Abstract
Purpose
To compare the clinical outcome and complications following total knee arthroplasty (TKA) in diabetic and non-diabetic patients, and to identify diabetes-related risk factors for negative outcomes.
Materials and Methods
222 primary TKAs in patients with diabetes were evaluated using Knee Society scores and Hospital for Special Surgery score. Postoperative complications were reviewed retrospectively. The mean follow-up was 53.2 months. The effect of diabetesrelated factors and comparison with a matched control group were analyzed statistically.
Results
Significant improvements were noted in all the scores after TKA (p < 0.05). There was no statistical difference in clinical sores between the diabetic and non-diabetic patients. In multivariate analysis associating age, gender and body mass index with pain and knee score at the latest follow-up, the average knee scores in normal and overweight group were found to be significantly higher than those in the obese group. The diabetic patients had an increased overall incidence of postoperative complications (17.6%) compared with the control group (8.1%) (p < 0.05). Particularly, the rate of wound complications such as skin necrosis, bulla formation or erythema with drainage was higher in the diabetic group (p < 0.05). Diabetes-related factors did not influence the incidence of complications. Associated diseases were the only significant risk factors correlated with wound complications and meniscal bearing dislodgement.
Conclusion
Patients with diabetes can benefit from TKA, even though diabetic patients are at an increased risk for overall postoperative and wound complications. Preoperative factors such as obesity and associated diseases may adversely affect the clinical outcome of TKA in diabetic patients.
Keywords: Diabetes mellitus, total knee arthroplasty, clinical outcome, complication, risk factor
INTRODUCTION
The prevalence of diabetes is rising as the elderly population increases. In Koreans, the age-adjusted prevalence of diabetes is 7.6%, corresponding to 2.7 million with diabetes. The prevalence of diabetes increases with age and reaches its peak in the oldest age-group; in population of 60 - 69 years of age the prevalence of diabetes is about 20%.1
Diabetes has been known to adversely affect the musculoskeletal system, to delay collagen synthesis,2,3 and to impair wound healing4 and phagocytosis.5 These factors may lead to higher risks of various infections and poorer wound healing after any surgical procedure. Some studies have shown an altered bone and mineral metabolism in diabetic animals and humans,6-10 and the fracture healing process may be delayed by this condition.11-14 Theoretically, these effects could contribute to prosthesis loosening in total joint replacement.
Total knee arthroplasty (TKA) is one of the most frequently performed orthopaedic operations in the elderly population. As TKA in diabetic patients has become more common, it has received increasing attention. Some authors have reported an increased rate of deep infection in diabetic patients,15-18 while others have raised concerns over wound complications.16-19
The aim of our present study was to compare the clinical outcome and complications following TKA in a consecutive series of diabetic and non-diabetic patients, and to identify diabetes-related risk factors for negative outcomes.
MATERIALS AND METHODS
Patient characteristics
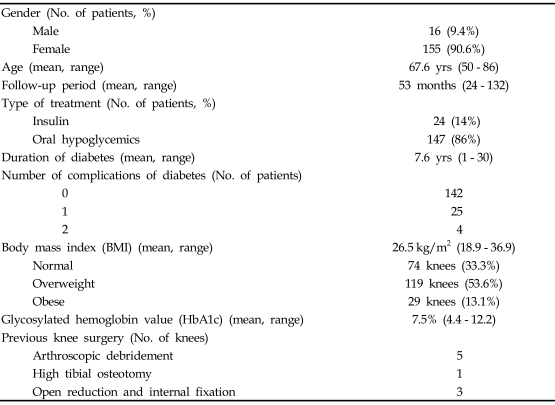
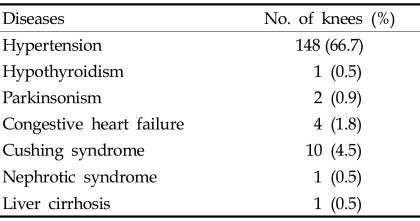
Between 1995 and 2004, 1581 primary TKA procedures were performed at our institution. Surgery was performed by one senior author. In this consecutive series, 222 primary TKAs (14%) in 171 patients with diabetes were available with a minimum follow-up period of 2 years. Only patients with osteoarthritis were included in the current study. The medical records of these patients were reviewed in detail to assess the demographic and medical features of each patient (Table 1). The study included 155 women (203 TKAs) and 16 men (19 TKAs) with a mean age of 67.6 years (range, 50 - 86 years) at the time of operation. Fifty-one patients had bilateral staged TKA. The mean duration