Abstract
Purpose
The purpose of the present study was to investigate whether electrical stimulation (ES) improves the paralytic effect of botulinum toxin type A (BTX-A) and evaluate the differences between low frequency (LF) and high frequency (HF) ES in children with spastic diplegic cerebral palsy (CP).
Materials and Methods
Twenty-three children with spastic diplegia CP who had BTX-A injections into both gastrocnemius muscles were assessed. Following the toxin injection, electrical stimulation was given to 1 side of the injected muscles and a sham-stimulation to the other side for 30 min a day for 7 consecutive days [HFES (25 Hz) to 11 children, LFES (4 Hz) to 12 children]. The compound motor action potentials (CMAP) from the gastrocnemius muscle were assessed before injection and at 5 time points (days 3, 7, 14, 21, and 30) after injection. The clinical assessments of spasticity were performed before and 30 days after injection.
Results
The CMAP area became significantly lower in both LFES and HFES sides from 3 days after injection compared to baseline values. In other words, the CMAP area of the sham-stimulated side showed a significant decrease at 7 or 14 days after injection. However, there were no significant differences in clinical assessment of spasticity between the stimulated and sham-stimulated sides.
Conclusion
Short-term ES in both LF and HF to the spastic muscles injected with BTX-A might induce earlier denervating action of BTX-A. However, it does not necessarily lead to clinical and electrophysiological benefits in terms of reduction of spasticity.
Keywords: Botulinum toxin type A, electrical stimulation, cerebral palsy, spasticity
INTRODUCTION
The spastic type is the most common form of CP.1,2 Spasticity has been considered to be a main contributor to both the impairment of function and decreased longitudinal muscle growth in children with spastic CP, which can lead to deformity.3-7 Thus, reduction of spasticity in children with CP is important in the management of the disease. Several treatment options have been used to reduce the spasticity and to improve function in children with CP. Among them, BTX-A injection has become a popular treatment for spasticity in the absence of fixed deformities in the pediatric population. Furthermore, the use of BTX-A is considered to be an effective and safe treatment for spasticity or dystonia.8
BTX-A produces a dose-dependent reversible chemodenervation of the injected muscle by blocking the presynaptic release of acetylcholine at the neuromuscular junction.9 Denervation of the spastic muscle allows muscle growth, prevention of contracture, and diminution of dynamic deformity. However, treatment is not always successful and the effects are often transient. Furthermore, variable clinical responses can be observed in patients with the same form of the disease.10,11
Since Hughes and Whaler12 in 1962 demonstrated that the repetitive ES of the axon increased BTX-A absorption in a frog diaphragm preparation model, it has been suggested that muscle activity may affect the paralytic effect of BTX-A. In recent experimental studies, performing a post-injection stretching exercise with ES was beneficial for maximizing the muscle paralysis effect of BTX-A on calf muscles in rabbits.13 Also, in normal human muscles, the effect of BTX-A on the muscle can be increased by ES immediately after injection.10,14 However, it remains unclear for spastic human muscle whether muscle activity induced by ES increases the effect of BTX-A on spasticity and functional improvement of patients.11,15,16
We designed this study to investigate whether ES improves the paralytic and anti-spastic effects of BTX-A and evaluate the differences between LF- and HFES in children with spastic CP using electrophysiological and clinical examination.
MATERIALS AND METHODS
Subjects
Among children with spastic CP who were admitted to our rehabilitation hospital, those who were planning to have BTX-A injections into both gastrocnemius muscles were included in this study. Exclusion criteria included previous orthopedic surgery, previous BTX-A injection within the past 12 months, and fixed joint contractures. This study was approved by the Institutional Review Board of our institution, and informed consent was obtained from the parents of children.
Twenty-three children (12 girls, 11 boys) participated in the study. The mean age was 46.0 ± 18.1 months. The types of CP included patients who were diplegic (n = 18) and quadriplegic (n = 5). The gross motor functional level of each patient was determined by the Gross Motor Function Classification System (GMFCS).17 They were classified as follows: 3 were considered level I (13%), 5 level II (22%), 8 level III (35%), and 7 level IV (30%). The physical and occupational therapy program was carried out during the hospital stay and included 6 sessions of physical therapy and 6 sessions of occupational therapy per week (30 minutes each per session).
