Abstract
Suppression of apoptosis is one of the hallmarks of carcinogenesis. Tumor cells endure apoptotic pressure by overexpressing several antiapoptotic proteins, and FLICE inhibitory protein (FLIP) is one of the important antiapoptotic proteins that have been shown to be overexpressed in various primary tumor cells. FLIP has two death-effector domains in tandem, mimicking the prodomain of procaspase-8. It is recruited to Fadd in death-inducing signaling complex, thereby preventing the activation of procaspase-8. To date, three isoforms of human cytosolic FLIP (c-FLIP) and six viral homologs (v-FLIP) have been identified. Recently, the crystal structure of v-FLIP MC159 was determined for the first time as an atomic-detail FLIP structure, which revealed that two death effector domains are packed tightly against each other mainly through conserved hydrophobic interactions. The overexpression of c-FLIP in tumor cells has been shown to be the determinant of the tumor's resistance to death ligands such as FasL and TRAIL. It has also been shown that the down-regulation of c-FLIP results in sensitizing resistant tumor cells. Therefore, the agents directly targeting c-FLIP at mRNA and protein levels are expected to be developed in near future and tested for the potential as a new class of anti-cancer drugs.
Keywords: FLICE inhibitory protein, death-inducing signaling complex, Fas, apoptosis, cancer
APOPTOSIS AND CANCER
Apoptosis is a programmed way of cell death which has been characterized by shrinking of cells, condensation of nuclei, and internucleosomal degradation of DNA.1,2 Within 24 hours after this program is switched on, the apoptotic cell divides into small blobs and is finally engulfed by neighboring cells.3 Since Dr. Stanley Korsmeyer had shown that apoptosis program is suppressed in B-cell lymphoma and its suppression enhances the development of B-cell lymphoma, thousands of studies have been accumulated to support the idea that the acquired resistance to apoptosis is a hallmark of most or perhaps all types of cancer.4 Moreover, a significant part of the benefits achieved by chemotheraphy relies on the induction of apoptosis in tumor cells,5 and cancers with alterations in proteins involved in apoptosis signaling are often resistant to chemotheraphy.6 Therefore, drugs designed to restore the apoptosis program might be effective against tumor cells. For selectivity, such drugs might induce cell death of only tumor cells because, unlike normal cells, they are under apoptotic stress and highly dependent on aberrations of the apoptosis signaling pathways to stay alive.6 For these reasons, apoptosis has been a very attractive phenomenon for the researchers who seek new strategies to fight against cancer.
Antiapoptotic proteins overexpressed in tumor cells have been recognized as targeting points for anti-cancer therapeutic interventions, and their inhibitors at the levels of mRNA and protein have been developed, which are mostly antisense oligonucleotides and small molecule inhibitors.6-8 Those drug candidate compounds are now mostly in the preclinical and early clinical stages. FLIP is an another important antiapoptotic protein overexpressed in various types of tumor cells,9 but the agents directly targeting it have not yet been reportedly developed.8 In this review, recent progress on FLIP research and its potential as an anti-cancer therapeutic target will be discussed.
INITIATOR CASPASE ACTIVATION IN INTRINSIC AND EXTRINSIC PATHWAYS
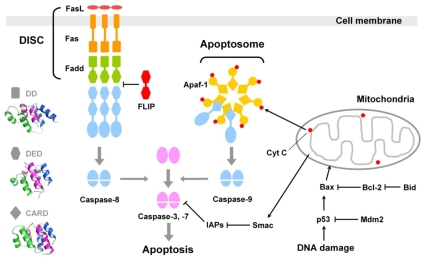
The central executioner of apoptosis is a set of cysteine proteases called caspases that are initially synthesized as inactive zymogens called procaspases. Upon the induction of apoptosis, procaspase is cleaved into p18 and p10 to form the active enzyme, which is a heterotetramer containing two p18/p10 heterodimers and two active sites.10 Based on their order of activation, caspases are classified into two families: initiator caspases and effector caspases.11 Initiator caspases (also known as apical caspases; caspase-8 & -9) are activated through autocatalytic cleavage on their own activation platform formed in response to upstream death signals. For example, caspase-8 is activated in death-inducing signaling complex (DISC) whose major components are Fas and Fadd.12-14 In caspase-9, the proteolytic activation is accomplished in apoptosome composed of Apaf-1 and cytochrom c (Fig. 1).11 Effector caspases (also known as executioner caspases) are proteolytically activated by initiator caspases. Once activated, effecter caspases (caspase-3 & -7) degrade more than 280 cellular proteins identified so far and consequently execute the cell death process.15
Fig. 1.
Apoptosis signaling and the caspase activation.
