Abstract
Purpose
The objective of this study was to examine the relation between anger management style and organ system-related somatic symptoms in depressive disorder and somatoform disorder patients.
Materials and Methods
The subjects included 73 patients with depressive disorders and 47 with somatoform disorders. Anger management styles were assessed by the Anger Expression Scale, while the severity of organ system-related somatic symptoms was evaluated using the Somatic Stress Response Scale (SSRS). The severity of depression and hostility was assessed by the Symptom Checklist-90-Revised (SCL-90-R) depression and hostility subscales.
Results
The results of multiple regression analyses showed that, in depressive disorder patients, the level of anger expression was significantly associated with the severity of somatic symptoms related to neuromuscular, cardiorespiratory and gastrointestinal systems. However, in these patients, the level of anger suppression was not significantly associated with the severity of somatic symptoms related to any specific organ systems. In patients with somatoform disorders, there was no significant association between the level of anger suppression or anger expression and the severity of the somatic symptoms related to any specific organ systems.
Conclusion
These results suggest that, in depressive disorder patients, anger expression is likely to be predominantly involved in the neuromuscular, cardiorespiratory and gastrointestinal organ systems. However, in each of depressive disorder and somatoform disorder patients, anger suppression is not likely to be associated with any specific organ systems.
Keywords: Anger expression, anger suppression, organ system symptoms, depressive disorder, somatoform disorder
INTRODUCTION
Anger is known to play a significant role in somatization.1,2 Previous studies have reported an association of anger suppression and somatic symptoms. Increased sympathetic nervous system activity induced by anger suppression has been linked to somatization.3 In one clinical study, depressed women who appeared to hold their anger in were judged to be more prone to somatic symptoms than those who were rarely angry or who expressed their anger.4
Hwa-byung is an anger syndrome specific to Korean culture, and it is characterized by a variety of somatic symptoms, such as a feeling of a mass in the epigastrium, hot sensation, palpitation, dyspnea, fatigue, and emotional symptoms such as a fear of impending death and dysphoria. All of these symptoms have been attributed to anger suppression.1,5-8
Depression and anger have long been associated. It was found that depressive disorder patients were more likely to experience anger than anxiety disorder or somatoform disorder patients.9 Patients with depressive disorders have been reported to have more anger attacks compared with both healthy volunteers10 and patients with anxiety disorders.11
Previous research has only linked anger suppression and somatic symptoms,3,4 and the relation between anger expression and somatic symptoms has not yet been examined. Moreover, the relationship between patients' anger management style and any organ systems has not been investigated. Therefore, this study will examine the effect of anger expression and anger suppression on the organ systems in depressive and somatoform disorders. Determining such relationships is expected to help predict which organ systems will be affected by anger expression or anger suppression.
In addition, unlike depression, hostility was not considered a main factor in the formation of functional somatic symptoms.12 However, the role of the two psychopathologies in somatic symptoms was not adequately examined in patients with depressive and somatoform disorders. Therefore, this study will include the examination of the effect of depression and hostility on the organ systems in each of the two disorders.
MATERIALS AND METHODS
Outpatients from the Department of Psychiatry at Severance Hospital (in Seoul, Korea) with diagnoses of depressive and somatoform disorders were enrolled in this study. One experienced psychiatrist (K.B.K.) made the diagnoses based on the DSM-IV13 criteria. Patients were eligible to participate if they were at least 18 years old. However, dually diagnosed subjects, including those with a combination of each of the above disorders and physical diseases or other psychiatric disorders, were excluded from this study. The subjects who had a past history of other mental disorders were also excluded.
Most of the subjects were unmedicated, but 17 patients who were within a few days after the initiation of medication were included in this study. They received antidepressants (selective serotonin reuptake inhibitors: SSRIs) such as fluoxetine or paroxetine. However, those who showed change in somatic and emotional symptoms after initiation of the medication were excluded.
The subjects were consecutively selected and interviewed. The therapist explained the purpose and procedure of the study to all the subjects, and written informed consent was obtained from all the participants. Only those patients who consented to the study completed the questionnaires, which included items regarding their sociodemographic characteristics and self-rating scales. All but three patients who were asked to participate consented and responded to the questionnaire.