of follow-up was 53.2 months (range, 24 - 132 months). Nine patients (5.3%) had previous knee procedures before definitive TKA; 5 received arthroscopic debridement, 1 high tibial osteotomy and 3 open reduction and internal fixation for distal femur or proximal tibial fracture. To identify diabetes-related factors that affect the outcome after TKA, several diabetes associated variables were investigated, including duration of diabetes, the number of major systemic complications of diabetes, body mass index (BMI) and glycosylated hemoglobin values (HbA1c). Twenty-four patients (31 knees; 14%) were treated with insulin, and 147 patients (191 knees; 86%) were treated with oral hypoglycemics. The mean time from the onset of diabetes to the time of surgery was 7.6 years (range, 1 - 30 years). Twenty-nine patients (37 knees; 17%) had major systemic complications of diabetes at the time of surgery. Twenty-five patients (32 knees) had one complication, and four patients (5 knees) had two complications. The distribution of complications from diabetes is listed in Table 2. Table 3 shows associations with various diseases; the most common was hypertension associated with 148 knees (66.7%). Body mass index (BMI) is the ratio of weight to height, calculated by dividing the weight by the square of the height (kg/m2). Patients were divided into three categories based on their BMI: individuals with a BMI of 20 - 25 are considered normal weight; 25 - 30 overweight; and > 30 obese.20-22 In the present study, the mean BMI was 26.5 kg/m2 (range, 18.9 - 36.9). Seventy-four knees (33.3%) were associated with normal weight, 119 knees (53.6%) with overweight and 29 knees (13.1%) with obese. HbA1c is a useful index of blood glucose control over a period of 4 - 5 weeks.23-25 HbA1c values, taken within 1 month before TKA to evaluate diabetes, were available for 185 knees with a mean HbA1c of 7.51% (range, 4.40 - 12.20). Antibiotic prophylaxis and a tourniquet were used in all patients. Prophylaxis for thromboembolism was not performed. All knees were approached through a standard medial parapatellar incision, and all patients received cefuroxime-impregnated cement (1 g of cefuroxime in 40 g of CMW) with mobile bearing low contact stress total knee system (Depuy, Warsaw, IN, USA). Full weight bearing ambulation and range of motion exercises were started on the first postoperative day.
Table 1.
Demographic Data
Table 2.
Preoperative Systemic Complications Associated with Diabetes Mellitus
Table 3.
Associated Diseases
Clinical evaluation
All the patients were identified only by the sequential number of TKA while administering rating scores and complications to stay blind to the patient's status as either a diabetes or control subjects. Patients were preoperatively evaluated according to the Knee Society clinical scoring system and then postoperatively at 6 weeks, 6 months, 1 year, and yearly thereafter.26 Values for knee and function score were obtained to assess prosthetic knee and patient function separately. Pain score was also obtained from the knee scores comprising pain, range of motion and stability. Ratings of 90 - 100 were considered excellent, 80 - 89 good, 70 - 79 fair, and less than 70 poor. We also evaluated Hospital for Special Surgery (HSS) knee-rating scores.27 Knees with a rating of 85 or more were designated as excellent, 70 - 84 good, 60 - 69 fair, and less than 60 poor. Perioperative and postoperative complications were noted retrospectively for all patients. Complications included wound complications, superficial or deep infection, meniscal bearing dislodgement, periprosthetic fracture, deep vein thrombosis (DVT), peroneal nerve palsy and other medical complications. Wound complications were defined as marginal skin necrosis, bulla formation or wound erythema with drainage that persisted longer than usual. To avoid observational bias, only the cases were determined to be adopted of which hospital stay was prolonged at least two days or more for wound management or additional use of intravenous antibiotics. Patients were clinically screened for DVT and it was confirmed with Doppler ultrasonography when diagnosis was suspicious.
