Abstract
Purpose
Plasma levels of renin, angiotensin II and aldosterone are increased during normal pregnancy. However, these values in preeclampsia are decreased to nearly that of a nonpregnant subject, and vascular sensitivity to angiotensin II is increased. In preeclampsia, aldosterone is decreased less than rennin. Therefore current studies were undertaken to determine the relationship between aldosterone to renin ratio (ARR) and uterine artery perfusion via RI value.
Materials and Methods
In this study, the relationship between plasma aldosterone and renin concentration was determined in 27 preeclamptic women and 50 normal pregnant women, whose gestational weeks were matched. The aldosterone to renin ratio was calculated and compared between the two groups. Doppler velocimetry of the uterine artery, which was used to calculate resistance index (RI), was performed on all subjects. The relationship between ARR and RI value was reviewed.
Results
In the preeclampsia group, RI value of the uterine artery was significantly higher than that of normal pregnant women. Both plasma renin and aldosterone concentrations were lower in the preeclampsia group. However, the ratio of these two parameters was significantly higher (38.3 vs. 16.1, p < 0.001); the greater ARR, the higher the RI of the uterine artery (r2=0.053, p = 0.048).
Conclusion
This study demonstrates that a high aldosterone to renin ratio may have a negative effect on perfusion of the uterine artery and play an important role in the pathophysiology of preeclampsia.
Keywords: Preeclampsia, aldosterone, renin, uterine artery
INTRODUCTION
Preeclampsia, which is characterized by hypertension and proteinuria during the latter half of pregnancy and can only be resolved by delivery1 is a major cause of maternal or perinatal morbidity and mortality.
Although the exact pathophysiology underlying these conditions remains unknown, several pathophysiological mechanisms have been implicated in the development of preeclampsia. These include genetic factors, immunologic disorders, endothelial dysfunctions, inflammatory pathways, oxidative stresses, activation of thrombosis and the renin-angiotensin-aldosterone system. From the view of endothelial dysfunction, preeclampsia is reliably associated with failure in the trophoblastic invasion of spiral arteries. Doppler studies have demonstrated that impedance of flow in the uterine arteries is increased under this condition.2
Plasma levels of renin, angiotensin II, and aldosterone are typically increased during normal pregnancy. This renin-aldosterone system is a major determinant of sodium balance in pregnancy, opposing the natriuretic effects of progesterone, arginine vasopressin, atrial natriuretic factor, prostaglandins, etc.3 In preeclampsia, these values trend toward a normal, non-pregnant range. However compared with normal pregnancy, a two fold increase is seen in ARR.4
Furthermore, it has recently been reported that plasma ARR may have an important role in aortic systolic pressure, and a relative increase of in aldosterone level enhaences the cardiovascular risks, ultimately resulting in end-organ damage.5
This study was undertaken to determine the relationship between plasma renin activity and plasma aldosterone concentration in normal pregnancy and preeclampsia. To study the effects of ARR on the pathophysiology of preeclampsia, we analyzed the relationship between ARR and uterine artery perfusion in both groups.
MATERIALS AND METHODS
Subjects
From March 2003 to July 2005, a total of 77 women receiving prenatal care at the Department of Obstetrics and Gynecology at Severance Hospital of Yonsei University Medical Center in Seoul, Korea were enrolled in this study. They were organized into two groups; a preeclampsia group (n=27) and a normotensive, normal pregnant group (n=50). Preeclampsia was defined as the development of systolic blood pressure > 140 mmHg and diastolic blood pressure > 90mmHg, proteinuria after 20 weeks of gestation with normal blood pressure prior to 20 weeks, no prior history of hypertension or renal disease and blood pressure returning to normal 12 weeks postpartum. Blood samples and uterine artery waveform were obtained form all patients. Exclusion criteria included multiple pregnancies, fetal anomalies, chromosomal anomalies, and maternal medical illnesses such as cardiovascular disease, renal disease and diabetes mellitus. The relationship between plasma aldosterone concentration and plasma renin activity was then determined with gestational weeks matched in all patients. ARR was calculated and compared between the two groups. By uterine artery waveform, RI was calculated and the relationship between ARR and RI value were reviewed. Informed consent was obtained from all patients.
Methods
Blood pressure measurements were performed with patients in a prone position on at least two separate occasions with a mercury sphygmomanometer.