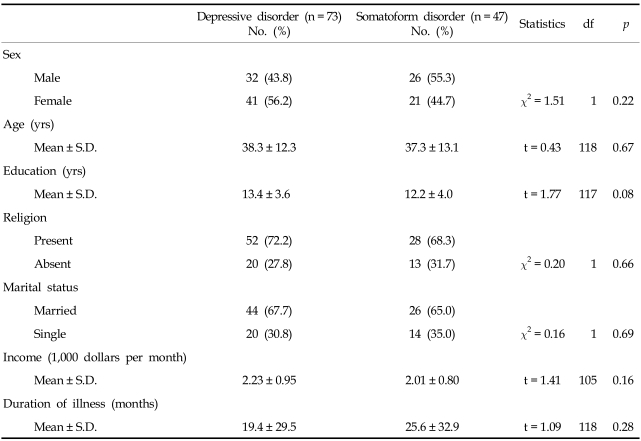
The depressive disorder group included 45 patients with a major depressive disorder and 28 with dysthymic disorder (32 men and 41 women). The somatoform disorder group included 22 patients with undifferentiated somatoform disorder, nine with somatization disorder, 11 with pain disorder, three with hypochondriasis, and two with conversion disorder (26 men and 21 women). The sociodemographic characteristics of the patients are described in Table 1.
Table 1.
Sociodemographic Characteristics
The psychological measures included the Korean version14 of the Anger Expression Scale,15 the Depression, Somatization and Hostility subscales of the Korean version16 of the Symptom Checklist-90-Revised (SCL-90-R),17 and the Somatic Stress Response Scale (SSRS).18 The Anger Expression Scale is a 22-item self-rating instrument designed to assess the levels of anger expression (anger-out) and anger suppression (anger-in). Anger-in refers to the inhibition of the overt expression of anger, while anger-out refers to an overt display of verbally and/or physically aggressive behaviors. In this study, anger was rated on a four-point scale ranging from 0 ("almost never") to 3 ("almost always"). The internal consistency of the Korean version of the Anger Expression Scale was significant for each of the two subscales (anger-in: Cronbach's α = 0.76 p < 0.001; anger-out: Cronbach's α = 0.83 p < 0.001). The test-retest reliability of the two subscale scores was fairly high (anger-in: r = 0.82, p < 0.01; anger-out: r = 0.87 p < 0.01). These correlation coefficients were obtained after an interval of two weeks.
The SCL-90-R is a 90-item self-rating instrument that was developed to assess the psychopathology of the previous week, and it includes nine subscales. The depression subscale of the SCL-90-R has 13 items, the somatization subscale has 12 items, and the hostility subscale has 6 items. The SSRS is a 32-item self-rating instrument that includes five subscales. Among the 32 items, 14 items were excluded because of the lack of differentiation of somatic symptoms based on organ systems, and only 18 items were clearly differentiated and subdivided into four groups, including neuromuscular system, cardiorespiratory system, gastrointestinal system and genitourinary system. The neuromuscular system included items such as "My face feels tense," "I have headaches," "I have a stiff neck," "I feel numb," and "I have tingling in my arms and legs." The cardiorespiratory system included items such as "I have palpitations," "I have chest pain," "I am out of breath," and "My chest feels tight." The gastrointestinal system included items such as "I have loose bowels," "I suffer from indigestion," 'I have an acidic stomach," "My stomach hurts," "I feel nauseated," "I feel throbbing in my stomach," and "I have constipation." Genitourinary organ system included items such as "I have a decreased sex drive," and "I urinate often." The research team, which was comprised of 10 psychiatrists and psychologists, all agreed on these 18 items. Each of these items was arranged in a Likert-type format as follows: "Not at all" (0 points), "Somewhat" (1 point), "Moderately" (2 points), "Very much" (3 points), and "Absolutely" (4 points). The internal consistency of the new version of the SSRS was significant for each of the four subscales (neuromuscular system: Cronbach's α = 0.74 p < 0.01; cardiorespiratory system: Cronbach's α = 0.81 p < 0.01; gastrointestinal system: Cronbach's α = 0.78 p < 0.01; genitourinary system: Cronbach's α = 0.49 p < 0.01). The test-retest reliability of the four subscale scores was fairly high (neuromuscular system: r = 0.85 p < 0.001; cardiorespiratory system: r = 0.80 p < 0.001; gastrointestinal system: r = 0.87 p < 0.001; genitourinary system: r = 0.88 p < 0.001). These correlation coefficients were obtained after an interval of two weeks.