BTX-A injection and ES
A lignocaine-based local anesthetic, EMLA cream (Astra, Kings Langley, UK), was applied to the skin prior to BTX-A injection. One hundred units of BTX-A (Botox, Allergan Inc., CA, USA) was diluted with 2 mL of 0.9% saline, and the same doses of the toxin were injected into the right and left gastrocnemius muscles (4 - 5 units/kg in each muscle).
Following the toxin injection, ES was given to 1 side of injected muscles and a sham stimulation was administered to the other side for 30 minutes a day for 7 consecutive days. The children were randomly assigned into 2 groups: 11 children (age, 40.2 ± 14.0 months) were assigned to the HFES (25 Hz) group, and 12 (age, 52.5 ± 20.4 months) to the LFES (4 Hz) group. Pairs of 90 × 50 mm self-adhesive surface electrodes (ValuTrode, Axelgaard Co., CA, USA) were attached close to a motor point. A 4-channel device (MegaXP, CyberMedic Corp., Iksan, Korea) with continuous trains of biphasic rectangular symmetric 0.25 ms current pulses was used for stimulation. The stimulation intensity was adjusted at threshold intensity for minimal gastrocnemius muscle twitching.
Electrophysiological assessment
In each gastrocnemius muscle, we used Synergy II (VIASYS Healthcare Inc., Madison, USA) to record the CMAP evoked by supramaximal ES of the tibial nerve at the popliteal area. The recording bandwidth was 3 - 10,000 Hz. CMAP was recorded with a self-adhesive electrode (Neuroline 710, Ambu A/S, Ballerup, Denmark). The active recording electrode was placed at mid-belly of the medial gastrocnemius muscle near the motor point. A reference electrode was placed at the Achilles tendon, and a ground electrode was placed between an active recording electrode and stimulator.18 The CMAPs from the gastrocnemius muscle were assessed before injection and at 5 discrete time points (days 3, 7, 14, 21, and 30) after injection. The attachment points of the active electrode were marked with an oil pen to ensure accurate recording of the CMAP at the same location at every time point. Peak-to-peak amplitude and the area of the CMAP were measured. The amplitude changes of CMAP (Amplitude %) and the area changes of CMAP (Area %) were analyzed.
Clinical assessment
The same investigator, who was blind to the treatment regimen, performed the clinical examination before the injection and at 30 days after injection. The triceps surae muscle tone was assessed using the Modified Ashworth Scale and Modified Tardieu Scale.19 The Modified Ashworth Scale is a 6-point rating scale used to gauge muscle resistance to passive movement. The scoring was as follows: 0 = no increase in muscle tone; 1 = slight increase in muscle tone as manifested by a "catch and release" or minimal resistance at the end of the range of motion; 2 = slight increase in muscle tone shown as a "catch" and followed by minimal resistance throughout less than half the range of motion; 3 = marked increase in muscle tone through most of the range of motion, the affected part being easily moved; 4 = considerable increase in muscle tone and difficult passive movement; 5 = affected part remained rigid. Two angles (R1 and R2) were determined in the Modified Tardieu Scale. The angle of muscle reaction (R1) was defined as the point in the joint range in which a velocity-dependent "catch" or clonus was felt during a quick stretch of the muscle. The angle of full range of motion (R2) was equivalent to the passive range of motion. Both R1 and R2 were measured relative to the neutral position of the ankle joints. The neutral position at the ankle joint (corresponding to angle zero) was the position with the ankle at 90 degrees to the lower leg. A negative value would be given if the ankle could not be dorsiflexed beyond angle zero. Modified Ashworth Scale and Modified Tardieu Scale were measured in both the knee flexion and extension positions.
Statistical analysis
To determine statistical differences between stimulated and sham-stimulated sides, Wilcoxon-signed rank test was used. To compare the post-injection value of CMAP at each time point to the pre-injection value for analyzing the onset of paralyzing effect, repeated measures ANOVA was used after performing normality test by Shapiro-Wilk test. Data for patients in the LFES and HFES groups were tested separately.