Death signals to activate the initiator caspases can occur internally from cytotoxic insults such as DNA damage or can be given externally in a form of cytokine collectively called as death ligands including Fas ligand (FasL) and TRAIL.7 In intrinsic pathway, DNA damage leads to the phosphorylation of p53, which then induces transcriptional activation of proapoptotic proteins such as Bax, Puma and Noxa.16 These proteins change the permeability of mytochondiral membrane, which results in the release of several proteins including cytochrome c. Cytochrome c in cytosol interacts with Apaf-1 and they form a heptameric complex called apoptosome where procaspase-9 is activated (Fig. 1).11 In extrinsic pathway, binding of the trimeric death ligand to the death receptor induces the oligomerization of the death receptor which leads to the formation of DISC where procaspase-8 is recruited and activated.12-14 Death receptors are a subfamily of the TNF receptor superfamily, and eight human death receptors have been identified; Fas (also known as Apo-1 and CD95), TNF-R1, DR-3 (Apo-3, TRAMP, WSL-1, LARD), TRAIL-R1 (DR-4), TRAIL-R2 (DR-5), DR-6, EDA-R and NGF-R.17 All death receptors have a death domain in their cytosolic part where the downstream signaling protein binds. Fas has been the most studied death receptor to date. Binding of FasL induces the oligomerization of Fas, and Fas recruits its downstream cytosolic adaptor protein Fadd, which in turn recruits procaspase-8.12-14 In addition to Fas, TRAIL-R1 and -R2 should be mentioned as members of special interests because their common ligand TRAIL was shown to induce apoptosis selectively in tumor cells but not in normal cells, highlighting its potential therapeutic application in cancer treatment.18,19
Protein-protein interactions in the activation of initiator caspases are mediated by three similar domains, which are death domain (DD), death-effecter domain (DED), and caspase-recruiting domain (CARD). In DISC for caspase-8 activation, DD in the cytosolic part of Fas interacts with DD of Fadd, and DED of Fadd in turn with DED of procaspase-8. In case of caspase-9 activation, Apaf-1 recruits procaspase-9 through CARD-CARD interaction. These three domains comprise the death domain superfamily, and they commonly adopt a simple globular fold of the characteristic hexahelical bundle in a Greek key topology. Even though these three domains are very similar in structure, their interactions are highly specific so that they interact only in a homotypic way (i.e. DD-DD, DED-DED, and CARD-CARD) and there is no established interaction across members at least to date.20 These homotypic interactions of DD, DED and CARD play an essential role in forming heteromultimeric platforms for the initiator caspase activation, i.e. DISC and apoptosome, as well as in recruiting the proenzyme of the initiator caspases to those platforms.
FLIP AS AN INHIBITOR OF RECEPTOR-MEDIATED APOPTOSIS
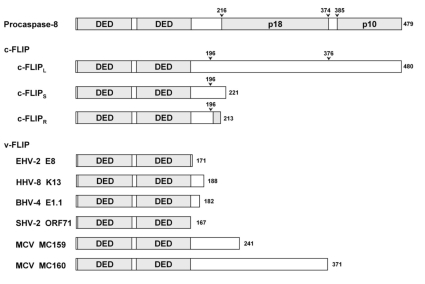
Procaspase-8 has two DEDs in the N-terminus of the catalytic domain, and Fadd has one DED in the N-terminus. In 1997, other DED-containing proteins have been identified in several γ-herpesviruses and the poxvirus MCV.21-23 They have been collectively called viral FLICE inhibitory proteins (v-FLIP) because they show inhibitory effects on apoptosis signaling of caspase-8 which is also called FLICE. Soon after the identification of v-FLIP, their human cellular homologs (cellular-FLIP, c-FLIP) were also identified (Fig. 2).24,25 Although 13 distinct splice variants of c-FLIP mRNA have been described,26 only three have been detected at the protein level; c-FLIPL, c-FLIPS, and c-FLIPR.24-27 The long isoform c-FLIPL has a domain structure similar to procaspase-8: two DED's in N-terminus and caspase-like domain in C-terminus. However, its caspase-like domain lacks the catalytic cysteine residue conserved in caspases and consequently it is catalytically inactive. All three isoforms of c-FLIP as well as v-FLIP have been reported to be recruited to DISC through its tandem DEDs interacting with DED of Fadd, and FLIP thereby excludes procaspase-8 from DISC. This may be the common mechanism of various FLIP's to inhibit the caspase-8 activation, although MC159 (v-FLIP from MCV) seems to act in a slightly different way.28
Fig. 2.
Various FLIPs and procaspase-8. Arrows denote the sites cleaved by caspase-8 or procaspase-8. Shadow region in the C-terminus of c-FLIP is its own unique 11-residue-long sequence which is not observed in other two isoforms, c-FLIPL and cFLIPS.
