Abstract
Purpose
Coexistence of different classes of β-lactamases in a single bacterial isolate may pose diagnostic and therapeutic challenges. We investigated a spread of Klebsiella pneumoniae isolates co-producing an AmpC β-lactamase and an extended-spectrum β-lactamase (ESBL) in a university hospital.
Materials and Methods
Over a three-month period, a total of 11 K. pneumoniae isolates, which exhibited resistance to cefotaxime, aztreonam, and cefoxitin, were isolated. These isolates showed positive to ESBLs by double disk tests. Minimal inhibitory concentrations (MICs) were determined by broth microdilution testing. All isolates were examined by isoelectric focusing, PCR and sequence analysis to identify blaSHV and blaDHA, and molecular typing by pulsed-field gel electrophoresis (PFGE).
Results
All 11 isolates were highly resistant (MIC, ≥128 µg/ml) to ceftazidime, aztreonam, and cefoxitin, while they were susceptible (MIC, ≤2 µg/ml) to imipenem. The blaSHV-12 and blaDHA-1 genes were detected by PCR and sequence analysis. PFGE revealed a similar pattern in 10 of the 11 strains tested.
Conclusion
This is the first outbreak report of K. pneumoniae in Korea which co-produced SHV-12 and DHA-1 β-lactamase, and we suggest a clonal spread of multidrug-resistant K. pneumoniae at a hospital.
Keywords: Outbreak, Klebsiella pneumoniae, SHV-12, DHA-1, Extended-spectrum β-lactamase, AmpC β-lactamase
INTRODUCTION
β-Lactamase production is the predominant mechanism for resistance to β-lactams in Enterobacteriaceae. Klebsiella pneumoniae isolates are often multidrug-resistant and are the major hosts for extended-spectrum β-lactamases (ESBLs).1 In particular, K. pneumoniae strains have also acquired plasmid-mediated AmpC enzymes. Unlike ESBLs, AmpCs are poorly inhibited by β-lactamase inhibitors and are less active against cefepime and cefpirome than ESBLs. AmpCs confer resistance to oxyimino- and 7-α-methoxy-cephalosporins.2 The prevalence of ESBLs and plasmid-mediated AmpC β-lactamases in K. pneumoniae has been rising in Korea. Of the ESBLs and the acquired AmpC enzymes, SHV-12 and DHA-1 are the most prevalent and widespread in Korea, respectively.3,4 Occurrence of ESBL and AmpC β-lactamase co-producing K. pneumoniae has been reported,5,6 but outbreaks of the isolates are extremely rare.
Over a three-month period in 2004, K. pneumoniae isolates resistant to cefotaxime, ceftazidime, cefepime, aztreonam, and cefoxitin were isolated from the patients hospitalized at a university hospital in Korea. The aim of our study was to analyze clinical and molecular epidemiology of the multidrug-resistant K. pneumoniae isolates.
MATERIALS AND METHODS
Patients and bacterial isolates
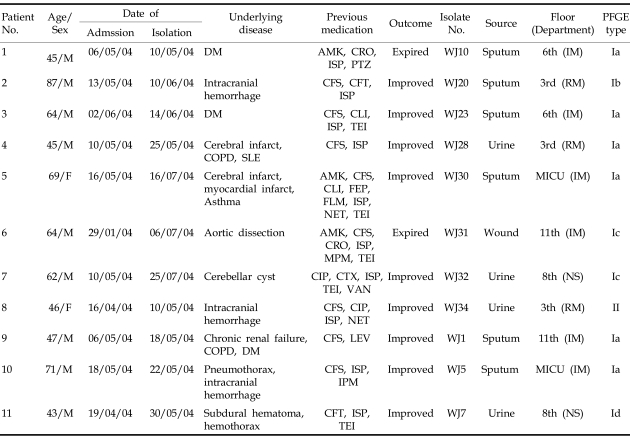
From May to July 2004, a total of 11 non-duplicated isolates of K. pneumoniae that were resistant to cefotaxime, ceftazidime, aztreonam, and cefoxitin, were identified by Clinical Laboratory and Standards Institute (CLSI) disk diffusion method.7 Ten of the 11 isolates were resistant to cefepime. All isolates were also resistant to aminoglycosides (amikacin, gentamicin, and tobramycin), ciprofloxacin, and trimethoprim-sulfamethoxazole. All isolates were suggested to produce ESBL based on the double disk test.8 Table 1 shows the clinical background of patients for each isolate and their respective treatment outcomes. The first isolate from each source of patient was included in the study.
Table 1.