Control group
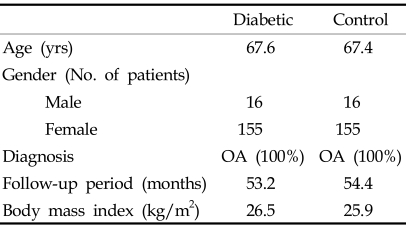
To compare the outcome after TKA in diabetic patients with that of non-diabetic patients, a randomized matching with controls was done from the same series using computer generated matching process. Non-diabetic patients were matched one to one with diabetic patients, based on the following criteria:17,19 (1) age at surgery (within 5 years), (2) gender, (3) diagnosis of arthritis, (4) length of follow-up period (within 1 year), (5) implant design, and (6) BMI (same BMI category). Characteristics of the control and diabetic groups are compared in Table 4. There were no statistical differences in any of the matching criteria between the two groups.
Table 4.
Comparison of Diabetic and Control Groups
OA, osteoarthritis.
Statistical assessment
Univariate analyses were performed to idenfity significant preoperative factors of the measured outcome. P values less than 0.05 were considered significant. The paired t-test was performed to determine whether there was a significant improvement in the clinical scores after TKA. Clinical scores were compared with those of the control group using the Student's t-test. Overall and individual complication rates were compared with the chi-square test. The relationship between clinical scores and continuous variables, such as duration of diabetes, BMI and the value of HbA1c, were analyzed using the Pearson correlation coefficients test. In addition, analysis of variance (ANOVA) was used when the comparison was performed with respect to BMI subgroups. However, in a number of major systemic complications of diabetes, the samples of group "2" did not fulfill the normality test, therefore, they were correlated with the clinical scores using the Kruskal-Wallis test as a nonparametric test. The chi-square test was used to analyze the effect of diabetes-related factors on complication rates.
Once possibly significant predictors of outcome had been identified by this method, multivariate analysis was carried out to identify any predictors that would independently alter outcome. Computation was performed with the Statistical Analysis System (SAS Institute, Cary, North Carolina, USA).
RESULTS
Clinical results
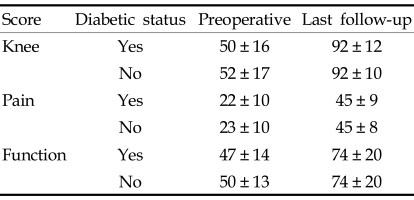
In the diabetic group, the average preoperative Knee Society knee score was 50 ± 16, and the average pain and function scores were 22 ± 10 and 47 ± 14, respectively. There was a significant improvement in all the scores (p < 0.0001) after TKA. At the latest follow-up, knee, pain, and function scores had increased to 92 ± 12, 45 ± 9 and 74 ± 20, respectively (Table 5). There was also improvement in the HSS score from 66 ± 10 to 89 ± 10 (p < 0.0001) (Table 6). The control group also showed a significant improvement in the Knee Society scores and HSS score (Tables 5 and 6). At the latest follow-up examination, there was no pain in 112 knees (50.5%), mild pain in 102 (45.9%), moderate pain in 6 (2.7%) and severe pain in 2 (0.9%). One hundred and seventy seven (79.7%) knees were rated excellent, 21 (9.5%) good, 16 (7.2%) fair, and 8 (3.6%) poor according to the Knee Society rating scale.
Table 5.
Average Knee Society Scores
Table 6.
Average Hospital for Special Surgery Scores
There were no statistically significant differences in the average preoperative Knee Society knee, pain and function scores between the diabetic and control groups (p > 0.05). Likewise, there were no statistical differences in the average postoperative knee, pain, and function scores between the two groups (Table 5). There were no significant differences in the average preoperative and postoperative HSS score between the two groups (Table 6).
Univariate analysis suggested that age, gender and BMI might affect the clinical outcomes. A significant relationship between other predictors and clinical outcomes could not be detected (p > 0.05). However, associated diseases and the number of diabetic complications were related with preoperative pain scores. For the number of diabetic complications, the median value was 10 for group 2, 20 for group 1, and 30 for group 0 (p = 0.0035), and 30 in patients without associated diseases, 20 with hypertension, 20 with congestive heart failure, and 15 with Cushing syndrome (p = 0.0096).
