the bioavailability of NO. This result supported our hypothesis that the transient peak of tissue Po2 was due to the formation of endogenous hydrogen peroxide.
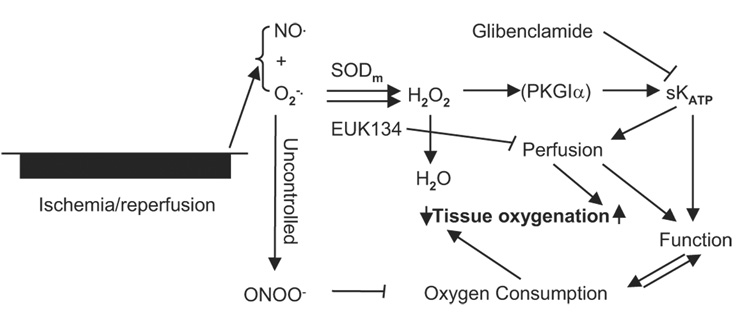
Figure 9 is the flow chart of our working hypotheses. In the uncontrolled situation with the burst formation of NO and superoxide, the oxidant peroxynitrite suppressed the mitochondrial oxygen consumption, degraded the contractile function, and therefore caused postischemic hyperoxygenation. Given that SODm selectively dismutates the superoxide burst at the beginning of reperfusion, the subsequently formed hydrogen peroxide may activate protein kinase G subunit 1α and eventually open the sKATP channels, which may increase blood perfusion to the risk area as well as enhance the cardiac contractile function. The increased blood perfusion at the beginning of reperfusion and enhanced cardiac function afterward may lead to the transient peak of tissue Po2 as depicted from the EPR oximetry measurement in the SODm group. When SODm was used together with glibenclamide, the inhibitor of the sKATP channels, the transient peak of tissue Po2 was attenuated. Because EUK134 has both SOD and catalase activities, the treatment of EUK134 dismutates superoxide and reduces hydrogen peroxide to water, thus, decreasing tissue perfusion. So with the treatment of EUK134, it is expected that there will be no transient variations of tissue Po2 but only the suppression of the hyperoxygenation status.
Fig. 9.
Flow chart of the proposed hypotheses of free radical-related signaling pathways. After ischemia reperfusion, there is a burst formation of ROS/RNS, including NO·, , and ONOO−. These reactive species interact with mitochondrial enzymes and, therefore, suppress the oxygen consumption, leading to tissue hyperoxygenation. Given that SODm is -specific while EUK134 has both SOD and catalase activities, with treatment of SODm, the burst is dismutated to H2O2. With treatment of EUK134, the burst is dismutated and reduced to water. The formation of H2O2 after treatment with SODm may further activate the sKATP channel, possibly through activation of protein kinase G subunit 1α (PKG1α). The activation of the sKATP channels may lead to increased blood perfusion as well as enhanced cardiac contractile function, which may explain why there is an initial increase and then a decrease (a transient peak) of tissue Po2 following increased blood flow and RPP values. When the sKATP channels are inhibited by glibenclamide, the increase of blood flow is attenuated and the RPP values are decreased gradually; therefore, the transient peak of Po2 is attenuated.