Clinical and PFGE Data of the Patients with SHV-12 and DHA-1 Co-Producing K. pneumoniae Isolates
MICU, medical intensive care unit; IM, internal medicine; RM, rehabilitation medicine; NS, neurosurgery; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; SLE, systemic lupus erythematosus AMK, amikacin; CFT, cefotiam; CFS, cefoperazone-sulbactam; CIP, ciprofloxacin; CLI, clindamycin; CRO, ceftriaxone; CTX, cefotaxime; FEP, cefepime; FLM, flomoxef; IPM, imipenem; ISP, isepamicin; LEV, levofloxacin; MPM, meropenem; NET, netilmicin; PTZ, piperacillin-tazobactam; TEI, teicoplanin; VAN, vancomycin.
Susceptibility testing and β-lactamase phenotype
Minimal inhibitory concentrations (MICs) were determined by the broth microdilution method according to the CLSI guidelines.9 The antimicrobial agents used were piperacillin (Sigma, Steinheim, Germany), tazobactam (Yuhan, Seoul, Korea), cefoxitin (Sigma), ceftazidime (Glaxo SmithKline, Harlow, UK), aztreonam (Bristol-Myers Squibb, Prinston, NJ, USA), cefepime (Boryung, Seoul, Korea), and imipenem (Merk, West Point, PA, USA). ESBL and AmpC phenotypes were investigated by the CLSI ESBL confirmatory disk diffusion test7 and the AmpC disk test,10 respectively.
Isoelectric focusing (IEF)
Crude β-lactamase preparations were obtained from the isolates by sonication and subjected to analytical IEF as described previously.11 β-Lactamase activity was detected by overlaying the gels with 0.5 mM nitrocefin in 0.1 M phosphate buffer, pH 7.0.
PCR amplification and DNA sequencing
Plasmids from the isolates were extracted by a rapid alkaline lysis procedure.12 PCR with blaDHA-13,14 and blaSHV-specific primers and subsequent sequencing of the PCR products were performed. An 1,071-bp fragment of the blaSHV gene was amplified with the primers SHV-F (5'-CGC CGG GTT ATT CTT ATT TG-3') and SHV-R (5'-CCA CGT TTA TGG CGT TAC CT-3'). Sequence alignment and analysis were performed on-line using the BLAST program of the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov).
Pulsed-field gel electrophoresis (PFGE)
PFGE was carried out with a CHEF-DRII System (Bio-Rad, Hercules, CA, USA). XbaI-digested genomic DNA was prepared according to the instruction of Bio-Rad, and fragments were separated for 20 h at 6 V/cm at 14℃, with initial and final pulse times of 5 and 40 s, respectively. DNA bands were visualized by staining the gel with ethidium bromide and photographed.
RESULTS
Bacterial strains and clinical characteristics
K. pneumoniae isolates were first found to show resistance to cefotaxime, ceftazidime, cefepime, aztreonam, and cefoxitin, evidenced by disk diffusion test. During a particular time period, a total of 11 isolates showing very similar antibiogram were isolated. These results strongly suggested that there is coexistence of ESBL and AmpC in these isolates. The isolates were obtained from 11 inpatients at a hospital; 43 to 87 years old, 9 males and 2 females, from May to July 2004. Of 11 isolates, 6 isolates were recovered from sputum, 4 isolates from urine, and 1 isolate from a wound. Isepamicin and cefoperazone-sulbactam had most frequently been administered in 10 and 9 patients, respectively. Two of these 11 patients died, and the infection in the remaining 9 patients improved with therapy (Table 1).
Antimicrobial susceptibility and β-lactamase phenotype
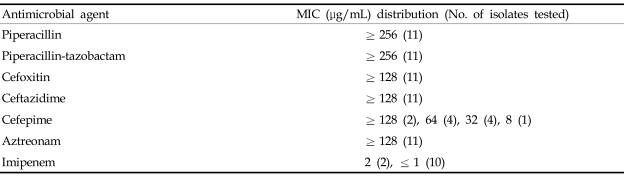
These 11 isolates showed very similar susceptibility profiles, characterized by elevated MICs (≥ 128 µ g/mL) of piperacillin, piperacillin-tazobactam, cefoxitin, ceftazidime, and aztreonam, while MICs of imipenem for all of them were ≤ 2 µ g/mL. The MICs of cefepime varied among the strains isolated (Table 2). All the isolates yielded positive to AmpC disk tests for AmpCs, double disk tests and CLSI ESBL confirmatory disk for ESBLs.
Table 2.
MIC Distributions of the 11 SHV-12 and DHA-1 Co-Producing K. pneumoniae Isolates
β-Lactamase genotype and PFGE profiles
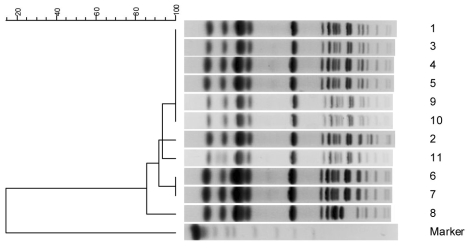
These isolates had β-lactamase bands at isoelectric points of 7.6 and 7.8. The blaSHV and blaDHA alleles were detected by PCR, and the sequences were identical to those of blaSHV-12 and blaDHA-1. The PFGE analysis identified one major profile with four subtypes (Ia, Ib, Ic, and Id) (Table 1, Fig. 1).