Blood sampling was performed after 1-2 min a prone position and samples were placed in a tube with ethylenediaminetetraacetic acid (EDTA). They were then immediately centrifuged at 2,000 rpm for 5 min at room temperature, stored at -70℃ and thawed once prior to plasma renin and aldosterone level assays.
Plasma renin concentration was measured by direct radioimmunoassay with a COBRA-II Gamma counter (PacKard, Meriden, Connecticut, USA), and plasma renin activity was determined by enzyme reaction and radioimmunoassay with a COBRA-II Gamma counter (SPL, Tokyo, Japan). All samples were analyzed at Seoul Clinical Laboratories in Seoul, Korea.
All uterine artery waveforms were obtained by using an Acuson (Secuoia 512, Mountianview, California, USA) or ALOKA (SSD 5500, Tokyo, Japan) attached to a 3.5-Mha curvilinear transducer with color and pulsed Doppler capacities. We used a modified method described by Thaler et al.6 to measure uterine artery Doppler wave forms.
Examinations were performed on patients in a semi-recumbent position while flat on their backs after 5 min of rest. Transducers were then placed in a longitudinal plane along the inguinal area. The wall filter and sample gate were set at 100 MHz and 2 mm, respectively. The quality of the flow velocity waveforms was maximized using the smallest possible angle of insonation, and only those waveforms with sharp and definite outlines were accepted. After a consecutive series of 5 or more uniform waveforms were obtained, the last waveform image was frozen and analyzed. S/D ratio was measured at peak systolic and end-diastolic flows.
Statistical analysis
Due to the sample size, Kruskal-Wallis Mann-Whitney tests were applied for statistical analysis to compare the groups. Spearman correlation coefficients were used to compare the relationship between the two parameters. A p value less than 0.05 was considered statistically significant.
RESULTS
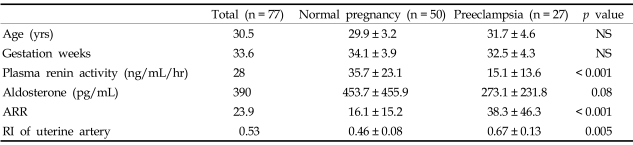
Actual age (29.9 vs. 31.7 years old) and gestational age (34.1 vs. 32.5 weeks) between the normotensive pregnancy and preeclampsia group were controlled (Table 1).
Table 1.
Subject Characteristics in Normal Pregnant Women and Women with Preeclampsia
Values are presented as (mean ± SD).
NS, non-significant.
In the preeclampsia group, both plasma renin activity and plasma aldosterone concentration were lower than those in a normotensive, normal pregnancy (Table 1, 35.7 vs. 15.1ng/mL/hr p < 0.001, 453.7 vs. 273.1pg/mL p = 0.08). But, in the preeclampsia group, plasma aldosterone decreased less than plasma renin activity, leading to a significant increase in ARR (Table 1, 16.1 vs. 38.3, p < 0.001). As demonstrated by previous studies, RI value of the uterine artery in the preeclampsia group was also much higher than that of the normotensive pregnant group (Table 1, 0.67 vs. 0.46, p = 0.005).
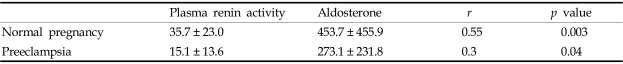
As gestational age increases, the increase in renin activity also increased but was not statistically significant (Table 2, Fig. 1, r2=0.03, p = 0.11). However, plasma aldosterone levels increase to a statistically significant degree with gestational age (Table 2, Fig. 2, p = 0.003). Aldosterone levels were stimulated by renin in both groups; however the sensitivity of aldosterone to renin was much higher in preeclampsia than in normal pregnancy (Table 3, Fig. 3 and 4, p = 0.003 vs. 0.04).
Table 2.
Correlation between Two Variables
Fig. 1.
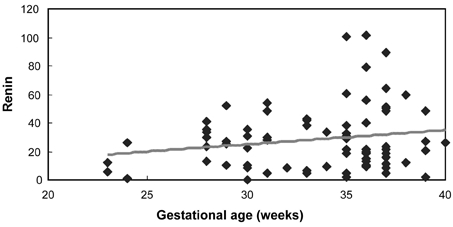
Correlation between gestational age and renin (r=0.18, p = 0.11).
