Abstract
Purpose
X-linked agammaglobulinemia (XLA) is a humoral immunodeficiency disease caused by a mutation in the Bruton tyrosine kinase (BTK) gene resulting in defective B cell differentiation. Because it is a relatively rare disorder, it is difficult for clinicians to have a comprehensive understanding of XLA due to a lack of exposure to the disease. Clinical presentations of patients with XLA were analyzed and discussed to improve care plans.
Materials and Methods
During a 20 year period, from January 1987 to June 2006, a total of 19 patients were diagnosed as XLA in the Department of Pediatrics at Severance Hospital, Seoul, Korea. A retrospective analysis of the clinical presentations of those patients was performed.
Results
The mean age of the XLA patients included in the study was 4.89 years, with a range of 6 months to 13 years. Twelve patients were diagnosed before age 5, while the other 7 patients were diagnosed after age 5. Recurrent infections observed in the patients included pneumonia, acute otitis media, septic arthritis, skin infection, sepsis, sinusitis, acute gastroenteritis, cervical lymphadenitis, epididymitis, meningitis, osteomyelitis, urinary tract infection and encephalitis. Frequency of admissions was variable from 0 to 12 times, depending on the time at which immunoglobulin therapy was started. Six cases had family histories positive for XLA. BTK gene mutations were found in 8 cases.
Conclusion
The overall prognosis of XLA is good as long as patients are diagnosed and treated early with regular intra venous gamma globulin therapy before the sequelae of recurrent infections appear.
Keywords: Agammaglobulinemiainfection, Bruton's tyrosine kinaseinfection, infection
INTRODUCTION
X-linked agammaglobulinemia (XLA) is a genetic disorder in which the development of B cells arrests during differentiation. It has been shown to be caused by a variety of mutations in the gene encoding Bruton tyrosine kinase (BTK).1-3 BTK is a signal-transducing protein expressed in all hematopoietic lineages, except in T cells and NK cells.2 It is composed of five distinct structural domains: the pleckstrin homology (PH), Tec homology (TH), Src homology (SH) 3, SH2, and catalytic kinase (SH1) domains.4 XLA is often characterized by recurrent bacterial infections due to a decrease in the number of B cells and a subsequent reduction in the level of serum immunoglobulin.2,3,5,6 The BTK gene has been mapped to Xq21.3-Xq22, encompassing 37.5 kb and containing 19 exons, and mutations in the gene are inherited in an X-linked recessive pattern.7
Since 1952 when the disease was first described by Bruton,8 the incidence of XLA has increased and has been recognized at an earlier age.9 Early diagnosis has been made possible by genetic analysis of the BTK gene, available since 1994, and thus administration of intravenous gamma globulin therapy (IVGG) can be started earlier. This treatment has decreased the prevalence of infections and frequency of hospital admissions.9,10
Because of the low incidence of this disease (around 1/200,000 live births11) there are few reports of XLA in a single institution with the exception of the study by Moin, et al., a retrospective study of 33 XLA patients over a period of 22 years at one of the largest referral centers in Iran.12 There are some reports of multicenter studies, such as those by Winkelstein, et al.9 in the US, and by Plebani, et al. in Italy.13 This study is the first attempt in South Korea for a systematic analysis of the natural clinical course and presentation of the disease. It was undertaken to increase awareness of XLA in physicians and to improve care plans through a better understanding of the clinical courses of the disease in patients at this institute, one of the largest tertiary referral centers in South Korea.
MATERIALS AND METHODS
We reviewed the records of 19 patients with XLA who had been referred to Severance Children's Hospital, South Korea from January 1987 to June 2006. The diagnosis of XLA was made based on standard criteria described below.14
Diagnostic Criteria
Definitive
A male patient with less than 2% CD19 B cells and at least one of the following findings is present.
Mutation in BTK gene.
Absence of BTK mRNA on northern blot analysis of neutrophils or monocytes.
Absence of BTK protein in monocytes or platelets.
Maternal cousins, uncles, or nephews with less than 2% CD19 B cells.
Probable
A male patient with less than 2% CD19 B cells in whom all of the following findings are present:
Recurrent bacterial infections in the first 5 years of life.
Serum IgG, IgM, and IgA more than 2 SD below normal for the patient's age.
Absent isohemagglutinins and/or poor response to vaccines.
Other causes of hypogammaglobulinemia have been excluded.
Possible
A male patient with less than 2% CD19 B cells in whom other causes of hypogammaglobulinemia have been excluded and at least one of the following findings is present:
Recurrent bacterial infections in the first 5 years of life.