Although c-FLIPL has been described in most reports as an inhibitor of caspase-8 activation in DISC, some other studies show that c-FLIPL can activate the caspase-8.29-33 Recently, it has been proposed that c-FLIPL can be either antiapoptotic or proapoptotic depending on its expression level,34 although all other FLIP's have been reported to be solely anti-apoptotic. At low expression levels, which are probably found in most cells, c-FLIPL enhances the caspase-8 activation. At intermediate expression levels, found in some cell types such as monocytes/macrophages and certain tumors, c-FLIPL acts as an inhibitor of caspase-8 activation. At very high non-physiological concentrations achieved by transient overexpression, c-FLIPL is cytotoxic by itself without the need for stimulation of Fas.34 Despite the dual role of c-FLIPL in caspase-8 activation, it should be emphasized that c-FLIPL acts as an antiapoptotic protein to inhibit caspase-8 activation at least in the range of physiological expression level in tumor cells.
NONAPOPTOTIC FUNCTIONS OF FLIP
In addition to its activity in apoptosis signaling pathway, c-FLIPL has been reported to activate NF-κB pathway, especially in lymphocytes.35-39 NF-κB activation by c-FLIPL requires the cleavages of c-FLIPL at Asp-376 by fully processed mature caspase-8, i.e. p102-p182 heterotetramer, and/or at Asp-196 by procaspase-8.37,38 Interestingly, mature caspase-8 and procaspase-8 showed mutually exclusive proteolytic specificity on two cleavage sites in c-FLIPL.38 These cleavages generate tandem-DED-containing N-terminal fragments of the molecular weight around 43 kDa and 22 kDa which are called p43-FLIP and p22-FLIP respectively. It has been shown that p22-FLIP can also be generated also from c-FLIPS.38 Both the FLIP N-terminal fragments activate NF-κB signaling via binding to the components of NF-κB signaling pathway; p43-FLIP binds to TRAF237 and p22-FLIP directly binds to IKK complex.38 Notably, caspase-8 has also been reported to be essential for NF-κB activation in T, B and NK cells.40,41 Involvement of c-FLIPL and caspase-8 in NF-κB activation may explain the dual role of c-FLIPL in caspase-8 activation discussed in the previous section.42 NF-κB activation by c-FLIPL and caspase-8 has been shown to play an important role in lymphocyte proliferation.37,38 In addition to NF-κB signaling, c-FLIPL has also been shown to activate Erk signaling pathway by binding to Raf-1.35,43
FLIP STRUCTURE IN ATOMIC DETAIL
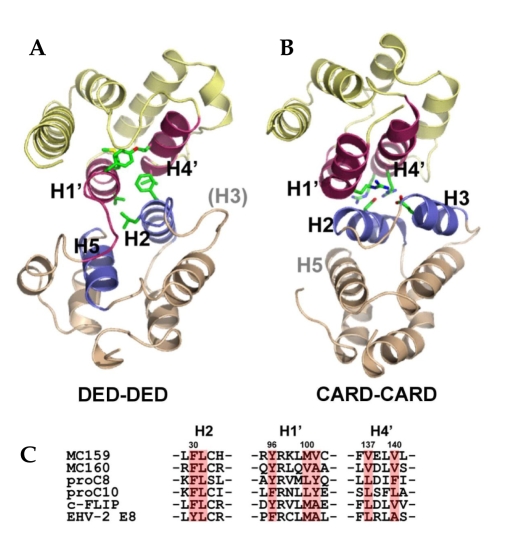
The structure of a viral FLIP, MC159, has recently been revealed by X-ray crystallography for the first time as a FLIP structure (Fig. 3A).28,44 It shows that DED1 significantly deviates from the canonical death fold observed in Fadd DED45 and Fas DD.46 MC159 DED1 lacks the helix 3, and there is instead a short loop structure. The crystal structure also revealed that two DEDs pack tightly against each other through hydrophobic interaction. The atomic-detail DED-DED interaction mode observed in MC159, although intramolecular, vividly contrasts to the previously reported intermolecular CARD-CARD interaction between procaspase-9 and Apaf-1 (Fig. 3A and 3B).47 Firstly, the CARD-CARD interface involves the helix 2/3 face (Apaf-1) and the helix 1/4 face (procaspase-9). In MC159 structure, however, the helix 2/5 face of DED1 meets the helix 1/4 face of DED2. Secondly, the interactions between two CARDs are mainly electrostatic. In contrast, the DED-DED interactions in MC159 are mainly hydrophobic. Interestingly with regards to the hydrophobic interface, the conserved hydrophobic patch in DED1 is buried in the interface, and the one in only DED2 is exposed. The hydrophobic patch is conserved in all known DEDs and has already been shown to be critical for the interaction of Fadd with other DED proteins such as procaspase-8.45 Other important observation is that the hydrophobic residues comprising this intramolecular DED-DED interface are conserved in other tandem-DED-containing proteins such as procaspase-8, -10, c-FLIP, and other v-FLIP's (Fig. 3C). It implies that two DEDs in those proteins might also pack against each other in the same way as in MC159. However, it cannot rule out the possibility that two DEDs, in some circumstances, might get apart and the hydrophobic patch in DED1 could be exposed to play a role in the interaction with their binding partners.