Dividing the BMI into 3 subgroups, the mean postoperative pain score was 44 ± 6 in the normal weight group, 46 ± 7 in the overweight group, and 41 ± 14 in the obese group (p = 0.0182). The mean postoperative knee score was 92 ± 10 in the normal weight group, 94 ± 8 in overweight group, and 86 ± 24 in obese group (p = 0.0109). Multivariate analysis associating age, gender and BMI with the pain and knee score at the latest follow-up was performed. Once the analysis was carried out, the differences in the knee scores were all significant, whereas only the difference in the pain score was significant between the overweight and obese groups.
Postoperative complications
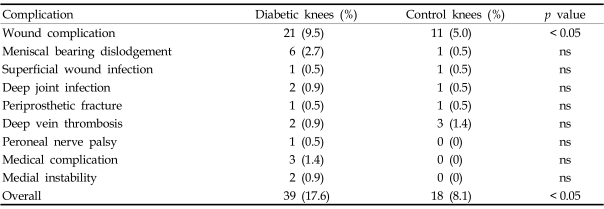
The diabetic group had an increased overall incidence of postoperative complications compared with the matched control group (17.6% vs. 8.1%; p = 0.0029). The rate of wound complications was significantly higher in patients with diabetes than in patients without diabetes (9.5% vs. 5.0%; p = 0.0461). The rate of dislodgement was 2.7% in diabetic versus 0.5% in non-diabetics and was found to be not significantly different (p = 0.0585). The rates of other complications were not statistically different (Table 7). Peroneal nerve palsy, serious medical complications and medial instability were not observed in the control group.
Table 7.
Postoperative Complications
ns, not significant.
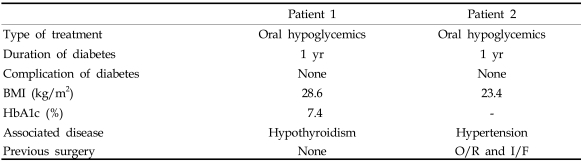
Six meniscal bearing dislodgements occurred postoperatively at an average of 18.8 months (range, 2 - 48) and were treated with meniscal bearing exchange. One each superficial wound infection occurred in the both groups, and they were treated with debridement of the wound and intravenous antibiotics. Two deep infections (0.9%) occurred in the diabetic group, whereas one deep infection (0.5%) in the control group. The deep infections in the diabetic group occurred 11 weeks and 33 months postoperatively, and the causative microorganisms were methicillin-resistant coagulase negative streptococcus and Escherichia coli, respectively. The deep infection in the control group occurred 36 months postoperatively, and the causative organism was Acinetobacter baumannii. These infections were treated with debridement of the joint and intravenous antibiotics, and the infected implants were retrieved and replaced with an antibiotic impregnated cement spacer. Definitive revisional total knee arthroplasty was performed 6 months later. Clinical features of the diabetic patients with deep infection are listed in Table 8. The patients were diagnosed as diabetes one year before surgery and had no complication of diabetes preoperatively. One diabetic patient with peroneal nerve palsy underwent electrical stimulation therapy and showed recovery of ankle dorsiflexion 3 weeks postoperatively and big toe extension one year postoperatively. There were no perioperative mortalities in our series.
Table 8.
Clinical Features of Diabetic Patients with Deep Infection
O/R, open reduction; I/F, internal fixation.
DISCUSSION
A few studies evaluated the effect of diabetes on outcome following TKA. Serna et al. investigated the effect of various diagnosis, the use of cement versus cementless fixation, preoperative deformity, the number of prior surgeries, patient weight greater than 80 kg, and Chanley functional class, and suggested the necessity to examine the degree of glucose control in terms of its effect on the final outcome. To our best knowledge, the current study is the first attempt to examine the effect of diabetes-related factors (duration of diabetes, number of complications of diabetes, body mass index, HbA1c value, type of treatment of diabetes, dose of insulin) on the clinical ratings and the incidence of postoperative complications rates.