Serum IgG, IgM, and IgA more than 2 SD below normal for the age.
Absence of isohemagglutinins.
To exclude other causes of hypogammaglobulinemia, such as transient hypogammaglobulinemia of infants, a close follow-up was done by checking serum IgG, IgA, and IgM levels, by examining B and T cell subset enumeration (CD19, CD3, CD4, and CD8) through 2 times of flow cytometric analysis in all patients, and by confirming the presence of tonsils. In 8 patients, gene sequencing was performed to detect BTK mutations using the method described by Futatani, et al.15
RT-PCR and DNA sequencing
Total RNA was prepared from PBMC using TRIzol reagent (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. cDNA was synthesized from RNA using reverse transcriptase. PCR amplification of the BTK cDNA was performed using seven overlapping PCR primers as described by Fututani, et al.15 The resulting PCR products were purified using a DNA purification system (Promega, Madison, WI). Direct sequencing of the amplified RT-PCR products by the dideoxynucleotide chain termination method was performed using the Sequenase 2.0 kit (USB, Cleveland, OH) and the BigDye terminator cycle sequencing kit (PE Applied Biosystems, Foster City, CA) along with an automated Applied Biosystems PRISM 310 genetic analyzer (PE Applied Biosystems). Forward and reverse primers for use in manual DNA sequencing were labeled with α-35S-dATP, and the reaction products were electrophoresed on denaturing 6% polyacrylamide gels. Mutated sequences were confirmed by opposite-strand sequencing or by sequencing genomic DNA fragments covering intron-exon boundaries (reference: U78027).
SSCP and RFLP analyses
For SSCP analysis, 3 µL of PCR product was mixed with 9 µL of sample loading buffer (95% formamide, 10 mM NaOH, 0.25% bromophenol blue, and 0.25% xylene cyanol). The samples were denatured for 5 min at 100℃ in 1 × sample buffer (33 mM Tris-sulfate and 7% glycerol, pH 8.3). The DNA was then resolved by 12% PAGE and stained using a Silver Stain Plus kit (Bio-Rad, Hercules, CA, USA). PCR-RFLP was performed using MboI restriction endonuclease site and analyzed by 2% agarose gel electrophoresis.
Statistical analysis
Pearson correlation coefficient between the age of the patient when IVGG treatment was started and the frequency of admission was calculated in patients with Bruton disease. The p value was two-sided, and values less than 0.05 were considered statistically significant.
RESULTS
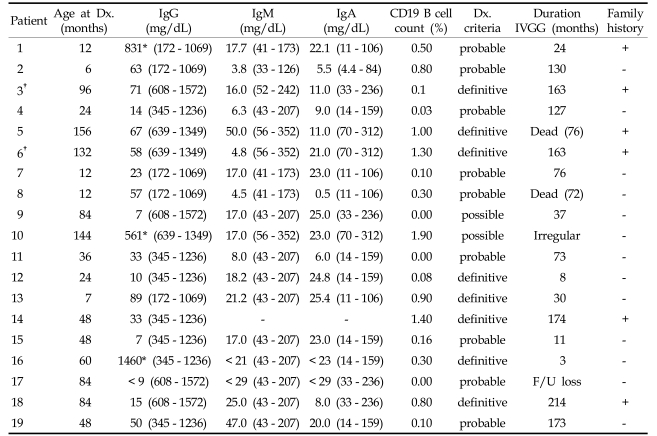
There were a total of 19 patients diagnosed as Bruton disease, with an age distribution at the time of diagnosis varying from 6 months to 13 years. The mean age at diagnosis was 4.89. Twelve patients were diagnosed before age 5. The other 7 patients had been transferred from other hospitals due to recurrent severe infections after age 5. Serum IgG concentrations ranged from 7 to 88.6 mg/dL, and B cell counts ranged from 0 to 1.9%. Patients 1, 10, and 16 received immunoglobulin prior to an immune work-up, so their baseline data were not available. The diagnostic laboratory results are listed in Table 1.
Table 1.
Clinical Features of X-linked Agammaglobulinemia Patients
*After immunoglobulin replacement values.
†Patients 3 and 6 are siblings.
Dx, diagnosis; F/U, follow up.
According to diagnostic criteria,14 all patients in the study were diagnosed as XLA. Eight of the patients (3, 5, 6, 12, 13, 14, 16, and 18) were diagnosed as definitive using genetic studies. Nine of them were diagnosed as probable, and 2 (9 and 10) as possible.