Data analysis
An independent t-test was conducted to compare the scores of each of the psychological measures and some of the sociodemographic data (age, income, duration of education, and duration of illness) between the two groups. Comparisons of the demographic data among the groups, such as gender, marital status (married vs. single) and religion (present vs. absent), were made using a chi-square test. The Pearson correlation test was used to examine the relation between the demographic data (age, level of education, income, and duration of illness) and the anger measure scores. A multiple regression analysis was done to determine the effect by the demographic variables, with the dependent variable being the SSRS total subscale score and the predictors being the sex, age, marital status, and anger-in or anger-out scores.
RESULTS
Sociodemographic data
The sociodemographic characteristics of the depressive and somatoform disorder groups are shown in Table 1. No significant differences were found in terms of sex, age, duration of education, income, marital status (married vs. single), religion (present vs. absent), or duration of illness between the two groups.
Comparison of anger management style and organ system symptoms between the depressive and somatoform disorder groups
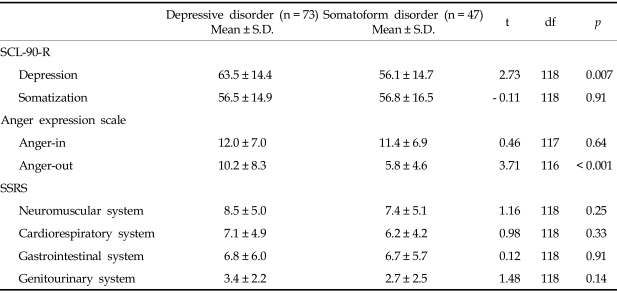
The depressive disorder group scored significantly higher on the anger-out subscale than the somatoform disorder group. However, no significant differences were found in scores of the anger-in subscale and the level of organ systems such as the subscale total scores of the SSRS between the two disorders (Table 2).
Table 2.
Scores of the SCL-90-R Depression and Somatization Subscales, the Anger Expression Scale and the SSRS in Each of the Psychiatric Patients
SCL-90-R, symptom checklist-90-revised; SSRS, somatic stress response scale.
The relationship between anger management style and the severity of organ system symptoms
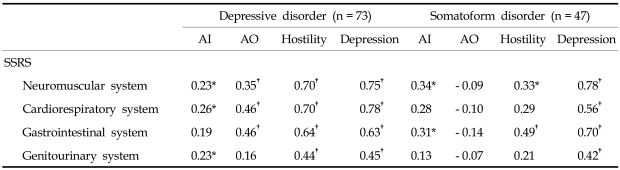
The depressive disorder group showed significant positive correlations between scores of the anger-out subscale and the level of organ system symptoms, such as neuromuscular system, cardiorespiratory system, and gastrointestinal system in the depressive disorder group. In these patients, scores of the anger-in subscale and the level of organ system symptoms, such as neuromuscular system, cardiorespiratory system, and genitourinary system (Table 3).
Table 3.
The Relationship between Anger-in, Anger-out, SCL-90-R Hostility and Depression Subscale Scores with the Level of Organ System Symptoms in Depressive Disorder and Somatoform Disorder Patients
The Pearson correlation test was used.
Results of the statistical analysis were described as Pearson correlation coefficients.
SSRS, somatic stress response ccale; SCL-90-R, symptom checklist-90-revised; AI, anger-in subscale of anger expression scale; AO, anger-out subscale of anger expression scale.
*p < 0.05.
†p < 0.01.