In vitro studies have shown that cytochrome c oxidase was reversibly inhibited by NO competing on the oxygen binding site (Lizasoain et al., 1996). The same study has also demonstrated that NADH dehydrogenase was irreversibly inhibited by peroxynitrite probably through protein tyrosine nitration. Yet another recent study has demonstrated that NO can also irreversibly inhibit cytochrome c oxidase at low oxygen concentrations, possibly through the formation of nitroxyl anions (Parihar et al., 2008). Our previous in vivo studies have demonstrated that both of these two enzymes were irreversibly inhibited after ischemia reperfusion (Zhu et al., 2007). In the current study, both of these two enzymes were also found to be inhibited after ischemia reperfusion, and treatment of SODm restored the activities, which indicated that the inhibition is probably due to superoxide-derived peroxynitrite. Both SODm and EUK134 can remove superoxide to protect ischemia-reperfusion heart, given the observation that in mitochondria there is no difference in activities of CcO and NADH-DH between the two treatments and the control group. SODm generates H2O2 via removing superoxide, increases cardiac function, and reperfuses the ischemic myocardium better. At the same time, SODm may induce tachycardia through generation of hydrogen peroxide depending on the dosage used. Overdose of SODm will certainly increase myocardial oxygen consumption abruptly in a short period of time; arrhythmia, circulatory derangement, or even death will occur. Although EUK134 reduces H2O2 to O2 and H2O, it gives a less increment in blood reperfusion and less reinforcement in heart function than that of SODm. In all, two SOD analogs were tested to afford protection to the ischemic and reperfused heart. With restricted dosage, SODm (4 mg/kg or less) enhanced cardiac function and increased postischemic reperfusion.
In conclusion, SOD mimetics suppressed the burst formation of superoxide at the beginning of reperfusion and preserved mitochondrial oxygen consumption. With specific SODm and the subsequent formation of hydrogen peroxide, myocardial tissue reperfusion was increased, and postischemic cardiac contractile function was enhanced. Our findings provide new insights into therapeutic strategies to target specific injurious ROS/RNS to prevent ischemia reperfusion injury and to salvage the myocardium at risk.
Acknowledgments
We thank Dr. Harold Swartz at the EPR Center for the Study of Viable Systems (Dartmouth Medical School, Hanover, NH) for support on the oximetry probes, Dr. Yeong-Renn Chen for support on the assay of mitochondrial enzyme activities, and MetaPhore Pharmaceuticals for support of the SOD mimetic M40403.
This work was supported by American Heart Association Grant 0435299N and National Heart, Lung, and Blood Institute, National Institutes of Health Grant HL-081630 (to G.H.).
ABBREVIATIONS
- ROS/RNS
reactive oxygen/nitrogen
- NADH-DH
NADH dehydrogenase
- SOD
superoxide dismutase
- SODm
SOD mimetic
- CcO
cytochrome c oxidase
- EPR
electron paramagnetic resonance
- Glib
glibenclamide
- LiPc
lithium phthalocyanine
- RPP
rate pressure product
- M40403
a manganese(II)-bis(cyclohexylpyridine)-substituted macrocyclic superoxide dismutase mimetic
- C21H35Cl2MnN5
EUK134 10006329 EUK 134 manganese 3-methoxy N, N1-bis(salicyclidene)ethylenediamine chloride
- sKATP
sarcolemmal ATP-dependent potassium channel
- mitoKATP
mitochondrial ATP-dependent potassium channel
- eNOS
endothelial nitric-oxide synthase
- PBS
phosphate-buffered saline
- LAD
left anterior descending coronary artery
- WT
wild type
- IR
ischemia reperfusion
- AAR
area at risk
- MABP
mean arterial blood pressure
- HR
heart rate
- TTC
triphenyl tetrazolium chloride
- LV
left ventricle
References