We used Mann-Whitney test to compare the Amplitude % and Area % of stimulated sides of the LFES and HFES groups at each time points. P-values less than 0.05 were considered statistically significant.
RESULTS
Electrophysiological assessment
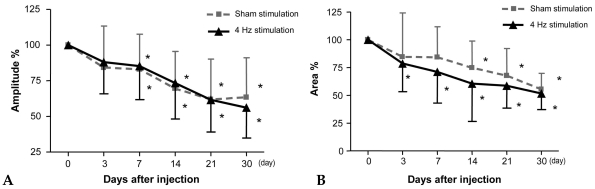
In the LFES group, Amplitude % decreased significantly from 7 days post-injection in both the stimulated and sham-stimulated side when compared to the pre-injection value of CMAP (Fig. 1A) (p < 0.05). In contrast, Area % decreased significantly from 3 days post-injection in the stimulated side and from 14 days post-injection in the sham-stimulated side, and remained low until 30 days after injection (Fig. 1B) (p < 0.05). However, there was no significant difference in Amplitude % and Area % between the stimulated and sham-stimulated sides at all 6 time points.
Fig. 1.
Comparison of amplitude and area of compound motor action potential between the 4 Hz frequency stimulated side and sham-stimulated side of gastrocnemius muscles in children with spastic CP (n = 12). *p < 0.05, before injection vs. each time point after injection.
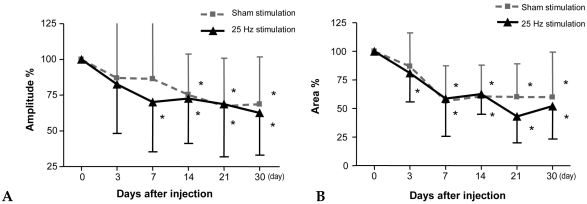
In the HFES group, Amplitude % decreased significantly from 7 days post-injection in the stimulated side and from 14 days post-injection in the sham-stimulated side, and remained low until 30 days after injection when compared to the pre-injection value of CMAP (Fig. 2A) (p < 0.05). Area % decreased significantly from 3 days post-injection in the stimulated side and from 7 days post-injection in the sham-stimulated side, and remained low until 30 days after injection (Fig. 2B) (p < 0.05). However, there was no significant difference in Amplitude % and Area % between the stimulated and sham-stimulated sides at all 6 time points.
Fig. 2.
Comparison of amplitude and area of compound motor action potential between the 25 Hz frequency stimulated side and sham-stimulated side of gastrocnemius muscles in children with spastic CP (n = 11). *p < 0.05, before injection vs. each time point after injection.
In addition, there were no significant differences in electrophysiological parameters assessed between the LFES and HFES groups.
Clinical assessment
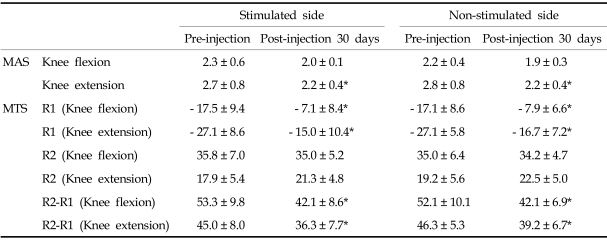
In the LFES group, Modified Ashworth Scale of ankle plantar-flexors in knee extension position, R1 in both knee flexion and extension positions, and R2-R1 in both knee flexion and extension positions improved in both the stimulated and sham-stimulated sides at 30 days post-injection (Table 1) (p < 0.05). However, there was no significant difference in improvement of spasticity between the stimulated and sham-stimulated sides.
Table 1.
Comparison of Clinical Assessment Data between 4 Hz Frequency-Stimulated Side and Non-stimulated Side of Gastrocnemius Muscles in Children with Spastic Cerebral Palsy (n = 12)
MAS, modified ashworth scale; MTS, modified tardieu scale; R1, angle of muscle reaction; R2, angle of full range of motion.
Values are means ± standard deviation.