The somatoform disorder group produced positive correlations between the anger-in subscale scores and the level of organ system symptoms, including neuromuscular system and gastrointestinal system. In contrast, the anger-out subscale scores were not significantly correlated with the level of organ systems (Table 3).
To control for demographic variables in the depressive and somatoform disorder groups, multiple regression analyses were conducted with the dependent variable being the subscale total score of the SSRS and the predictors being sex, age, marital status, and score of either anger-out or anger-in subscale. It was discovered that, regardless of the demographic variables, the anger-out subscale score was significantly associated with the scores of the neuromuscular system symptoms (R2 = 0.19 F = 5.24 p = 0.003 B = 0.24 S.E. = 0.07 ß = 0.40 p = 0.001), cardiorespiratory system symptoms (R2 = 0.26 F = 7.94 p < 0.001 B = 0.30 S.E. = 0.06 ß = 0.50 p < 0.001) and gastrointestinal system symptoms (R2 = 0.29 F = 9.32 p < 0.001 B = 0.33 S.E. = 0.08 ß = 0.46 p < 0.001) in depressive disorder patients. However, the anger-in subscale score was not significantly associated with the scores of the neuromuscular system symptoms (R2 = 0.08 F = 1.94 p = 0.13 B = 0.15 S.E. = 0.09 ß = 0.21 p = 0.07), cardiorespiratory system symptoms (R2 = 0.08 F = 2.07 p = 0.11 B = 0.18 S.E. = 0.08 ß = 0.25 p = 0.03) and genitourinary system symptoms (R2 = 0.05 F= 1.32 p = 0.28 B = 0.07 S.E. = 0.04 ß = 0.23 p = 0.05) in depressive disorder patients. Multiple regression analyses also showed that the anger-in subscale score was not significantly associated with the scores of the neuromuscular system symptoms (R2 = 0.12 F = 1.88 p = 0.15 B = 0.25 S.E. = 0.11 ß = 0.34 p = 0.03) and gastrointestinal system symptoms (R2 = 0.13 F = 2.01 p = 0.13 B = 0.23 S.E. = 0.13 ß = 0.28 p = 0.07) in somatoform disorder patients.
The relationship between depression or hostility and the severity of organ systems
The SCL-90-R depression and hostility subscale scores correlated significantly with the scores of all the SSRS subscales in the depressive group. The SCL-90-R depression subscale scores also correlated significantly with the scores of all the SSRS subscales in the somatoform disorder group. However, the SCL-90-R hostility subscale score correlated significantly with the scores of neuromuscular system and gastrointestinal system in the somatoform disorder group (Table 3).
The relationship between the sociodemographic variables and organ system symptoms in each of the disorder groups
In the depressive disorder group, there were no significant differences in each of the total SSRS subscale scores between males and females (neuromuscular system 7.6 ± 5.3 vs. 9.2 ± 4.7 t = -1.38 df = 71 p = 0.17, cardiorespiratory system 6.7 ± 4.6 vs. 7.4 ± 5.1 t = - 0.66 df = 71 p = 0.51, gastrointestinal system 7.1 ± 6.5 vs. 6.6 ± 5.6 t = 0.40 df = 71 p = 0.69, genitourinary system 3.3 ± 2.1 vs. 3.4 ± 2.3 t = - 0.30 df = 71 p = 0.77). In addition, single patients scored significantly higher on gastrointestinal system than married patients (9.0 ± 6.6 vs. 5.4 ± 5.3 t = 2.28 df = 62 p = 0.03). However, no significant differences were found in the total scores of other SSRS subscales between married and single patients (neuromuscular system 8.2 ± 4.7 vs. 8.6 ± 5.1 t = - 0.25 df = 62 p = 0.81, cardiorespiratory system 6.7 ± 5.1 vs. 7.7 ± 4.7 t = - 0.74 df = 62 p = 0.46, genitourinary system 3.7 ± 2.2 vs. 2.9 ± 2.2 t = 1.30 df = 62 p = 0.20). Age had a significant and negative correlation with gastrointestinal system (r = - 0.30 p = 0.01). However, the level of income and the duration of illness had no significant correlations with the total score on each of other SSRS subscales (r = - 0.18~0.08 p > 0.05).