Fig. 3.
Structure of v-FLIP and comparison of DED-DED interface with CARD-CARD interface. (A) Structure of v-FLIP MC159 and the intramolecular DED-DED interaction. (B) CARD-CARD interaction observed between Apaf-1 and procaspase-9. (C) Hydrophobic residues in DED-DED interface are conserved among the proteins containing tandem DEDs.
RxDL sequence motif in the beginning of helix 6 is highly conserved among proteins containing a DED or tandem DEDs.48 Mutation on the motif in Fadd DED abolished its self-association which is essential for its apoptosis signaling activity,49 and this motif is essential also for the antiapoptotic activity of v-FLIP MC159.50 The crystal structure of v-FLIP MC159 revealed that the arginine residue in the RxDL motif interacts with two acidic residues; an aspartate in the motif and a glutamate in helix 2 (Fig. 4). The three charged residues from helix 2 and 6 are highly conserved among DED proteins, and the sequence motif characteristic of DED was rephrased into E/D-RxDL motif. The triad of these three charged residues seems to play a structural role to hold the helix 2 and 6 together in correct positions, and consequently to help stabilize the entire DED fold. The functional defect of the mutants on this motif reported previously is more likely a secondary effect caused by the structural disturbance. This idea is supported by recent study on Fadd DED. Even though Fadd R72A mutant (corresponding to R in E/D-RxDL motif) shows the same circular dichroism spectra as the wild type, its NMR peaks exchange broaden and none of the DED resonances are visible.51 It implies that the Fadd R72A mutant has the same helical content as the wild type but its structural integrity of DED fold is disturbed to some extent. Therefore, E/D-RxDL motif conserved in DEDs must play a structural role to help maintain the stability of DED fold.
Fig. 4.
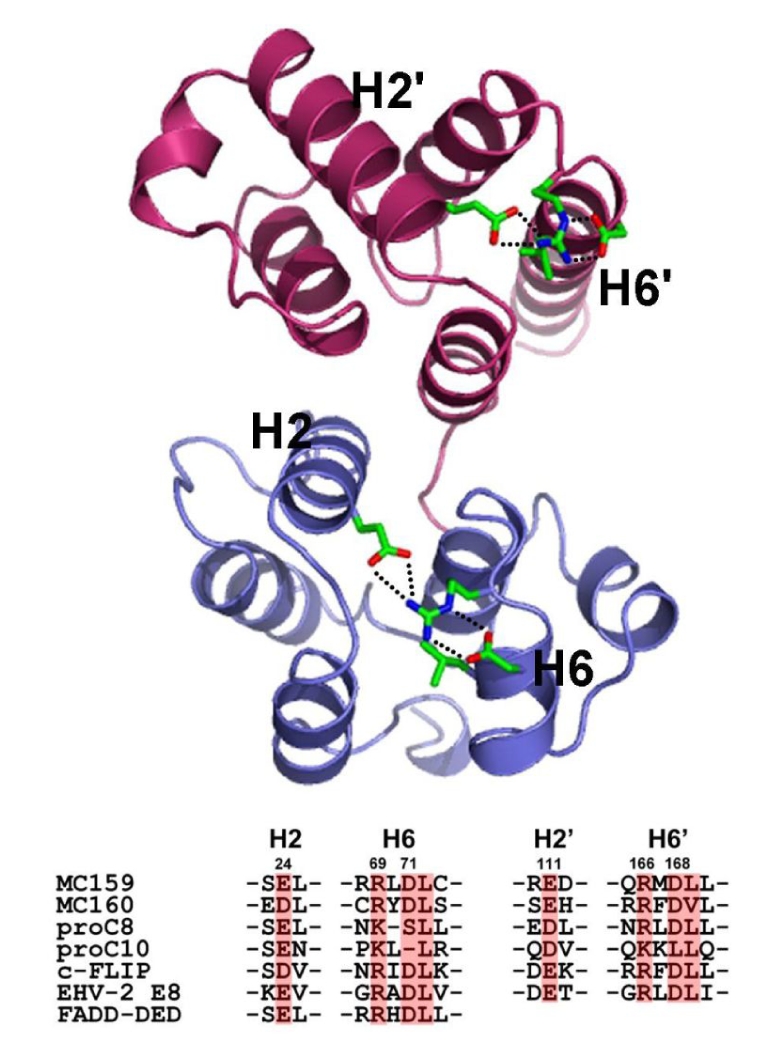
Conserved E/D-RxDL motif in DED fold.