*p < 0.05 when compared between pre-injection and post-injection 30 days data.
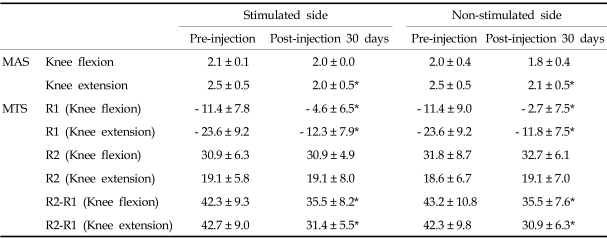
In the HFES group, Modified Ashworth Scale of ankle plantar-flexors in knee extension position, R1 in both knee flexion and extension positions, and R2-R1 in both knee flexion and extension positions also improved in both the stimulated and sham-stimulated sides at 30 days post-injection (Table 2) (p < 0.05). However, there was also no significant difference in improvement of spasticity between the stimulated and sham-stimulated sides.
Table 2.
Comparison of Clinical Assessment Data between 25 Hz Frequency-Stimulated Side and Non-stimulated Side of Gastrocnemius Muscles in Children with Spastic Cerebral Palsy (n = 11)
MAS, modified ashworth scale; MTS, modified tardieu scale; R1, angle of muscle reaction; R2, angle of full range of motion.
Values are means ± standard deviation.
*p < 0.05 when compared between pre-injection and post-injection 30 days data.
In addition, there were no significant differences in the clinical parameters for spasticity between the LFES and HFES groups.
DISCUSSION
BTX-A has complex protein structures containing 150 kDa neurotoxin molecule. It binds to specific external high-affinity receptors on the membranes of cholinergic neurons and is internalized by endocytosis into vesicular compartments.20 In order to be active, the 150 kDa neurotoxins must be nicked by proteases into 2 polypeptide fragments; a 100 kDa heavy chain and 50 kDa light chain that are held together by an inter-chain disulfide bond.21 The light chain, which is translocated across the endosomal membrane into the cytosol, is a zinc-endopeptidase responsible for intracellular toxin activity.20 In animal experiments, it is suggested that muscle inactivity may delay this activation process of BTX-A while ES influences the uptake and onset latency. Hughes and Whaler12 demonstrated that repetitive ES of the axon increased BTX-A absorption in a frog diaphragm preparation model, and Simpson22 identified that ES accelerates the lytic step for internalization of BTX-A into the motor nerve terminal, thereby facilitating its inhibitory effect on acetylcholine release.
In human muscles, some researchers tried to clarify the effectiveness of ES applied to muscles following BTX-A injection using neurophysiologic quantification with nerve conduction studies.10,13,14,18 Eleopra et al.10 injected low dose BTX-A into normal adult extensor digitorum brevis muscles and delivered the electrical current to the injected muscles at a 4 Hz frequency. They compared the Amplitude % of CMAP-which was the parameter we also used in this study-and reported that the effect of the induced neuromuscular blockade was significantly greater in the electrically stimulated side. Flasson et al.14 also reported that the short-term ES to the injected muscles induces a rapid and persistent reduction of CMAP recorded at the extensor digitorum brevis muscles in patients with spastic paraplegia. However, the extensor digitorum brevis muscles at which they assessed the changes of CMAP in both studies are not the sites where the BTX-A injections are usually performed in clinical practice; the gastrocnemius muscles are the muscles most commonly treated with BTX-A injection in children with spastic CP. For that reason, we selected the gastrocnemius muscles to assess the CMAP. Short-term ES was given only to these gastrocnemius muscles to find out the effect of ES on the injected muscle. In this study, a decrease in Area % of CMAP became significant from 3 days post-injection on the stimulated side and 7 or 14 days on the sham-stimulated side in both the LFES and HFES groups. These findings suggest that ES might induce the earlier denervating action of BTX-A on the gastrocnemius muscles for correcting the equinus deformity in children with spastic CP. This earlier onset of denervation can be explained by the foregoing mechanism; ES facilitates the lytic step of BTX-A for internalization. Although the onset of decrease in the CMAP area is shortened in the stimulated sides, there was no significant difference in Amplitude % and Area % between the stimulated and sham-stimulated sides at all 6 time points in both the LFES and HFES groups. In addition, decrease in amplitude % of CMAP became significant from post-injection 7 days in both the stimulated and sham-stimulated sides of the LFES group. Therefore, it is not appropriate to state that the combined treatment of BTX-A and ES is superior to BTX-A alone in electrophysiological analysis.