Fig. 2.
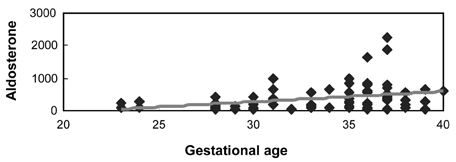
Correlation between gestational age and aldosterone (r=0.33, p = 0.003).
Table 3.
Correlation between Plasma Renin Activity and Aldosterone
Values are presented as (mean ± SD).
Fig. 3.
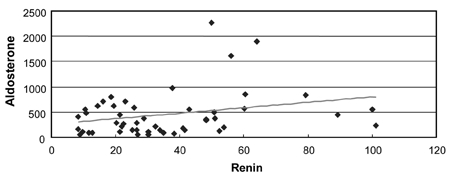
Correlation between renin and aldosterone in normal pregnancy (r=0.3, p = 0.04).
Fig. 4.
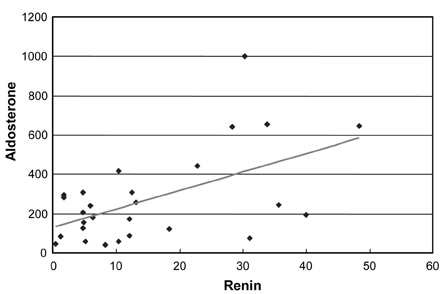
Correlation between renin and aldosterone in preeclampsia (r=0.55, p = 0.003).
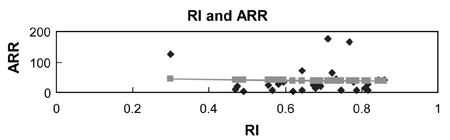
Most importantly, ARR positively correlates with RI levels of uterine artery (Table 2, Fig. 5, p = 0.048, r2=0.053). However, in each control and preeclampsia group, the correlation was not statistically significant (Fig. 6 and 7, p = 0.718, p = 0.868, respectively).
Fig. 5.
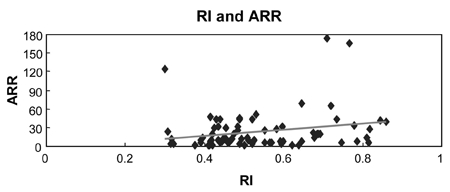
Correlation between RI and ARR (r=0.23, p = 0.048).
Fig. 6.
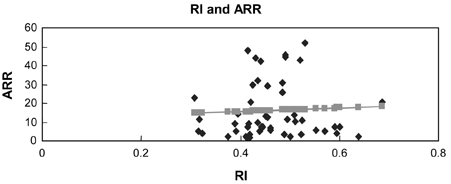
Control group (r=0.052, p = 0.718).
Fig. 7.
Preeclampsia group (r=0.033, p = 0.868).
DISCUSSION
Our study demonstrated that the ARR correlated positively with RI of the uterine artery but in each control and preeclampsia group, the correlation was not statistically significant. It is highly likely that because ARR and RI value are significantly different between the control and preeclampsia group, the inclusion of both groups in correlation analyse may affect the correlation between ARR and RI values. The pregnant group demonstrated that the higher ARR, the higher the RI value. Therefore, a high ARR was inversely associated with uterine artery blood circulation. Furthermore a relative increase of aldosterone might possibly increase the sensitivity of the kidney to angiotensin II. Thus, aldosterone increased with gestational age without causing preeclampsia.
Preeclampsia may involve various manifestations in multiple systems: however, it is most consistently associated with renal involvement.7 Among many mechanisms of preeclampsia, the renin-angiotensin-aldosterone system is thought to be the etiology of kidney involvement.8 Normotensive pregnancy is characterized by an early increase of circulating maternal plasma renin. The origin of this increase is the ovaries and placenta in addition to the kidney.8,9 With this increase in plasma renin activity, plasma angiotensin and aldosterone were also increased potentially contributing to sodium retention and resultant water retention in pregnancy. Angiotensin II also acts as a potent systemic vasoconstrictor and although it is expected to increase blood pressure under normal circumstances, blood pressure was not increased in normal pregnancy. This is because the systemic vasculature is refractory to angiotensin II during normal pregnancy,10,11 a phenomenon ascribed partly to progesterone and prostacycline.12 In fact, according to Forcier et al. and Brochu et al., angiotensin I receptor expression on rat adrenals remains unchanged throughout pregnancy.13 In preeclampsia, the renin-angiotensin-aldosterone system differs from a normal pregnancy. Preeclamptic patients plasma renin activity and aldosterone are suppressed to normal, non-pregnancy levels but there is a relative increase in aldosterone for the given level of renin, suggesting increased adrenal sensitivity to angiotensin II.