TARGETING FLIP FOR CANCER THERAPY
Elevated expression of c-FLIP has been found in various types of tumor cells which are often resistant to death-receptor-mediated apoptosis. Those tumors include colorectal carcinoma,52-55 gastric carcinoma,56-58 pancreatic carcinoma,59,60 Hodgkin's lymphoma,61-63 B cell chronic lymphocytic leukemia,64,65 melanoma,24,66,67 ovarian carcinoma,68-70 cervical carcinoma,71 bladder urothelial carcinoma,72 and prostate carcinoma.73 The expression of c-FLIP has been proven to be one of the major determinants of the resistance to death ligands such as FasL and TRAIL, and numerous reports have shown that down-regulation of c-FLIP results in sensitizing a various types of resistant tumor cells.52,57,60,62,63,66,69,70,74-76 Conversely, forced expression of c-FLIP renders cells resistant to Fas and/or TRAIL. These observations collectively imply that c-FLIP may be an attractive therapeutic target against at least the above mentioned kinds of tumors of which malignancy and resistance have been shown to be strongly dependent upon c-FLIP overexpression. In addition, v-FLIP K13 of human herpesvirus 8 (HHV8, also called Kaposi's sarcoma-associated herpesvirus, KSHV) has also been shown to act as a tumor progression factor by inhibiting receptor-mediated apoptosis.77
To date, several kinds of small molecules have been known to lower c-FLIP expression and to sensitize the resistant tumor cell to death-receptor-mediated apoptosis.9 They include DNA-damaging agents (cisplatin and doxorubicin), RNA synthesis inhibitor (actinomycin D), protein synthesis inhibitor (cycloheximide), topoisomerase I inhibitors (camptothecin, 9-NC, topotecan), and histone deacetylase inhibitors (Trichostatin A).9 In addition, the inhibitors of several kinases (MEK1/ 2, PKC and PI3K) also lower FLIP expression through blocking the corresponding signaling pathways for the transcriptional activation of FLIP. If it is considered that the above-mentioned tumors depend upon FLIP overexpression for the resistance to TRAIL,18,78,79 then the combination of TRAIL with these agents might be an attractive therapeutic strategy to kill those tumor cells. However, it should be noted that the agents directly targeting FLIP at mRNA or protein levels has not yet been developed.8
Antiapoptotic proteins overexpressed in tumor cells have been recognized as attractive targets for anti-cancer therapeutic intervention. Compounds targeted to Bcl-2, IAP, and MDM2 at either mRNA or protein levels have been developed and are now in the stages of preclinical and early clinical trials.6,8,80 Various antisense oligonucleotides are targeted to their mRNA's and small molecule inhibitors are designed to bind those proteins. The small molecule inhibitors are designed primarily based on their crystal structure in complex with their corresponding proapoptotic proteins; Bcl-XL complexed with Bad,81 XIAP complexed with Smac,82,83 and MDM2 complexed with p53.84 In development of the inhibitors of antiapoptotic proteins, FLIP seems to be left behind in comparison to Bcl-2, IAP and MDM2. It is probably because FLIP was recognized more recently and the detailed information on its structure, especially in complex with Fadd, has not been available. With regard to FLIP structure, it is notable that the crystal structure of v-FLIP MC159 has recently been reported for the first time as an atomic-detail three-dimensional structure of FLIP.28,44 The antisense oligonucleotides and small molecule inhibitors directly targeting FLIP at the levels of mRNA and protein are expected to be developed in near future and tested for the potential as a new class of anti-cancer drugs.
Footnotes
This work was supported by the Soongsil University Research Fund to JKY.