It is known that the effects of ES can be modulated by changing the stimulation frequency.23,24 In the study by Frasson et al.14 to measure CMAP at extensor digitorum brevis muscles after BTX-A injection and ES, 2 stimulation frequencies (LF, 4 Hz; and HF, 25 Hz) were used, and they reported that Amplitude % of CMAP was significantly lower on the 4 Hz stimulated side than on the non-stimulated side 4 days after injection, and it remained until 60 days after injection. Conversely, in patients receiving 25 Hz ES, no significant difference was found between the stimulated and non-stimulated muscle. The above authors suggested that prolonged HFES could reduce the excitability at the site of nerve fiber stimulation, and this might be associated with the frequency-dependent effects of ES. In our findings, the CMAP area of the gastrocnemius showed that both 4 and 25 Hz of stimulation hastened the onset of denervation induced by BTX-A compared to the sham-stimulated side. Additionally, there were no significant differences in the electrophysiological parameters assessed between the LFES and HFES groups. The reasons why our study did not show frequency-dependent changes and did not accord with previous observations remain unexplained. However, in the study by Frasson et al.,14 they also found spontaneous denervation potentials appearing early in needle electromyographic examination of extensor digitorum brevis muscles at both stimulation frequencies. As a result, they acknowledged that they could not explain why ES in their study induced frequency-dependent changes. Therefore, further research is needed to clarify whether various stimulation frequencies used could differentially affect the absorption of BTX-A.
Several studies have been performed to investigate whether additional ES enhances the clinical outcome after BTX-A injection therapy. Hesse and colleagues11 reported that ES of the lower limb muscles following BTX-A injection resulted in greater improvement of gait velocity and a reduction of muscle tone in randomized controlled studies of patients with spastic hemiplegia after stroke. For the upper limb of hemiplegic patients after stroke, they reported that ES reduced the muscle tone of the elbow joint and improved the cleaning activity of the affected palm.16 However, in their studies, they applied ES not only to the injected muscles but also to antagonist muscles. When ES is applied to antagonist muscle, it has an inhibitory effect on the activity of the injected muscle through the polysynaptic inhibiting pathway, reducing its spasticity.25 In addition, it may have a positive action on the rheological properties through stretching of the injected muscles.26 Stretching the spastic muscles after BTX-A injection has been utilized to enhance the effect of BTX-A.10,13,27 It is hypothesized that the prolonged stretching of a muscle could result in a greater internalization of BTX-A or microstructural modifications of the muscles, which reduces the spasticity.26 A recent study showed that optimal stretching with taping could lead to higher and faster hypertonus reduction compared to ES to the injected muscles and splinting.26 From this prospective, the beneficial effect of electrical simulation in these studies may be the consequence of the effect of ES on the antagonist to an injected muscle or the effect of ES on both the antagonist and injected muscles. As for children with CP, only 1 study on the effect of ES on the clinical outcome following BTX-A injection has been carried out by Detrembleur et al.15 They applied ES only to the injected muscles and used relatively HF current pulses (20 Hz) for stimulation, and used 3-dimensional gait analysis and clinical assessment data including passive joint range of motion, Modified Ashworth Scale, and physician rating scale without electrophysiological assessment. The result showed no evidence of superiority of the addition of ES to the BTX-A injection therapy. For clinical assessment of spasticity of the triceps surae muscle, we used Modified Ashworth Scale and Modified Tardieu Scale. Modified Ashworth Scale is the most commonly used clinical scale for the measurement of spasticity. However, their validity has recently been questioned because they do not address the velocity-dependent aspect of the phenomenon of spasticity.28 On the other hand, Modified Tardieu Scale measures the passive range of movement at different movement velocity (slow and fast velocity passive stretches) and determines the dynamic component of the muscle contracture.29 Therefore, this scale has been suggested as a more suitable tool for spasticity measurement compared to Modified Ashworth Scale.30,31 In our findings, both scales for clinical assessment of spasticity did not differ between the stimulated and sham-stimulated sides in both the LFES and HFES groups before and 30 days after BTX-A injection. Because we did not investigate earlier changes of spasticity before 30 days post-injection, we could not ascertain whether reduction of spasticity was induced more rapidly in the stimulated sides. However, it is difficult in our clinical analysis to establish superiority of the combined treatment of BTX-A and ES over BTX-A alone.