- Al-Obaidi MK, Etherington PJ, Barron DJ, Winlove CP, Pepper JR. Myocardial tissue oxygen supply and utilization during coronary artery bypass surgery: evidence of microvascular no-reflow. Clin Sci (Lond) 2000;98:321–328. [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic Biol Med. 2002;33:1440–1450. doi: 10.1016/s0891-5849(02)01112-7. [DOI] [PubMed] [Google Scholar]
- Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- Chen YR, Chen CL, Chen W, Zweier JL, Augusto O, Radi R, Mason RP. Formation of protein tyrosine ortho-semiquinone radical and nitrotyrosine from cytochrome c-derived tyrosyl radical. J Biol Chem. 2004;279:18054–18062. doi: 10.1074/jbc.M307706200. [DOI] [PubMed] [Google Scholar]
- Clementi E, Brown GC, Foxwell N, Moncada S. On the mechanism by which vascular endothelial cells regulate their oxygen consumption. Proc Natl Acad Sci U S A. 1999;96:1559–1562. doi: 10.1073/pnas.96.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JF, Swartz HM. In vivo electron paramagnetic resonance oximetry with particulate materials. Methods. 2003;30:159–166. doi: 10.1016/s1046-2023(03)00077-x. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- Grover GJ, Dzwonczyk S, Sleph PG, Sargent CA. The ATP-sensitive potassium channel blocker glibenclamide (glyburide) does not abolish preconditioning in isolated ischemic rat hearts. J Pharmacol Exp Ther. 1993;265:559–564. [PubMed] [Google Scholar]
- Grover GJ, Newburger J, Sleph PG, Dzwonczyk S, Taylor SC, Ahmed SZ, Atwal KS. Cardioprotective effects of the potassium channel opener cromakalim: stereoselectivity and effects on myocardial adenine nucleotides. J Pharmacol Exp Ther. 1991;257:156–162. [PubMed] [Google Scholar]
- He G, Shankar RA, Chzhan M, Samouilov A, Kuppusamy P, Zweier JL. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci U S A. 1999;96:4586–4591. doi: 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M, McDonald MC, Sharpe MA, Chatterjee PK, Thiemermann C. Superoxide dismutase mimetics with catalase activity reduce the organ injury in hemorrhagic shock. Shock. 2002;18:230–235. doi: 10.1097/00024382-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Kokusho Y, Komaru T, Takeda S, Takahashi K, Koshida R, Shirato K, Shimokawa H. Hydrogen peroxide derived from beating heart mediates coronary microvascular dilation during tachycardia. Arterioscler Thromb Vasc Biol. 2007;27:1057–1063. doi: 10.1161/ATVBAHA.0000261570.85983.4f. [DOI] [PubMed] [Google Scholar]
- Kreutzer U, Jue T. Critical intracellular O2 in myocardium as determined by 1H nuclear magnetic resonance signal of myoglobin. Am J Physiol. 1995;268:H1675–H1681. doi: 10.1152/ajpheart.1995.268.4.H1675. [DOI] [PubMed] [Google Scholar]
- Kuppusamy P, Zweier JL. EPR imaging of free radicals in the perfused heart. Curr Top Biophys. 1994;18:3–13. [Google Scholar]
- Lizasoain I, Moro MA, Knowles RG, Darley-Usmar V, Moncada S. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem J. 1996;314:877–880. doi: 10.1042/bj3140877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke KE, McConnell PI, Tuzman JM, Shesely EG, Smith CJ, Stackpole CJ, Thompson CI, Kaley G, Wolin MS, Hintze TH. Endogenous endothelial nitric oxide synthase-derived nitric oxide is a physiological regulator of myocardial oxygen consumption. Circ Res. 1999;84:840–845. doi: 10.1161/01.res.84.7.840. [DOI] [PubMed] [Google Scholar]
- Masini E, Cuzzocrea S, Mazzon E, Marzocca C, Mannaioni PF, Salvemini D. Protective effects of M40403, a selective superoxide dismutase mimetic, in myocardial ischaemia and reperfusion injury in vivo. Br J Pharmacol. 2002;136:905–917. doi: 10.1038/sj.bjp.0704774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari MS, Schaffer SW. Effect of pressure overload on cardioprotection of mitochondrial KATP channels and GSK-3beta: interaction with the MPT pore. Am J Hypertens. 2008;21:570–575. doi: 10.1038/ajh.2008.25. [DOI] [PubMed] [Google Scholar]