References
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol. 2001;1:50–58. doi: 10.1038/35095508. [DOI] [PubMed] [Google Scholar]
- 3.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Wajant H. Targeting the FLICE Inhibitory Protein (FLIP) in cancer therapy. Mol Interv. 2003;3:124–127. doi: 10.1124/mi.3.3.124. [DOI] [PubMed] [Google Scholar]
- 6.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 7.Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- 8.Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–418. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka T. The caspase-8 modulator c-FLIP. Crit Rev Immunol. 2005;25:31–58. doi: 10.1615/critrevimmunol.v25.i1.30. [DOI] [PubMed] [Google Scholar]
- 10.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y. Caspase activation: revisiting the induced proximity model. Cell. 2004;117:855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 13.Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, et al. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 14.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer U, Jänicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.French LE, Tschopp J. Protein-based therapeutic approaches targeting death receptors. Cell Death Differ. 2003;10:117–123. doi: 10.1038/sj.cdd.4401185. [DOI] [PubMed] [Google Scholar]
- 18.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 19.MacFarlane M. TRAIL-induced signalling and apoptosis. Toxicol Lett. 2003;139:89–97. doi: 10.1016/s0378-4274(02)00422-8. [DOI] [PubMed] [Google Scholar]
- 20.Lahm A, Paradisi A, Green DR, Melino G. Death fold domain interaction in apoptosis. Cell Death Differ. 2003;10:10–12. doi: 10.1038/sj.cdd.4401203. [DOI] [PubMed] [Google Scholar]
- 21.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 22.Bertin J, Armstrong RC, Ottilie S, Martin DA, Wang Y, Banks S, et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci U S A. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu S, Vincenz C, Buller M, Dixit VM. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J Biol Chem. 1997;272:9621–9624. doi: 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- 24.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 25.Hu S, Vincenz C, Ni J, Gentz R, Dixit VM. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J Biol Chem. 1997;272:17255–17257. doi: 10.1074/jbc.272.28.17255. [DOI] [PubMed] [Google Scholar]
- 26.Djerbi M, Darreh-Shori T, Zhivotovsky B, Grandien A. Characterization of the human FLICE-inhibitory protein locus and comparison of the anti-apoptotic activity of four different flip isoforms. Scand J Immunol. 2001;54:180–189. doi: 10.1046/j.1365-3083.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- 27.Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005;280:14507–14513. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- 28.Yang JK, Wang L, Zheng L, Wan F, Ahmed M, Lenardo MJ, et al. Crystal structure of MC159 reveals molecular mechanism of DISC assembly and FLIP inhibition. Mol Cell. 2005;20:939–949. doi: 10.1016/j.molcel.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 30.Goltsev YV, Kovalenko AV, Arnold E, Varfolomeev EE, Brodianskii VM, Wallach D. CASH, a novel caspase homologue with death effector domains. J Biol Chem. 1997;272:19641–19644. doi: 10.1074/jbc.272.32.19641. [DOI] [PubMed] [Google Scholar]
- 31.Han DK, Chaudhary PM, Wright ME, Friedman C, Trask BJ, Riedel RT, et al. MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death. Proc Natl Acad Sci U S A. 1997;94:11333–11338. doi: 10.1073/pnas.94.21.11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inohara N, Koseki T, Hu Y, Chen S, Núñez G. CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis. Proc Natl Acad Sci U S A. 1997;94:10717–10722. doi: 10.1073/pnas.94.20.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu HB, Halpin DR, Goeddel DV. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity. 1997;6:751–763. doi: 10.1016/s1074-7613(00)80450-1. [DOI] [PubMed] [Google Scholar]
- 34.Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 36.Hu WH, Johnson H, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem. 2000;275:10838–10844. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 37.Kataoka T, Tschopp J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol. 2004;24:2627–2636. doi: 10.1128/MCB.24.7.2627-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golks A, Brenner D, Krammer PH, Lavrik IN. The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation. J Exp Med. 2006;203:1295–1305. doi: 10.1084/jem.20051556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhary PM, Eby MT, Jasmin A, Kumar A, Liu L, Hood L. Activation of the NF-kappaB pathway by caspase 8 and its homologs. Oncogene. 2000;19:4451–4460. doi: 10.1038/sj.onc.1203812. [DOI] [PubMed] [Google Scholar]