Fig. 1.
PFGE dendrograms of 11 SHV-12 and DHA-1 co-producing K. pneumoniae isolates. The scale indicates the percent of genetic similarity. The molecular size marker includes yeast chromosomes, Saccharomyces cerevisiae (Bio-Rad Laboratories, Hercules, CA, USA).
DISCUSSION
Expanded-spectrum cephalosporin-resistant organisms due to ESBL production have been reported in many countries.15 ESBL production among K. pneumoniae isolates in Korea was 28.4% during 2002 - 2003,16 which is significantly higher than the United States, Taiwan, or Hong Kong.1,6 SHV-12, SHV-2a, and TEM-52 were common in the late 1990s in Korea.17 In 2002, an SHV-12-like enzyme was prevalent, but SHV-2a and TEM-52 were found in a few isolates in Korea.18 DHA-1, an inducible plasmid-mediated AmpC that was first identified in Salmonella enteritidis isolated in Saudi Arabia in 1992,19 has been reported in several other countries.5,20 DHA-1 enzyme is the most frequently identified among the AmpC β-lactamase-producing K. pneumoniae isolates in Korea.4 Genes encoding both ESBLs and plasmid-mediated AmpC β-lactamases are usually located on large multidrug resistance plasmids. Therefore, both types of enzymes are typically associated with resistance to multiple antibiotics, thus leaving few therapeutic options.21
In our study, the isolates that co-produced SHV-12 and DHA-1 enzymes showed high MICs of ceftazidime, cefepime, aztreonam, and cefoxitin. Four (8.7%) of 46 plasmid-mediated AmpC-producing K. pneumoniae isolates also had an ESBL at a Korean hospital.14 Furthermore, the outbreak of K. pneumoniae isolates co-producing SHV-2a ESBL and DHA-1 AmpC has been reported in Korea.22 The nosocomial infections caused by DHA-1 and SHV-12 β-lactamase producers occurred in Japan.23 However, this is the first report of its occurrence in Korea.
In this study, K. pneumoniae strains were recovered from patients with a mean age of 58 years. These data agree with a previous study showing usually older with ESBL.24 However, the mean age (55 years old) of the other 164 patients who were infected with broad-spectrum cephalosporins-susceptible K. pneumoniae was older in age (data not shown). The extended-spectrum cephalosporins had preferentially been used as first-line drugs at the hospital. Among 11 patients, all of them had received the extended-spectrum cephalosporins (9 had received cefoperazone-sulbactam). The emergence of multi-resistant organisms, characterized by a heavy use of antimicrobial agents, may provide nosocomial transmission.25 In our present study, the emergence of K. pneumoniae isolates co-producing SHV-12 and DHA-1 could be correlated with heavy use of extended-spectrum cephalosporins. PFGE of XbaI-digested genomic DNA revealed that 10 out of the 11 isolates in this study, likely corresponded to a single clone, whereas one isolate (WJ34) was not clonally related.
In conclusion, the first outbreak caused by K. pneumoniae isolates co-producing SHV-12 ESBL and DHA-1 AmpC β-lactamase occurred at a university hospital in Korea. The spreads was caused by an endemic clone of the resistance gene.
ACKNOWLEDGEMENT
The authors are thankful to Tae-Jae Lee for providing excellent technical support.