In the present study, there was a significant improvement in HSS and Knee Society scores between preoperative and postoperative values for both control and diabetic groups, and these improvements of the scores were associated with pain relief and reflected changes in walking distance and gait aids. In diabetic patients, sensory and autonomic neuropathy can exist at subclinical levels,28 and the resulting loss of pain perception may contribute to higher Knee Society knee and pain scores in patients with diabetes. In addition, combined chronic complications of diabetes and medical comorbidities may have an adverse effect on the function and mobility of patients. These ideas are supported by Meding et al.,29 who reported higher knee and pain scores and lower function scores in patients with diabetes. Papagelopoulos et al.19 also reported lower function scores in diabetic patients; whereas the Knee Society knee score and HSS scores were lower in patients with diabetes in their series. Likewise, Serna et al.17 reported lower HSS scores in patients with diabetes. Those authors recognized that diabetes might influence clinical outcome. However, the influence of diabetes-related factors on clinical outcome was not addressed in their study. We demonstrated that there was no difference in clinical scores between the diabetic and non-diabetic patients, and univariate analysis revealed that BMI was the single significant diabetes-related predictor on clinical outcome. It appears, therefore, that the lower knee score in the obese group is largely due to the lower pain score. Low postoperative pain scores have been attributed to the increased rates of postoperative patellofemoral symptoms among obese patients.30-32 There have been a number of reports on the outcomes of TKA among obese patients, and there is a general consensus that obese patients have satisfactory clinical and radiological results in the long-term.32-34 Adverse outcomes have been reported in highly or morbidly obese patients,34,35 however the current study did not include morbidly obese patients and involved only one patient with a BMI greater than 35 kg/m2. This may explain the lack of difference in postoperative complication rates between the subgroups of BMI in the current study.
There was higher overall postoperative complication rate in diabetic patients, but all the individual complication rates were not statistically different. The most common complications were wound complications (9.5%) and meniscal bearing dislodgement (2.7%). Wound complications after TKA in diabetic patients has been described by several authors;15-19 however, the definitions of wound complications are somewhat different in each study. Meding et al. defined it as skin necrosis, superficial infection, and wound dehiscence, while Papagelopoulos et al. defined it as wound dehiscence, hematoma, and wound discharge. Other groups defined wound complications as superficial infection.16-18 Furthermore, the published rate of wound complications varies from 1.2% to 12%. The rate observed in the current study (9.5%) is not directly comparable with other studies, because it represents marginal skin necrosis, bulla formation, or wound erythema with drainage that persisted longer than usual. There were 14 cases of erythema, 5 cases of necrosis, and 2 cases of bulla formation. No wound dehiscence was observed. These are minor complications compared with those reported by other authors, and none of them caused significant morbidity.
The incidence of meniscal bearing dislodgement has been reported to vary from 0.83% to 9.3%.36,37 The current study revealed a dislodgement rate of 2.7%. Joint sepsis has been a matter of concern after TKA in diabetic patients. England et al.16 reported a deep infection rate of 7% and a wound complication rate of 12% in diabetic patients after TKA. In that series, the rate of deep joint infection in diabetic patients was 10 times higher than the reported incidence of sepsis in non-diabetic patients, therefore they suggested the use of antibiotic-impregnated cement. Chiu et al.15 performed a prospective randomized study to evaluate the role of cefuroxime-impregnated cement in the prevention of deep infection at primary TKA in patients with diabetes. They found no cases of deep infection in the group with cefuroxime-impregnated cement compared with a deep infection rate of 13.5% in the control group, and concluded that cefuroxime-impregnated cement was effective in the prevention of deep infection in patients with diabetes and suggested routine addition of cefuroxime. Its thermal stability and biological effectiveness in cement as well as good clinical result have been well documented, therefore we have routinely been using cefuroxime-impregnated cement for primary TKA, vindicating the idea that prophylactic use of antibiotic bone cement should be the standard of practice for primary TKA.38 Meding et al. also added cefuroxime to cement and reported a deep infection rate of 1.2% in diabetic patients and 0.7% in nondiabetic patients, which are comparable with ours. In their study, all deep infections occurred in patients with insulin-dependent diabetes, whereas all deep infections in the current study occurred in patients who were treated with oral hypoglycemics (Table 8). Therefore, diabetes-related factors appear to have little influence on the rate of deep infection.