In the somatoform disorder group, females scored significantly higher on genitourinary system than males (1.8 ± 2.2 vs. 3.8 ± 2.5 t = - 2.87 df = 45 p = 0.006). However, there were no significant differences in the total scores of other SSRS subscales between males and females (neuromuscular system 7.5 ± 5.5 vs. 7.3 ± 4.8 t = 0.08 df = 45 p = 0.93, cardiorespiratory system 5.6 ± 4.2 vs. 7.0 ± 4.3 t = -1.12 df = 45 p = 0.27, gastrointestinal system 5.8 ± 5.4 vs. 7.8 ± 6.0 t = - 1.18 df = 45 p = 0.25). Patients who were married also scored significantly higher on genitourinary system (3.2 ± 2.3 vs. 1.6 ± 2.3 t = 2.13 df = 38 p = 0.04) than single patients. However, no significant differences were found in the total score of other SSRS subscales between married and single patients (neuromuscular system 7.4 ± 4.9 vs. 7.4 ± 5.9 t = - 0.003 df = 38 p = 0.99, cardiorespiratory system 6.4 ± 3.8 vs. 6.4 ± 4.7 t = 0.05 df = 38 p = 0.96, gastrointestinal system 7.2 ± 5.6 vs. 5.8 ± 5.8 t = 0.76 df = 38 p = 0.45). Age had a significant and positive correlation with the severity of genitourinary system symptoms (r = 0.35 p = 0.02), but the level of education, income, and the duration of the illness had no significant correlations with the total score of other SSRS subscales (r = - 0.12~0.27 p > 0.05).
DISCUSSION
This study found that the level of depression was associated with all the SSRS subscales, whereas the level of anger suppression or anger expression was associated with only some of the SSRS subscales in each of the depressive disorder and somatoform disorder patients. Therefore, depression is likely to be associated with somatic symptoms more widely than either anger suppression or anger expression in each of the two disorder groups. In previous research, a consistent association of depression with somatic symptoms has also been reported.19,20
In this study, the level of hostility, such as a SCL-90-R hostility subscale score, was correlated with all the SSRS subscales in depressive disorders, whereas the level of hostility was significantly correlated with some of the SSRS subscales, such as the neuromuscular system and gastrointestinal system in somatoform disorders. It was previously suggested that hostility may not be a main factor in the formation of functional somatic symptoms.12 However, this was not the case in depressive disorders as mentioned above.
Multiple regression analyses was conducted with the dependent variable being the level of the organ system symptoms that showed significant correlations with the level of either anger-in or anger-out and the predictors being the sex, age, marital status, and anger-in or anger-out scores. The results of the multiple regression analyses showed that in depressive disorder patients, the level of anger expression was significantly associated with the severity of somatic symptoms related to neuromuscular, cardiorespiratory and gastrointestinal organ systems. However, multiple regression analyses found that the level of anger suppression was not significantly associated with the severity of somatic symptoms of neuromuscular, cardiorespiratory and genitourinary systems in these patients. These results suggest that, in depressive disorder patients, anger expression is more likely to be predominantly involved in specific organ system-related somatic symptoms than is anger suppression.
In contrast, multiple regression analyses showed that the level of anger suppression was not significantly associated with the severity of somatic symptoms related to the neuromuscular and gastrointestinal systems in somatoform disorder patients. Therefore, anger suppression is not likely to be involved in any specific organ systems in somatoform disorder patients, although an association of inhibited anger and somatic symptoms has been reported in previous studies.3,4
In conclusion, these results suggest that, in depressive disorder patients, anger expression is likely to be predominantly involved in the neuromuscular, cardiorespiratory and gastrointestinal organ systems. However, in each of depressive disorder and somatoform disorder patients, anger suppression is not likely to be associated with any specific organ systems.