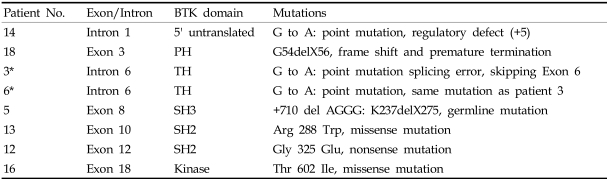
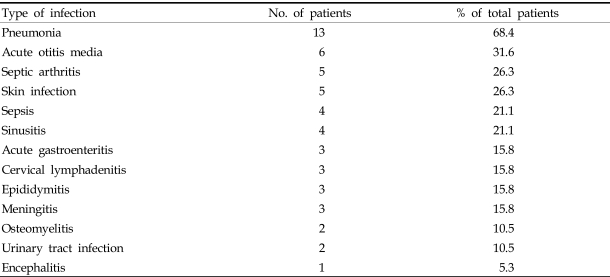
Most of the patients were admitted due to recurrent bacterial infections; only one case (patient 3) was revealed to be Bruton disease by genetic screening because his sibling had been diagnosed as XLA (patient 6). In the case of patient 13, his mother had undergone chorionic villus sampling during her second pregnancy, and it revealed that the fetus had a BTK gene mutation. The results of gene sequencing are found in Table 2. A total of 8 cases were part of the BTK gene study. The most frequent infections, in order of descending frequencies, were pneumonia, acute otitis media, septic arthritis, skin infection, sepsis, sinusitis, acute gastroenteritis, cervical lymphadenitis, epididymitis, meningitis, osteomyelitis, urinary tract infection and encephalitis (Table 3). About 25% of patients had more than one episode of septic arthritis. At first, primary care physicians (PCPs) suspected these patients had juvenile rheumatoid arthritis, and they were treated with NSAIDs. The PCPs later referred them to the tertiary care center because there was no clinical improvement. Synovial fluid cultures revealed the patients had staphylococcal, pseudomonal, and mycobacterial infections. Recurring and unusual clinical courses of pneumonia, gastroenteritis, and meningitis by enteroviral infection led pediatricians to suspect immunodeficiency, and after several tests the patients were diagnosed as XLA. A prolonged course of illness and the development of serious complications like bronchiectasis, gastrointestinal bleeding, and meningoencephalitis prompted these suspicions. For patients below age one, it is difficult to diagnose XLA caused by sporadic mutations until fulminant sepsis, atypical pneumonia, and encephalitis by enterovirus occur. Ten percent of our XLA patients were below age one. One case was revealed as XLA by immune studies due to recurrent acute gastroenteritis and pneumonia presented with a prolonged clinical course. Another case was diagnosed due to a past medical history of encephalitis and sepsis and a recent diagnosis of Q-fever after a full work-up for fever of unknown origin (FUO). Each clinical course of XLA patients and antibiotic therapies given to each patient is listed in Table 4.
Table 2.
Summary of Mutations in the Bruton Tyrosine Kinase Gene of the Patients
PH, pleckstrin homology; SH, Src homology; TH, Tec homology.
*Patients 3 and 6 are siblings.
Table 3.
Infections Leading to Hospitalization in Patients with XLA
Table 4.
Clinical Courses of the Patients with XLA
INAH, isoniazid; RFP, rifampin; PZA, pyrazinamide; FUO, fever of unknown origin; AOM, acute otitis media; AGE, acute gastroenteritis; UTI, urinary tract infection.
There were 2 cases (patients 4 and 19) in which chronic lung diseases (CLD) developed due to recurrent pneumonia. Despite receiving IVGG therapy regularly since diagnosis, the patients' CLDs have been remained and continued to be major health problems.
Microorganisms isolated from the patients included S. aureus, S. pneumoniae, P. aeruginosa, E. coli, Enterovirus, Epstein-Barr virus, Aspergillus fumigatus, Candida albicans, and M. tuberculosis.
For their routine immunizations, all XLA patients included in our study received the live vaccine for polio, tuberculosis, measles, mumps, and rubella before diagnosis.
The frequency of admission varied from 0 to 12 times, but with earlier IVGG therapy there were significantly fewer admissions (r = 0.624, p < 0.01). There were no cases of fatal pneumonia in the group receiving IVGG therapy, although mild otitis media and sinusitis did occur in this group.