A higher than expected aseptic loosening rate in patients with diabetes has been reported by several authors.19,29 With a mean follow-up period of 4.3 years, Meding et al. demonstrated that the rate of aseptic loosening was significantly higher in patient with diabetes (3.6%) than with non-diabetic patients (0.4%). After a mean follow-up of 8 years, Papagelopoulos et al. reported the aseptic loosening rate of 7.4%, which was higher than the control group. However, there are also various studies showing contradictory outcomes. With the mean follow-up of 4.2 years. Chiu et al.15 reported no aseptic loosening, whereas others16-18 have reported the rate of aseptic loosening, ranging from 2 to 3% with the mean follow-up from 3.6 to 4.5 years, however only 1 or 2 aseptic loosenings were included in the series. Similarly, there was no aseptic loosening after a mean follow-up of 4.4 years in our series. After all, diabetes theoretically could contribute to implant loosening, nevertheless, reported incidences are not consistent: There seem to be other factors such as demographic differences besides diabetes in developing aseptic loosening.
The current study represents a retrospective review of clinical outcomes and postoperative complications in diabetes following TKA. While BMI standards reported by WHO expert committee were used in this study, several authors have raised a concern for its inability to detect differences and account for changes by age, gender,39 and ethnic group.40-43 However, the purpose of those studies were to precisely estimate the true association between adiposity and disease risk. Judging from the fact that the outcomes after TKA are affected by mechanical aspect rather than the possibility of cardiovascular disease, we think that it is a logical way to make BMI standard as an international classification to compare with existing studies.
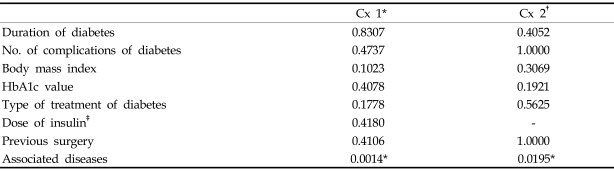
Univariate analysis to identify a significant predictor of postoperative complications was possible only for wound complications and meniscal bearing dislodgement, because the number of patients with other complications was too small. As listed in Table 9, preoperative factors associated with diabetes did not affect the rate of wound complication and meniscal bearing dislodgement. The only significant preoperative factor affecting the complication rates was associated disease. Patients who had iatrogenic Cushing syndrome before TKA had an odds ratio of 19 for wound complications and 38 for meniscal bearing dislodgement compared with patients without associated disease, and power analysis showed a power of 0.95 and 0.80, respectively, validating the ratios.
Table 9.
Statistical Analysis of the Relationships between Preoperative Factors and Postoperative Complications
*wound complications.
†meniscal bearing dislodgement.
‡the statistical analysis was done with Student's t-test for dose of insulin, chi-square test for others.
In conclusion, our present results indicate that patients with diabetes have preoperative clinical conditions similar to non-diabetic patients and benefit equally well from TKA. However, the diabetic patients were at an increased risk for overall postoperative complications and wound complications. Preoperative factors such as BMI and associated diseases may adversely affect the clinical outcome of TKA in diabetic patients. Therefore, it is necessary to balance the benefits of TKA against the risks and exercise extra caution in patient selection.
References
- 1.Kim SM, Lee JS, Lee J, Na JK, Han JH, Yoon DK, et al. Prevalence of diabetes and impaired fasting glucose in Korea: Korean National Health and Nutrition Survey 2001. Diabetes Care. 2006;29:226–231. doi: 10.2337/diacare.29.02.06.dc05-0481. [DOI] [PubMed] [Google Scholar]
- 2.Goodson WH, 3rd, Hunt TK. Studies of wound healing in experimental diabetes mellitus. J Surg Res. 1977;22:221–227. doi: 10.1016/0022-4804(77)90137-8. [DOI] [PubMed] [Google Scholar]