In conclusion, the results of this study suggested that short-term ES in both LF and HF to spastic muscles after BTX-A injection might induce the earlier response of BTX-A. However, there was no definite evidence in this study that the adjuvant ES after BTX-A injection was superior to BTX-A alone in alleviating spasticity in terms of both electrophysiological and clinical assessment.
Footnotes
This study was supported by a faculty research grant from Yonsei University College of Medicine for 2005 (6-2005-0061).
References
- 1.Mongan D, Dunne K, O'Nuallain S, Gaffney G. Prevalence of cerebral palsy in the West of Ireland 1990-1999. Dev Med Child Neurol. 2006;48:892–895. doi: 10.1017/S0012162206001952. [DOI] [PubMed] [Google Scholar]
- 2.Platt MJ, Cans C, Johnson A, Surman G, Topp M, Torrioli MG, et al. Trends in cerebral palsy among infants of very low birthweight (< 1500 g) or born prematurely (< 32 weeks) in 16 European centres: a database study. Lancet. 2007;369:43–50. doi: 10.1016/S0140-6736(07)60030-0. [DOI] [PubMed] [Google Scholar]
- 3.Corry IS. Use of a motion analysis laboratory in assessing the effects of botulinum toxin in a cerebral palsy [dissertation] Belfast (North Ireland): The Queens University; 1995. Thesis for doctor of medicine in the faculty of medicine. [Google Scholar]
- 4.Cosgrove AP, Graham HK. Botulinum toxin A prevents the development of contractures in the hereditary spastic mouse. Dev Med Child Neurol. 1994;36:379–385. doi: 10.1111/j.1469-8749.1994.tb11863.x. [DOI] [PubMed] [Google Scholar]
- 5.Dunne JW, Heye N, Dunne SL. Treatment of chronic limb spasticity with botulinum toxin A. J Neurol Neurosurg Psychiatry. 1995;58:232–235. doi: 10.1136/jnnp.58.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lance JW. Pathophysiology of spasticity and clinical experience of baclofen. In: Feldman RG, Young RR, Koella WP, editors. Spasticity: disordered motor control. Chicago: Year Book Medical Publishers; 1980. pp. 185–203. [Google Scholar]
- 7.Ziv I, Blackburn N, Rang M, Koreska J. Muscle growth in normal and spastic mice. Dev Med Child Neurol. 1984;26:94–99. doi: 10.1111/j.1469-8749.1984.tb04412.x. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed KA, Moore AP, Rosenbloom L. Adverse events following repeated injections with botulinum toxin A in children with spasticity. Dev Med Child Neurol. 2001;43:791. doi: 10.1017/s0012162201211426. [DOI] [PubMed] [Google Scholar]
- 9.Aoki KR, Guyer B. Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions. Eur J Neurol. 2001;8 Suppl 5:21–29. doi: 10.1046/j.1468-1331.2001.00035.x. [DOI] [PubMed] [Google Scholar]
- 10.Eleopra R, Tugnoli V, De Grandis D. The variability in the clinical effect induced by botulinum toxin type A: the role of muscle activity in humans. Mov Disord. 1997;12:89–94. doi: 10.1002/mds.870120115. [DOI] [PubMed] [Google Scholar]
- 11.Hesse S, Jahnke MT, Luecke D, Mauritz KH. Short-term electrical stimulation enhances the effectiveness of Botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci Lett. 1995;201:37–40. doi: 10.1016/0304-3940(94)12124-9. [DOI] [PubMed] [Google Scholar]
- 12.Hughes R, Whaler BC. Influence of nerve-ending activity and of drugs on the rate of paralysis of rat diaphragm preparations by Cl. botulinum type A toxin. J Physiol. 1962;160:221–233. doi: 10.1113/jphysiol.1962.sp006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HS, Hwang JH, Jeong ST, Lee YT, Lee PK, Suh YL, et al. Effect of muscle activity and botulinum toxin dilution volume on muscle paralysis. Dev Med Child Neurol. 2003;45:200–206. doi: 10.1017/s0012162203000380. [DOI] [PubMed] [Google Scholar]