XLA patients were treated with IVGG (400 mg/kg/dose) monthly to maintain a serum concentration of IgG of over 500 mg/dL.13 In spite of IVGG therapy, there were incidents of pneumonia, pulmonary mycobacterial infections, and upper respiratory infections in the patients, but overall clinical features were not severe.
Several patients also presented extra manifestations. Autoimmune diseases were observed in 6 of the patients, 3 were diagnosed as juvenile rheumatoid arthritis, and 3 suffered from autoimmune epididymitis. Hepatocellular carcinoma (HCC) was also observed in one patient.
Two patients in the study died. Patient 5 died of HCC which developed from liver cirrhosis due to chronic Hepatitis B. Patient 8 was not followed-up and was found to be dead after a phone call inquiry. Patient 17 was neither followed-up and thus was dropped from our survey after 2003.
Extended family histories were evaluated in 6 cases. Having a family member who had been diagnosed as XLA was considered to have a positive family history. Excluded from this designation were cases in which an early death of a family member occurred due to severe recurrent infections.10 In two families, an older brother had been previously diagnosed, prompting an evaluation of the other member of the family. The patient's sibling was then diagnosed as XLA by BTK gene sequencing, and, as a result, the younger brother received IVGG therapy earlier than any other patient in our study and did not experience any severe infections. A total of 3 families received gene screening with PCR-SSCP. As a result, in two families, the patients' mothers exhibited a typical mosaic pattern and in the third family, the patient's maternal grandmother was normal while one maternal aunt had the same carrier state.
DISCUSSION
An early diagnosis of XLA with immediate initiation of therapy is crucial for ensuring good outcomes for the affected patients.10 Delayed diagnosis could lead to long-lasting sequelae such as bronchiectasis, hearing loss, or liver cirrhosis due to chronic hepatitis. BTK gene sequencing is very useful in diagnosing XLA in cases of overlapping clinical features like common variable immunodeficiency, transient hypogammaglobulinemia, and mixed neutropenia situations. Early diagnoses are now being made using gene sequencing techniques before overwhelming infections occur. Though missense mutations affecting nonconserved residues in the amino acid sequence for each domain and mutations in noninvariant splicing positions are less severe mutations,10 the genotype-phenotype correlation has not been well-characterized.4,16 The main feature of XLA is recurrent bacterial infections due to humoral immunodeficiency. The disease occasionally manifests with diverse clinical spectrums such as autoimmune diseases or cancer. It is known to have an X chromosome-linked inheritance pattern. However, according to several reports, there is a positive family history in only 30 - 50% of all XLA patients, with the remaining cases developing as sporadic mutations.9,10 According to the BTK gene database (http://bioinf.uta.fi/BTKbase/types_domains.html), a total of 620 gene mutations from 974 unrelated families have been registered. Patients assumed to have XLA have BTK gene mutations in 90 - 95% of the cases and, if a mutation in BTK is revealed, the diagnosis is confirmed.14 This gene study is the most useful in atypical XLA patients, whose serum IgG concentrations are above the diagnostic threshold and whose resulting clinical manifestations are subtle; these patients usually have a delayed diagnosis.17 According to references, in general, around 15 - 59% of patients have undergone gene studies to look for BTK mutations.9,12 In our institution, 42% of XLA patients were genetically examined. The accepted standard diagnosis method, based on hypogammaglobulinemia and a B cell count under 2%, accounts for only 17% of the total of XLA diagnoses.9 Around 20% of XLA patients were diagnosed on the basis of family history, recurrent bacterial infections, and BTK gene mutation when other laboratory data did not fulfill diagnostic criteria.9 Another diagnostic clue is the absence of tonsils and scanty cervical lymph nodes. The marked paucity of cervical lymph nodes and tonsilar tissue with recurrent otitis media is unusual and should alert primary physicians to the possibility of immunodeficiency.10
Fortunately, none of the patients in our study had any negative consequences due to the live vaccines they received as standard care prior to diagnosis. This phenomenon might be explained by the fact that T cell function in XLA is not impaired, so partial immune defense mechanisms can compensate for impaired humoral immunity. Recently, the live polio vaccine was replaced by an inactivated, killed vaccine, so there is now no risk that XLA patients might develop polio infection.12,18,19
A history of septic arthritis can be another diagnostic clue of XLA. If physicians suspect this disorder, they should take a thorough family history and order an immune work-up. If patients with septic arthritis and XLA are treated with antibiotics alone, they show transient improvement because they cannot clear the infection without immunoglobulin replacement therapy. In this study, patient 3 was diagnosed as XLA earlier than other patients, so the IVGG therapy began earlier, and the septic arthritis did not recur. He did not suffer any functional defects as a result. In contrast, patient 10 was diagnosed when he was 12 years old, and IVGG therapy was delayed, so the septic arthritis escalated to recurrent osteomyelitis, leaving this patient with a functional defect.