References
- 1.Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC-type β-lactamases. Antimicrob Agents Chemother. 2002;46:1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryoo NH, Kim EC, Hong SG, Park YJ, Lee K, Bae IK, et al. Dissemination of SHV-12 and CTX-M-type extended-spectrum β-lactamases among clinical isolates of Escherichia coli and Klebsiella pneumoniae and emergence of GES-3 in Korea. J Antimicrob Chemother. 2005;56:698–702. doi: 10.1093/jac/dki324. [DOI] [PubMed] [Google Scholar]
- 4.Lee K, Lee M, Shin JH, Lee MH, Kang SH, Park AJ, et al. Prevalence of plasmid-mediated AmpC β-lactamases in Escherichia coli and Klebsiella pneumoniae in Korea. Microb Drug Resist. 2006;12:44–49. doi: 10.1089/mdr.2006.12.44. [DOI] [PubMed] [Google Scholar]
- 5.Yan JJ, Ko WC, Wu HM, Tsai SH, Chuang CL, Wu JJ. Complexity of Klebsiella pneumoniae isolates resistant to both cephamycins and extended-spectrum cephalosporins at a teaching hospital in Taiwan. J Clin Microbiol. 2004;42:5337–5340. doi: 10.1128/JCM.42.11.5337-5340.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moland ES, Hanson ND, Black JA, Hossain A, Song W, Thomson KS. Prevalence of newer β-lactamases in gram-negative clinical isolates collected in the United States from 2001 to 2002. J Clin Microbiol. 2006;44:3318–3324. doi: 10.1128/JCM.00756-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement . Wayne, PA: Clinical and Laboratory Standards Institute; 2007. M100-S17. [Google Scholar]
- 8.Brun-Buisson C, Legrand P, Philippon A, Montravers F, Ansquer M, Duval J. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet. 1987;2:302–306. doi: 10.1016/s0140-6736(87)90891-9. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard. 7th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. M7-A7. [Google Scholar]
- 10.Black JA, Moland ES, Thomson KS. AmpC disk test for detection of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking chromosomal AmpC β-lactamases. J Clin Microbiol. 2005;43:3110–3113. doi: 10.1128/JCM.43.7.3110-3113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathew A, Harris AM, Marshall MJ, Rose GW. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol. 1975;88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi S, Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984;20:608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez-Pérez FJ, Hanson ND. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002;40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song W, Kim JS, Kim HS, Yong D, Jeong SH, Park MJ, et al. Increasing trend in the prevalence of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking chromosomal ampC gene at a Korean university hospital from 2002 to 2004. Diagn Microbiol Infect Dis. 2006;55:219–224. doi: 10.1016/j.diagmicrobio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Livermore DM, Yuan M. Antibiotic resistance and production of extended-spectrum beta-lactamases amongst Klebsiella spp. from intensive care units in Europe. J Antimicrob Chemother. 1996;38:409–424. doi: 10.1093/jac/38.3.409. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Kwon Y, Pai H, Kim JW, Cho DT. Survey of Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J Clin Microbiol. 1998;36:1446–1449. doi: 10.1128/jcm.36.5.1446-1449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai H. The characteristics of extended-spectrum β-lactamases in Korean isolates of Enterobacteriaceae. Yonsei Med J. 1998;39:514–519. doi: 10.3349/ymj.1998.39.6.514. [DOI] [PubMed] [Google Scholar]
- 18.Yum JH, Kim S, Lee H, Yong D, Lee K, Cho SN, et al. Emergence and wide dissemination of CTX-M-type ESBLs, and CMY-2 and DHA-1-type AmpC β-lactamases in Korean respiratory isolates of Klebsiella pneumoniae. J Korean Med Sci. 2005;20:961–965. doi: 10.3346/jkms.2005.20.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaillot O, Clément C, Simonet M, Philippon A. Novel transferable β-lactam resistance with cephalosporinase characteristics in Salmonella enteritidis. J Antimicrob Chemother. 1997;39:85–87. doi: 10.1093/jac/39.1.85. [DOI] [PubMed] [Google Scholar]
- 20.Verdet C, Benzerara Y, Gautier V, Adam O, Ould-Hocine Z, Arlet G. Emergence of DHA-1-producing Klebsiella spp. in the Parisian region: genetic organization of the ampC and ampR genes originating Morganella morganii. Antimicrob Agents Chemother. 2006;50:607–617. doi: 10.1128/AAC.50.2.607-617.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moland ES, Black JA, Ourada J, Reisbig MD, Hanson ND, Thomson KS. Occurrence of newer β-lactamases in Klebsiella pneumoniae isolates from 24 U.S. hospitals. Antimicrob Agents Chemother. 2002;46:3837–3842. doi: 10.1128/AAC.46.12.3837-3842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song W, Lee KM, Kim HS, Kim JS, Kim J, Jeong SH, et al. Clonal spread of both oxyimino-cephalosporin- and cefoxitin-resistant Klebsiella pneumoniae isolates co-producing SHV-2a and DHA-1 β-lactamase at a burns intensive care unit. Int J Antimicrob Agents. 2006;28:520–524. doi: 10.1016/j.ijantimicag.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Muratani T, Kobayashi T, Matsumoto T. Emergence and prevalence of β-lactamase-producing Klebsiella pneumoniae resistant to cephems in Japan. Int J Antimicrob Agents. 2006;27:491–499. doi: 10.1016/j.ijantimicag.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 24.De Gheldre Y, Struelens MJ, Glupczynski Y, De Mol P, Maes N, Nonhoff C, et al. National epidemiologic surveys of Enterobacter aerogenes in Belgian hospitals from 1996 to 1998. J Clin Microbiol. 2001;39:889–896. doi: 10.1128/JCM.39.3.889-896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson KS, Prevan AM, Sanders CC. Novel plasmid-mediated β-lactamases in enterobacteriaceae: emerging problems for new β-lactam antibiotics. Curr Clin Top Infect Dis. 1996;16:151–163. [PubMed] [Google Scholar]