- 3.Brenner RE, Riemenschneider B, Blum W, Mörike M, Teller WM, Pirsig W, et al. Defective stimulation of proliferation and collagen biosynthesis of human bone cells by serum from diabetic patients. Acta Endocrinol (Copenh) 1992;127:509–514. doi: 10.1530/acta.0.1270509. [DOI] [PubMed] [Google Scholar]
- 4.McMurry JF., Jr Wound healing with diabetes mellitus. Better glucose control for better wound healing in diabetes. Surg Clin North Am. 1984;64:769–778. doi: 10.1016/s0039-6109(16)43393-1. [DOI] [PubMed] [Google Scholar]
- 5.Robertson HD, Polk HC., Jr The mechanism of infection in patients with diabetes mellitus: a review of leukocyte malfunction. Surgery. 1974;75:123–128. [PubMed] [Google Scholar]
- 6.Einhorn TA, Boskey AL, Gundberg CM, Vigorita VJ, Devlin VJ, Beyer MM. The mineral and mechanical properties of bone in chronic experimental diabetes. J Orthop Res. 1988;6:317–323. doi: 10.1002/jor.1100060303. [DOI] [PubMed] [Google Scholar]
- 7.Schneider LE, Schedl HP, McCain T, Haussler MR. Experimental diabetes reduces circulating 1,25-dihydroxyvitamin D in the rat. Science. 1977;196:1452–1454. doi: 10.1126/science.141098. [DOI] [PubMed] [Google Scholar]
- 8.Shires R, Teitelbaum SL, Bergfeld MA, Fallon MD, Slatopolsky E, Avioli LV. The effect of streptozotocin-induced chronic diabetes mellitus on bone and mineral homeostasis in the rat. J Lab Clin Med. 1981;97:231–240. [PubMed] [Google Scholar]
- 9.Weiss RE, Gorn AH, Nimni ME. Abnormalities in the biosynthesis of cartilage and bone proteoglycans in experimental diabetes. Diabetes. 1981;30:670–677. doi: 10.2337/diab.30.8.670. [DOI] [PubMed] [Google Scholar]
- 10.Weiss RE, Reddi AH. Influence of experimental diabetes and insulin on matrix-induced cartilage and bone differentiation. Am J Physiol. 1980;238:E200–E207. doi: 10.1152/ajpendo.1980.238.3.E200. [DOI] [PubMed] [Google Scholar]
- 11.Cozen L. Does diabetes delay fracture healing? Clin Orthop Relat Res. 1971;82:134–140. [PubMed] [Google Scholar]
- 12.Herbsman H, Powers JC, Hirschman A, Shaftan GW. Retardation of fracture healing in experimental diabetes. J Surg Res. 1968;8:424–431. doi: 10.1016/0022-4804(68)90058-9. [DOI] [PubMed] [Google Scholar]
- 13.Loder RT. The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop Relat Res. 1988;232:210–216. [PubMed] [Google Scholar]
- 14.Macey LR, Kana SM, Jingushi S, Terek RM, Borretos J, Bolander ME. Defects of early fracture-healing in experimental diabetes. J Bone Joint Surg Am. 1989;71:722–733. [PubMed] [Google Scholar]
- 15.Chiu FY, Lin CF, Chen CM, Lo WH, Chaung TY. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus. A prospective, randomised study. J Bone Joint Surg Br. 2001;83:691–695. doi: 10.1302/0301-620x.83b5.11737. [DOI] [PubMed] [Google Scholar]
- 16.England SP, Stern SH, Insall JN, Windsor RE. Total knee arthroplasty in diabetes mellitus. Clin Orthop Relat Res. 1990;260:130–134. [PubMed] [Google Scholar]
- 17.Serna F, Mont MA, Krackow KA, Hungerford DS. Total knee arthroplasty in diabetic patients: Comparison to a matched control group. J Arthroplasty. 1994;9:375–379. doi: 10.1016/0883-5403(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 18.Yang K, Yeo SJ, Lee BP, Lo NN. Total knee arthroplasty in diabetic patients: a study of 109 consecutive cases. J Arthroplasty. 2001;16:102–106. doi: 10.1054/arth.2001.19159. [DOI] [PubMed] [Google Scholar]
- 19.Papagelopoulos PJ, Idusuyi OB, Wallrichs SL, Morrey BF. Long term outcome and survivorship analysis of primary total knee arthroplasty in patients with diabetes mellitus. Clin Orthop Relat Res. 1996;330:124–132. doi: 10.1097/00003086-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Böstman OM. Prevalence of obesity among patients admitted for elective orthopaedic surgery. Int J Obes Relat Metab Disord. 1994;18:709–713. [PubMed] [Google Scholar]
- 21.James WP. The epidemiology of obesity. Ciba Found Symp. 1996;201:1–16. 32–36. doi: 10.1002/9780470514962.ch1. [DOI] [PubMed] [Google Scholar]
- 22.Sichieri R, Everhart JE, Hubbard VS. Relative weight classifications in the assessment of underweight and overweight in the United States. Int J Obes Relat Metab Disord. 1992;16:303–312. [PubMed] [Google Scholar]