- 40.Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 41.Su H, Bidère N, Zheng L, Cubre A, Sakai K, Dale J, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 42.Peter ME. The flip side of FLIP. Biochem J. 2004;382(Pt 2):e1–e3. doi: 10.1042/BJ20041143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SJ, Kim YY, Ju JW, Han BG, Park SI, Park BJ. Alternative splicing variants of c-FLIP transduce the differential signal through the Raf or TRAF2 in TNF-induced cell proliferation. Biochem Biophys Res Commun. 2001;289:1205–1210. doi: 10.1006/bbrc.2001.6086. [DOI] [PubMed] [Google Scholar]
- 44.Li FY, Jeffrey PD, Yu JW, Shi Y. Crystal structure of a viral FLIP: insights into FLIP-mediated inhibition of death receptor signaling. J Biol Chem. 2006;281:2960–2968. doi: 10.1074/jbc.M511074200. [DOI] [PubMed] [Google Scholar]
- 45.Eberstadt M, Huang B, Chen Z, Meadows RP, Ng SC, Zheng L, et al. NMR structure and mutagenesis of the FADD (Mort1) death-effector domain. Nature. 1998;392:941–945. doi: 10.1038/31972. [DOI] [PubMed] [Google Scholar]
- 46.Huang B, Eberstadt M, Olejniczak ET, Meadows RP, Fesik SW. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature. 1996;384:638–641. doi: 10.1038/384638a0. [DOI] [PubMed] [Google Scholar]
- 47.Qin H, Srinivasula SM, Wu G, Fernandes-Alnemri T, Alnemri ES, Shi Y. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature. 1999;399:549–557. doi: 10.1038/21124. [DOI] [PubMed] [Google Scholar]
- 48.Tibbetts MD, Zheng L, Lenardo MJ. The death effector domain protein family: regulators of cellular homeostasis. Nat Immunol. 2003;4:404–409. doi: 10.1038/ni0503-404. [DOI] [PubMed] [Google Scholar]
- 49.Muppidi JR, Lobito AA, Ramaswamy M, Yang JK, Wang L, Wu H, et al. Homotypic FADD interactions through a conserved RXDLL motif are required for death receptor-induced apoptosis. Cell Death Differ. 2006;13:1641–1650. doi: 10.1038/sj.cdd.4401855. [DOI] [PubMed] [Google Scholar]
- 50.Garvey TL, Bertin J, Siegel RM, Wang GH, Lenardo MJ, Cohen JI. Binding of FADD and caspase-8 to molluscum contagiosum virus MC159 v-FLIP is not sufficient for its antiapoptotic function. J Virol. 2002;76:697–706. doi: 10.1128/JVI.76.2.697-706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrington PE, Sandu C, Wei Y, Hill JM, Morisawa G, Huang T, et al. The structure of FADD and its mode of interaction with procaspase-8. Mol Cell. 2006;22:599–610. doi: 10.1016/j.molcel.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 52.Hernandez A, Wang QD, Schwartz SA, Evers BM. Sensitization of human colon cancer cells to TRAIL-mediated apoptosis. J Gastrointest Surg. 2001;5:56–65. doi: 10.1016/s1091-255x(01)80014-7. [DOI] [PubMed] [Google Scholar]
- 53.Ryu BK, Lee MG, Chi SG, Kim YW, Park JH. Increased expression of cFLIP(L) in colonic adenocarcinoma. J Pathol. 2001;194:15–19. doi: 10.1002/path.835. [DOI] [PubMed] [Google Scholar]
- 54.Korkolopoulou P, Saetta AA, Levidou G, Gigelou F, Lazaris A, Thymara I, et al. c-FLIP expression in colorectal carcinomas: association with Fas/FasL expression and prognostic implications. Histopathology. 2007;51:150–156. doi: 10.1111/j.1365-2559.2007.02723.x. [DOI] [PubMed] [Google Scholar]
- 55.Ullenhag GJ, Mukherjee A, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. Overexpression of FLIPL is an independent marker of poor prognosis in colorectal cancer patients. Clin Cancer Res. 2007;13:5070–5075. doi: 10.1158/1078-0432.CCR-06-2547. [DOI] [PubMed] [Google Scholar]
- 56.Lee SH, Kim HS, Kim SY, Lee YS, Park WS, Kim SH, et al. Increased expression of FLIP, an inhibitor of Fas-mediated apoptosis, in stomach cancer. APMIS. 2003;111:309–314. doi: 10.1034/j.1600-0463.2003.1110203.x. [DOI] [PubMed] [Google Scholar]
- 57.Nam SY, Jung GA, Hur GC, Chung HY, Kim WH, Seol DW, et al. Upregulation of FLIP(S) by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci. 2003;94:1066–1073. doi: 10.1111/j.1349-7006.2003.tb01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou XD, Yu JP, Liu J, Luo HS, Chen HX, Yu HG. Overexpression of cellular FLICE-inhibitory protein (FLIP) in gastric adenocarcinoma. Clin Sci (Lond) 2004;106:397–405. doi: 10.1042/CS20030238. [DOI] [PubMed] [Google Scholar]
- 59.Elnemr A, Ohta T, Yachie A, Kayahara M, Kitagawa H, Fujimura T, et al. Human pancreatic cancer cells disable function of Fas receptors at several levels in Fas signal transduction pathway. Int J Oncol. 2001;18:311–316. doi: 10.3892/ijo.18.2.311. [DOI] [PubMed] [Google Scholar]