Additionally, this increased sensitivity to angiotensin II may develop before the clinical manifestations of the disease.12 It was reported that plasma renin activity and urine aldosterone levels decreased from previously normal levels in women with chronic hypertension as preeclampsia developed. In uncomplicated chronic hypertensive pregnancies, plasma renin activity and urine aldosterone excretion remain elevated throughout gestation, in a fashion analogous to a normotensive pregnancy.14
Preeclampsia has been also reliably associated with failures in the trophoblastic invasion of spiral arteries.
Currently, ultrasonographic Doppler velocimetry is used in the obstetric field as a noninvasive method for evaluating placental circulation. Many studies have utilized this technology to predict blood flow and complications in pregnancy.15
Pulsatile index (PI) and RI in Doppler velocimetry can directly reflect downstream circulation of the uterine artery. The basic principle consists of determining the pulsatility of the Doppler waveform in a virtually angle-independent manner. This is performed by deriving Doppler indices or ratios from various combinations of the peak systolic, end-diastolic, and temporal mean values of the maximum frequency shift envelope.16 Thus, these tests can be used as the main approach for Doppler-based fetal circulatory assessment in addition to predicting preeclampsia from the view of endothelial placenta dysfunction.
This study shows that both plasma renin activity and plasma aldosterone were lower in the preeclampsia group. Plasma ARR was significantly higher than in normal pregnant women. These results that circulating rennin-angiotensin-aldosterone system suppressed in pregnancy-induced hypertension correlate to another study.4,17 However, other studies demonstrated that plasma renin activity was not different between normal pregnancy and preeclamptic patients.7
According to recent studies, ARR may play an important role in determining arterial stiffness, particularly wave reflection, and aortic systolic pressure in subclinical essential hypertension. It is also of predictive value for the efficacy of spironolactone, an aldosterone antagonist valuable in the treatment of hypertension.5
High blood pressure in preeclampsia appears to be related to an amplified sensitivity of the blood vessels to increases in angiotensin II. As aldsterone synthase expression increases in rat adrenals throughout pregnancy,18 we could also speculate that renin secretion is suppressed in hypertensive pregnancies whlile aldosterone production is maintained but not totally related to the rennin secretion due to an increased availability of aldosterone synthase. Whether this is due to heightened sensitivity at the adrenal gland or is directly influenced by the relative increase of aldosterone is a topic for future discussion. Furthermore, it should be noted that, although the value of renin and aldosterone was determined, norepinephrine, a major factor in determining blood pressure, was excluded from this study.
In conclusion, our study demonstrates that women with preeclampsia have decreased plasma renin activity, but relatively greater stimulation of aldosterone than normal pregnancy. The study shows that a high ARR has a negative effect on perfusion of the uterine artery. High aldosterone may play an important role in the pathophysiology of preeclampsia. ARR serve as a vital screening test for the predictive value and severity of preeclampsia. According to a recent report, measurements of ARR may also facilitate the choice of drug therapy in essential hypertension and potential use of aldosterone antagonists in reducing aortic systolic BP and arterial stiffness5 : In preeclamptic patients unable to induce labor due to prematurity, spironolactone, an aldosterone antagonist, may be an effective therapeutic agent. A large-scale study on aldosterone and preeclampsia should be carried out in the future.