In addition to septic arthritis, XLA patients often suffer chronic enteroviral infections, persistent rotaviral infection, chronic diarrhea by Giardia lamblia, and other persistent infections as a result of incomplete eradication of antigen due to humoral immunodeficiency. In patients 13, 14, and 18, there were several histories of admissions because of this kind of problems, and in patient 5, an enteroviral infection progressed to encephalitis. All of these episodes could lead to a differential diagnosis including XLA.19
Comparing this study with recent reports in the US, 58% of XLA patients have a family history of the disease, with about 34% of these patients diagnosed without symptoms through investigations indicated by a positive family history.9 By contrast, in this study, about 30% of XLA patients had a family history of XLA and broad genetic screenings could not have been performed on cousins. In only for a select few cases genetic screenings were performed. The reluctance to consent to the screening could be due to a fear of stigma against the maternal side of the family, failure to understand the disease, and core family trends.
The most common infections seen in XLA patients differ slightly from country to country. In the US, early diagnoses based on family history, recurrent bacterial infections, and neutropenia are prevalent, so the most frequent clinical feature is otitis media. This is a relatively mild symptom compared with severe life threatening infections suffered by other XLA patients.9 Other common infections are pneumonia, sinusitis, and gastroenteritis. In the cases in this study, fulminant pneumonia and septic arthritis were the most common. Other common infections were skin infections and otitis media. Immune work-ups were done in cases of unusual recurrent infections such as pseudomonal sepsis, staphylococcal sepsis, and perianal abscess.10
Early IVGG replacement therapy decreased the rates of admission and morbidity for chronic complications, such as bronchiectasis and chronic lung disease, and prevented fatal complications like meningoencephalitis. According to one report, appropriate IV immunoglobulin replacement therapy should be started at 6 to 8 weeks of age because around 25% of the XLA patients show clinical symptoms before 4 months of age.20 After the infant period, more than 3 occurrences of otitis media and sinusitis, the absence of tonsils, and the presence of scanty cervical lymph nodes are indications to check immunoglobulin serum levels. If the levels of more than two types of immunoglobulin are decreased, XLA should be suspected.9 Otolaryngologists should also be mindful of this disease when they are caring for otitis media and sinusitis, the most frequent bacterial infections in childhood.
The incidence of XLA seems to be increasing throughout the world.9 In our study, from 1985 - 2000, 11 cases were observed; from 2000 - 2006, 8 cases were observed. This phenomenon may be due to increasing rates of diagnosis since the implementation of genetic analysis. One patient in our study had only recurrent upper respiratory infections and otitis media as symptoms of the underlying problem and had no other serious infections. However, his observant primary care physician ordered the immunologic screening test and referred him to this hospital.
According to a recent study done in the US in 2006,9 a total of 201 XLA patients were entered in the registry. Seventeen of those patients died. Fourteen of the patients were dead at the time they were entered in the registry (before 1999), and the additional 3 patients died (3.75%) during the follow-up period (1999 - 2004). This is a relatively low rate compared with those of other cellular immunodeficiencies. The most common cause of death was chronic enteroviral infection, followed by CLD and hepatitis. In this study, one patient died of hepatocellular carcinoma secondary to Hepatitis B virus (HBV). HBV infection is emerging as a life-threatening infection in long term follow-up of XLA patients. If the patient has a family member who is an HBV carrier, routine monitoring of serum HBV surface antigen is necessary, especially in areas like South Korea where HBV is endemic.
Respiratory tract infections are prominent clinical problem observed during follow-up despite immunoglobulin replacement therapy. The overall probability of developing CLD reached about 80% after 17 years of follow-up.13 In our study, following patients over 20 years, almost all XLA patients have experienced intermittent mild sinusitis and bronchitis despite IVGG. No severe CLD was found in our study.
XLA is a well-known immunodeficiency disease for which early diagnosis and proper management is possible if physicians have a high index of suspicion for this disease. Although gene defects are known, clinical phenotypes are different for the same mutation and several diseases can be presented combining with XLA, so further studies should tell us about these findings. To advance the study of XLA, including gene analysis and new treatment modalities, the joint efforts of several referral centers would be required.