- 60.Mori T, Doi R, Toyoda E, Koizumi M, Ito D, Kami K, et al. Regulation of the resistance to TRAIL-induced apoptosis as a new strategy for pancreatic cancer. Surgery. 2005;138:71–77. doi: 10.1016/j.surg.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Thomas RK, Kallenborn A, Wickenhauser C, Schultze JL, Draube A, Vockerodt M, et al. Constitutive expression of c-FLIP in Hodgkin and Reed-Sternberg cells. Am J Pathol. 2002;160:1521–1528. doi: 10.1016/S0002-9440(10)62578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dutton A, O'Neil JD, Milner AE, Reynolds GM, Starczynski J, Crocker J, et al. Expression of the cellular FLICE-inhibitory protein (c-FLIP) protects Hodgkin's lymphoma cells from autonomous Fas-mediated death. Proc Natl Acad Sci U S A. 2004;101:6611–6616. doi: 10.1073/pnas.0400765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathas S, Lietz A, Anagnostopoulos I, Hummel F, Wiesner B, Janz M, et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J Exp Med. 2004;199:1041–1052. doi: 10.1084/jem.20031080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olsson A, Diaz T, Aguilar-Santelises M, Osterborg A, Celsing F, Jondal M, et al. Sensitization to TRAIL-induced apoptosis and modulation of FLICE-inhibitory protein in B chronic lymphocytic leukemia by actinomycin D. Leukemia. 2001;15:1868–1877. doi: 10.1038/sj.leu.2402287. [DOI] [PubMed] [Google Scholar]
- 65.MacFarlane M, Harper N, Snowden RT, Dyer MJ, Barnett GA, Pringle JH, et al. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene. 2002;21:6809–6818. doi: 10.1038/sj.onc.1205853. [DOI] [PubMed] [Google Scholar]
- 66.Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- 67.Bullani RR, Huard B, Viard-Leveugle I, Byers HR, Irmler M, Saurat JH, et al. Selective expression of FLIP in malignant melanocytic skin lesions. J Invest Dermatol. 2001;117:360–364. doi: 10.1046/j.0022-202x.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- 68.Xiao CW, Yan X, Li Y, Reddy SA, Tsang BK. Resistance of human ovarian cancer cells to tumor necrosis factor alpha is a consequence of nuclear factor kappaB-mediated induction of Fas-associated death domain-like interleukin-1beta-converting enzyme-like inhibitory protein. Endocrinology. 2003;144:623–630. doi: 10.1210/en.2001-211024. [DOI] [PubMed] [Google Scholar]
- 69.Abedini MR, Qiu Q, Yan X, Tsang BK. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004;23:6997–7004. doi: 10.1038/sj.onc.1207925. [DOI] [PubMed] [Google Scholar]
- 70.Mezzanzanica D, Balladore E, Turatti F, Luison E, Alberti P, Bagnoli M, et al. CD95-mediated apoptosis is impaired at receptor level by cellular FLICE-inhibitory protein (long form) in wild-type p53 human ovarian carcinoma. Clin Cancer Res. 2004;10:5202–5214. doi: 10.1158/1078-0432.CCR-03-0537. [DOI] [PubMed] [Google Scholar]
- 71.Wang W, Wang S, Song X, Sima N, Xu X, Luo A, et al. The relationship between c-FLIP expression and human papillomavirus E2 gene disruption in cervical carcinogenesis. Gynecol Oncol. 2007;105:571–577. doi: 10.1016/j.ygyno.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 72.Korkolopoulou P, Goudopoulou A, Voutsinas G, Thomas-Tsagli E, Kapralos P, Patsouris E, et al. c-FLIP expression in bladder urothelial carcinomas: its role in resistance to Fas-mediated apoptosis and clinicopathologic correlations. Urology. 2004;63:1198–1204. doi: 10.1016/j.urology.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Jin TG, Yang H, DeWolf WC, Khosravi-Far R, Olumi AF. Persistent c-FLIP(L) expression is necessary and sufficient to maintain resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in prostate cancer. Cancer Res. 2004;64:7086–7091. doi: 10.1158/0008-5472.CAN-04-1498. [DOI] [PubMed] [Google Scholar]
- 74.Siegmund D, Hadwiger P, Pfizenmaier K, Vornlocher HP, Wajant H. Selective inhibition of FLICE-like inhibitory protein expression with small interfering RNA oligonucleotides is sufficient to sensitize tumor cells for TRAIL-induced apoptosis. Mol Med. 2002;8:725–732. [PMC free article] [PubMed] [Google Scholar]
- 75.Ricci MS, Jin Z, Dews M, Yu D, Thomas-Tikhonenko A, Dicker DT, et al. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol. 2004;24:8541–8555. doi: 10.1128/MCB.24.19.8541-8555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim K, Fisher MJ, Xu SQ, el-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000;6:335–346. [PubMed] [Google Scholar]
- 77.Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, Grandien A. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 1999;190:1025–1032. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 80.Arkin M. Protein-protein interactions and cancer: small molecules going in for the kill. Curr Opin Chem Biol. 2005;9:317–324. doi: 10.1016/j.cbpa.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 82.Liu Z, Sun C, Olejniczak ET, Meadows RP, Betz SF, Oost T, et al. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–1008. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 83.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 84.Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996;274:948–953. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]