References
- 1.Gladstone IM, Katz VL. The morbidity of the 34-to 35-week gestation: should we reexamine the paradigm? Am J Perinatol. 2004;21:9–13. doi: 10.1055/s-2004-820503. [DOI] [PubMed] [Google Scholar]
- 2.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 3.Weir RJ, Brown JJ, Fraser R, Kraszewski A, Lever AF, McIlwaine GM, et al. Plasma renin, renin substrate, angiotensin II, and aldosterone in hypertensive disease of pregnancy. Lancet. 1973;1:291–294. doi: 10.1016/s0140-6736(73)91540-7. [DOI] [PubMed] [Google Scholar]
- 4.Brown MA, Zammit VC, Mitar DA, Whitworth JA. Renin-aldosterone relationships in pregnancy-induced hypertension. Am J Hypertens. 1992;5(6 Pt 1):366–371. doi: 10.1093/ajh/5.6.366. [DOI] [PubMed] [Google Scholar]
- 5.Mahmud A, Feely J. Aldosterone-to-renin ratio, arterial stiffness, and the response to aldosterone antagonism in essential hypertension. Am J Hypertens. 2005;18:50–55. doi: 10.1016/j.amjhyper.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Thaler I, Weiner Z, Itskovitz J. Systolic or diastolic notch in uterine artery blood flow velocity waveforms in hypertensive pregnant patients: relationship to outcome. Obstet Gynecol. 1992;80:277–282. [PubMed] [Google Scholar]
- 7.Elsheikh A, Creatsas G, Mastorakos G, Milingos S, Loutradis D, Michalas S. The rennin-aldosterone system during normal and hypertensive pregnancy. Arch Gynecol Obstet. 2001;264(4):182–185. doi: 10.1007/s004040000104. [DOI] [PubMed] [Google Scholar]
- 8.Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am J Physiol Renal Physiol. 2005;288:F614–F625. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 9.Dzau VJ, Gonzalez D, Ellison K, Churchill S, Emmett N. Characterization of purified rabbit uterine renin: influence of pregnancy on uterine inactive renin. Endocrinology. 1987;120:358–364. doi: 10.1210/endo-120-1-358. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan NM, Lieberman E, Neal W. Kaplan's clinical hypertension. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2002. Hypertension with pregnancy and the pill; pp. 404–433. Chapt 11. [Google Scholar]
- 11.Ochi H, Matsubara K, Kusanagi Y, Taniguchi H, Ito M. Significance of a diastolic notch in the uterine artery flow velocity waveform induced by uterine embolisation in the pregnant ewe. Br J Obstet Gynaecol. 1998;105:1118–1121. doi: 10.1111/j.1471-0528.1998.tb09946.x. [DOI] [PubMed] [Google Scholar]
- 12.Gant NF, Worley RJ, Everett RB, MacDonald PC. Control of vascular responsiveness during human pregnancy. Kidney Int. 1980;18:253–258. doi: 10.1038/ki.1980.133. [DOI] [PubMed] [Google Scholar]
- 13.Forcier I, St-Louis J, Brochu M. Angiotensin II receptor subtypes in the adrenals of pregnant rats. Mol Cell Endocrinol. 1995;114:177–186. doi: 10.1016/0303-7207(95)96798-m. [DOI] [PubMed] [Google Scholar]
- 14.August P, Lenz T, Ales KL, Druzin ML, Edersheim TG, Hutson JM, et al. Longitudinal study of the renin-angiotensin-aldosterone system in hypertensive pregnant women: deviations related to the development of superimposed preeclampsia. Am J Obstet Gynecol. 1990;163(5 Pt 1):1612–1621. doi: 10.1016/0002-9378(90)90639-o. [DOI] [PubMed] [Google Scholar]
- 15.Chung JE, Cho JS, Han SS, Park YW, Kim JW. Uterine artery Doppler velocimetry in the prediction of adverse obstetric outcomes in unexplained MSAFP elevations. Yonsei Med J. 2000;41:17–21. doi: 10.3349/ymj.2000.41.1.17. [DOI] [PubMed] [Google Scholar]
- 16.Maulik D. Hemodynamic interpretation of the arterial Doppler waveform. Ultrasound Obstet Gynecol. 1993;3:219–227. doi: 10.1046/j.1469-0705.1993.03030219.x. [DOI] [PubMed] [Google Scholar]
- 17.Israel A, Peceño A. Renin-angiotensin-aldosterone system in pregnancy-induced hypertension. J Hum Hypertens. 2000;14 (Suppl 1):S36–S39. doi: 10.1038/sj.jhh.1000985. [DOI] [PubMed] [Google Scholar]
- 18.Brochu M, Lehoux JG, Picard S. Effects of gestation on enzymes controlling aldosterone synthesis in the rat adrenal. Endocrinology. 1997;138:2354–2358. doi: 10.1210/endo.138.6.5198. [DOI] [PubMed] [Google Scholar]