References
- 1.Koh KB. Stress and psychosomatic medicine. Seoul, Korea: Ilchokak; 2002. pp. 231–249. [Google Scholar]
- 2.Kellner R. Psychosomatic syndrome and somatic symptoms. Washington, DC: Am Psychiatric Press; 1991. pp. 189–213. [Google Scholar]
- 3.Kellner R, Hernandez J, Pathak D. Self-rated inhibited anger, somatization and depression. Psychother Psychosom. 1992;57:102–107. doi: 10.1159/000288582. [DOI] [PubMed] [Google Scholar]
- 4.Harris ID. Mood, anger, and somatic dysfunction. J Nerv Ment Dis. 1951;113:152–158. [PubMed] [Google Scholar]
- 5.Mezzich JE, Lin K, Hughes CC. Acute and transient psychotic disorders and culture-bound syndromes. In: Sadock BJ, Sadock VA, editors. Comprehensive textbook of psychiatry. 7th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000. pp. 1264–1276. [Google Scholar]
- 6.Lin KM. Hwa-Byung: a Korean culture-bound syndrome? Am J Psychiatry. 1983;140:105–107. doi: 10.1176/ajp.140.1.105. [DOI] [PubMed] [Google Scholar]
- 7.Min SK, Lee MH, Shin JH. A diagnostic study on hwabyung. J Korean Med Assoc. 1986;29:653–661. [Google Scholar]
- 8.Min SK. A study of the concept of hwabyung. J Korean Neuropsychiatr Assoc. 1989;28:604–616. [Google Scholar]
- 9.Koh KB, Kim CH, Park JK. Predominance of anger in depressive disorders compared with anxiety disorders and somatoform disorders. J Clin Psychiatry. 2002;63:486–492. doi: 10.4088/jcp.v63n0604. [DOI] [PubMed] [Google Scholar]
- 10.Fava M, Rosenbaum JF, McCarthy M, Pava J, Steingard R, Bless E. Anger attacks in depressed outpatients and their response to fluoxetine. Psychopharmacol Bull. 1991;27:275–279. [PubMed] [Google Scholar]
- 11.Gould RA, Ball S, Kaspi SP, Otto MW, Pollack MH, Shekhar A, et al. Prevalence and correlates of anger attacks: a two site study. J Affect Disord. 1996;39:31–38. doi: 10.1016/0165-0327(96)00017-1. [DOI] [PubMed] [Google Scholar]
- 12.Kellner R, Slocumb J, Wiggins RG, Abbott PJ, Winslow WW, Pathak D. Hostility, somatic symptoms, and hypochondriacal fears and beliefs. J Nerv Ment Dis. 1985;173:554–560. doi: 10.1097/00005053-198509000-00006. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 14.Koh KB, Kim SJ. Comparison of anger between patients with essential hypertension and normal controls. Korean J Psychosom Med. 1995;3:19–27. [Google Scholar]
- 15.Spielberger CD, Johnson EH, Russell SF, Crane R, Jacob GA, Worden TJ. The experience and expression of anger: construction and validation of an anger expression scale. In: Chesney MA, Rosenman RH, editors. Anger and hostility in cardiovascular and behavioral disorders. Washington, DC: Hemisphere; 1985. [Google Scholar]
- 16.Kim KI, Kim JH, Won HT. Korean manual of symptom checklist-90-revision. Seoul, Korea: Chung Ang Aptitude Publishing Co; 1984. [Google Scholar]
- 17.Derogatis LR, Rickels K, Rock AF. The SCL-90 and the MMPI: a step in the validation of a new self-report scale. Br J Psychiatry. 1976;128:280–289. doi: 10.1192/bjp.128.3.280. [DOI] [PubMed] [Google Scholar]
- 18.Koh KB, Park JK, Cho S. Development of the somatic stress response scale and its application in clinical practice. Yonsei Med J. 2005;46:614–624. doi: 10.3349/ymj.2005.46.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katon W, Kleinman A, Rosen G. Depression and somatization: a review, part 1. Am J Med. 1982;72:127–135. doi: 10.1016/0002-9343(82)90599-x. [DOI] [PubMed] [Google Scholar]
- 20.Katon W, Kleinman A, Rosen G. Depression and somatization: a review, part II. Am J Med. 1982;72:241–247. doi: 10.1016/0002-9343(82)90816-6. [DOI] [PubMed] [Google Scholar]