References
- 1.Parolini O, Hejtmancik JF, Allen RC, Belmont JW, Lassiter GL, Henry MJ, et al. Linkage analysis and physical mapping near the gene for X-linked agammaglobulinemia at Xq22. Genomics. 1993;15:342–349. doi: 10.1006/geno.1993.1066. [DOI] [PubMed] [Google Scholar]
- 2.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 4.Chan KW, Chen T, Jiang L, Fok SF, Lee TL, Lee BW, et al. Identification of Bruton tyrosine kinase mutations in 12 Chinese patients with X-linked agammaglobulinaemia by long PCR-direct sequencing. Int J Immunogenet. 2006;33:205–209. doi: 10.1111/j.1744-313X.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosen FS, Cooper MD, Wedgwood RJ. The primary immunodeficiencies (1) N Engl J Med. 1984;311:235–242. doi: 10.1056/NEJM198407263110406. [DOI] [PubMed] [Google Scholar]
- 6.Campana D, Farrant J, Inamdar N, Webster AD, Janossy G. Phenotypic features and proliferative activity of B cell progenitors in X-linked agammaglobulinemia. J Immunol. 1990;145:1675–1680. [PubMed] [Google Scholar]
- 7.Kwan SP, Kunkel L, Bruns G, Wedgwood RJ, Latt S, Rosen FS. Mapping of the X-linked agammaglobulinemia locus by use of restriction fragment-length polymorphism. J Clin Invest. 1986;77:649–652. doi: 10.1172/JCI112351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9:722–728. [Google Scholar]
- 9.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 10.Conley ME, Howard V. Clinical findings leading to the diagnosis of X-linked agammaglobulinemia. J Pediatr. 2002;141:566–571. doi: 10.1067/mpd.2002.127711. [DOI] [PubMed] [Google Scholar]
- 11.Vihinen M, Kwan SP, Lester T, Ochs HD, Resnick I, Valiaho J, et al. Mutations of the human BTK gene coding for bruton tyrosine kinase in X-linked agammaglobulinemia. Hum Mutat. 1999;13:280–285. doi: 10.1002/(SICI)1098-1004(1999)13:4<280::AID-HUMU3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Moin M, Aghamohammadi A, Farhoudi A, Pourpak Z, Rezaei N, Movahedi M, et al. X-linked agammaglobulinemia: a survey of 33 Iranian patients. Immunol Invest. 2004;33:81–93. doi: 10.1081/imm-120027687. [DOI] [PubMed] [Google Scholar]
- 13.Plebani A, Soresina A, Rondelli R, Amato GM, Azzari C, Cardinale F, et al. Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: an Italian multicenter study. Clin Immunol. 2002;104:221–230. doi: 10.1006/clim.2002.5241. [DOI] [PubMed] [Google Scholar]
- 14.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 15.Futatani T, Miyawaki T, Tsukada S, Hashimoto S, Kunikata T, Arai S, et al. Deficient expression of Bruton's tyrosine kinase in monocytes from X-linked agammaglobulinemia as evaluated by a flow cytometric analysis and its clinical application to carrier detection. Blood. 1998;91:595–602. [PubMed] [Google Scholar]
- 16.Jo EK, Song CH, Park JK, Lee JH, Kim DS. Protein and genetic analysis of Bruton's tyrosine kinase (Btk) in three Korean x-linked agammaglobulinemia (XLA) families. J Korean Pediatr Soc. 2002;45:44–54. [Google Scholar]
- 17.Bykowsky MJ, Haire RN, Ohta Y, Tang H, Sung SS, Veksler ES, et al. Discordant phenotype in siblings with X-linked agammaglobulinemia. Am J Hum Genet. 1996;58:477–483. [PMC free article] [PubMed] [Google Scholar]
- 18.Minor PD. Biosafety consequences of eradication of wild-type polioviruses. Lancet. 2001;358:166–168. doi: 10.1016/s0140-6736(01)05399-5. [DOI] [PubMed] [Google Scholar]
- 19.Halliday E, Winkelstein J, Webster AD. Enteroviral infections in primary immunodeficiency (PID): a survey of morbidity and mortality. J Infect. 2003;46:1–8. doi: 10.1053/jinf.2002.1066. [DOI] [PubMed] [Google Scholar]
- 20.Lederman HM, Winkelstein JA. X-linked agammaglobulinemia: an analysis of 96 patients. Medicine (Baltimore) 1985;64:145–156. [PubMed] [Google Scholar]