- Pandian RP, Parinandi NL, Ilangovan G, Zweier JL, Kuppusamy P. Novel particulate spin probe for targeted determination of oxygen in cells and tissues. Free Radic Biol Med. 2003;35:1138–1148. doi: 10.1016/s0891-5849(03)00496-9. [DOI] [PubMed] [Google Scholar]
- Parihar A, Vaccaro P, Ghafourifar P. Nitric oxide irreversibly inhibits cytochrome oxidase at low oxygen concentrations: evidence for inverse oxygen concentration-dependent peroxynitrite formation. IUBMB Life. 2008;60:64–67. doi: 10.1002/iub.12. [DOI] [PubMed] [Google Scholar]
- Pasdois P, Beauvoit B, Costa AD, Vinassa B, Tariosse L, Bonoron-Adele S, Garlid KD, Dos Santos P. Sarcoplasmic ATP-sensitive potassium channel blocker HMR1098 protects the ischemic heart: implication of calcium, complex I, reactive oxygen species and mitochondrial ATP-sensitive potassium channel. J Mol Cell Cardiol. 2007;42:631–642. doi: 10.1016/j.yjmcc.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Rapapaport E. Free radicals in reperfusion infury. In: Rapapaport E, editor. Early Interventions in Acute Myocardial Infarction. Philadelphia: Kluwer Academic Publishers; 1989. [Google Scholar]
- Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Dunn JF. Measurements of oxygen in tissues: overview and perspectives on methods. Adv Exp Med Biol. 2003;530:1–12. doi: 10.1007/978-1-4615-0075-9_1. [DOI] [PubMed] [Google Scholar]
- Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol. 2003;285:H2255–H2263. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- Venkatesh N, Stuart JS, Lamp ST, Alexander LD, Weiss JN. Activation of ATP-sensitive K+ channels by cromakalim. Effects on cellular K+ loss and cardiac function in ischemic and reperfused mammalian ventricle. Circ Res. 1992;71:1324–1333. doi: 10.1161/01.res.71.6.1324. [DOI] [PubMed] [Google Scholar]
- Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, et al. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YW, Kaminski PM, Wolin MS. Inhibition of rat cardiac muscle contraction and mitochondrial respiration by endogenous peroxynitrite formation during posthypoxic reoxygenation. Circ Res. 1998;82:891–897. doi: 10.1161/01.res.82.8.891. [DOI] [PubMed] [Google Scholar]
- Yada T, Shimokawa H, Morikawa K, Takaki A, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Role of Cu,Zn-SOD in the synthesis of endogenous vasodilator hydrogen peroxide during reactive hyperemia in mouse mesenteric microcirculation in vivo. Am J Physiol Heart Circ Physiol. 2008;294:H441–H448. doi: 10.1152/ajpheart.01021.2007. [DOI] [PubMed] [Google Scholar]
- Zhao X, He G, Chen YR, Pandian RP, Kuppusamy P, Zweier JL. Endothelium-derived nitric oxide regulates postischemic myocardial oxygenation and oxygen consumption by modulation of mitochondrial electron transport. Circulation. 2005;111:2966–2972. doi: 10.1161/CIRCULATIONAHA.104.527226. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Richman A, Storey C, Radford NB, Pantano P. In situ fiber-optic oxygen consumption measurements from a working mouse heart. Anal Chem. 1999;71:3887–3893. doi: 10.1021/ac9903003. [DOI] [PubMed] [Google Scholar]
- Zhu X, Liu B, Zhou S, Chen YR, Deng Y, Zweier JL, He G. Ischemic preconditioning prevents in vivo hyperoxygenation in postischemic myocardium with preservation of mitochondrial oxygen consumption. Am J Physiol Heart Circ Physiol. 2007;293:H1442–H1450. doi: 10.1152/ajpheart.00256.2007. [DOI] [PubMed] [Google Scholar]
- Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]
- Zweier JL, Kuppusamy P, Lutty GA. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988;85:4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT. Measurement and characterization of postischemic free radical generation in the isolated perfused heart. J Biol Chem. 1989;264:18890–18895. [PubMed] [Google Scholar]