- 23.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin Alc in diabetes mellitus. N Engl J Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 24.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310:341–346. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 25.Larsen ML, Hørder M, Mogensen EF. Effect of long-term monitoring of glycosylated hemoglobin levels in insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:1021–1025. doi: 10.1056/NEJM199010113231503. [DOI] [PubMed] [Google Scholar]
- 26.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 27.Insall JN, Ranawat CS, Aglietti P, Shine J. A comparison of four models of total knee-replacement prostheses. J Bone Joint Surg Am. 1976;58:754–765. [PubMed] [Google Scholar]
- 28.Horowitz SH. Diabetic neuropathy. Clin Orthop Relat Res. 1993;296:78–85. [PubMed] [Google Scholar]
- 29.Meding JB, Reddleman K, Keating ME, Klay A, Ritter MA, Faris PM, et al. Total knee replacement in patients with diabetes mellitus. Clin Orthop Relat Res. 2003;416:208–216. doi: 10.1097/01.blo.0000093002.90435.56. [DOI] [PubMed] [Google Scholar]
- 30.Griffin FM, Scuderi GR, Insall JN, Colizza W. Total knee arthroplasty in patients who were obese with 10 years follow-up. Clin Orthop Relat Res. 1998;356:28–33. doi: 10.1097/00003086-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Healy WL, Wasilewski SA, Takei R, Oberlander M. Patellofemoral complications following total knee arthroplasty. Correlation with implant design and patient risk factors. J Arthroplasty. 1995;10:197–201. doi: 10.1016/s0883-5403(05)80127-5. [DOI] [PubMed] [Google Scholar]
- 32.Stern SH, Insall JN. Total knee arthroplasty in obese patients. J Bone Joint Surg Am. 1990;72:1400–1404. [PubMed] [Google Scholar]
- 33.Mont MA, Mathur SK, Krackow KA, Loewy JW, Hungerford DS. Cementless total knee arthroplasty in obese patients. A comparison with a matched control group. J Arthroplasty. 1996;11:153–156. doi: 10.1016/s0883-5403(05)80009-9. [DOI] [PubMed] [Google Scholar]
- 34.Winiarsky R, Barth P, Lotke P. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80:1770–1774. doi: 10.2106/00004623-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Miric A, Lim M, Kahn B, Rozenthal T, Bombick D, Sculco TP. Perioperative morbidity following total knee arthroplasty among obese patients. J Knee Surg. 2002;15:77–83. [PubMed] [Google Scholar]
- 36.Bert JM. Dislocation/subluxation of meniscal bearing elements after New Jersey low-contact stress total knee arthroplasty. Clin Orthop Relat Res. 1990;254:211–215. [PubMed] [Google Scholar]
- 37.Buechel FF, Pappas MJ. Long-term survivorship analysis of cruciate spraing versus cruciate sacrificing knee prosthesis using meniscal bearings. Clin Orthop Relat Res. 1990;260:162–169. [PubMed] [Google Scholar]
- 38.Bourne RB. Prophylactic use of antibiotic bone cement: an emerging standard-in the affirmative. J Arthroplasty. 2004;19(4 Suppl 1):69–72. doi: 10.1016/j.arth.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Adams TD, Heath EM, LaMonte MJ, Gress RE, Pendleton R, Strong M, et al. The relationship between body mass index and per cent body fat in the severely obese. Diabetes Obes Metab. 2007;9:498–505. doi: 10.1111/j.1463-1326.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 40.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 41.Cho J, Juon HS. Assessing overweight and obesity risk among Korean Americans in California using World Health Organization body mass index criteria for Asians. Prev Chronic Dis. 2006;3:A79. [PMC free article] [PubMed] [Google Scholar]
- 42.Goh VH, Tain CF, Tong TY, Mok HP, Wong MT. Are BMI and other anthropometric measures appropriate as indices for obesity? A study in an Asian population. J Lipid Res. 2004;45:1892–1898. doi: 10.1194/jlr.M400159-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Wildman RP, Gu D, Reynolds K, Duan X, He J. Appropriate body mass index and waist circumference cutoffs for categorization of overweight and central adiposity among Chinese adults. Am J Clin Nutr. 2004;80:1129–1136. doi: 10.1093/ajcn/80.5.1129. [DOI] [PubMed] [Google Scholar]