- 14.Frasson E, Priori A, Ruzzante B, Didone G, Bertolasi L. Nerve stimulation boosts botulinum toxin action in spasticity. Mov Disord. 2005;20:624–629. doi: 10.1002/mds.20395. [DOI] [PubMed] [Google Scholar]
- 15.Detrembleur C, Lejeune TM, Renders A, Van Den Bergh PY. Botulinum toxin and short-term electrical stimulation in the treatment of equinus in cerebral palsy. Mov Disord. 2002;17:162–169. doi: 10.1002/mds.1282. [DOI] [PubMed] [Google Scholar]
- 16.Hesse S, Reiter F, Konrad M, Jahnke MT. Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: a randomized, double-blind, placebo-controlled trial. Clin Rehabil. 1998;12:381–388. doi: 10.1191/026921598668275996. [DOI] [PubMed] [Google Scholar]
- 17.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 18.Seo JH, Kim SS, Kim NK, Lee JH. Measuring compound muscle action potentials after botulinum toxin a injection for the quantification of effects. J Korean Acad Rehabil Med. 1998;22:1225–1231. [Google Scholar]
- 19.Yam WK, Leung MS. Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. J Child Neurol. 2006;21:1031–1035. doi: 10.1177/7010.2006.00222. [DOI] [PubMed] [Google Scholar]
- 20.Pellizzari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos Trans R Soc Lond B Biol Sci. 1999;354:259–268. doi: 10.1098/rstb.1999.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das Gupta BR, Sugiyama H. Role of a protease in natural activation of Clostridium botulinum neurotoxin. Infect Immun. 1972;6:587–590. doi: 10.1128/iai.6.4.587-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson LL. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1980;212:16–21. [PubMed] [Google Scholar]
- 23.Kesar T, Chou LW, Binder-Macleod SA. Effects of stimulation frequency versus pulse duration modulation on muscle fatigue. J Electromyogr Kinesiol. 2007;18:662–671. doi: 10.1016/j.jelekin.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesar T, Binder-Macleod S. Effect of frequency and pulse duration on human muscle fatigue during repetitive electrical stimulation. Exp Physiol. 2006;91:967–976. doi: 10.1113/expphysiol.2006.033886. [DOI] [PubMed] [Google Scholar]
- 25.Johnson C, Wood DE, Swain ID, Tromans AM, Strike P, Burridge JH. A pilot study to investigate the combined use of botulinum neurotoxin type a and functional electrical stimulation, with physiotherapy, in the treatment of spastic dropped foot in subacute stroke. Artif Organs. 2002;26:263–266. doi: 10.1046/j.1525-1594.2002.06948.x. [DOI] [PubMed] [Google Scholar]
- 26.Carda S, Molteni F. Taping versus electrical stimulation after botulinum toxin type A injection for wrist and finger spasticity. A case-control study. Clin Rehabil. 2005;19:621–626. doi: 10.1191/0269215505cr879oa. [DOI] [PubMed] [Google Scholar]
- 27.Rodriquez AA, McGinn M, Chappell R. Botulinum toxin injection of spastic finger flexors in hemiplegic patients. Am J Phys Med Rehabil. 2000;79:44–47. doi: 10.1097/00002060-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Pandyan AD, Johnson GR, Price CI, Curless RH, Barnes MP, Rodgers H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin Rehabil. 1999;13:373–383. doi: 10.1191/026921599677595404. [DOI] [PubMed] [Google Scholar]
- 29.Love SC, Valentine JP, Blair EM, Price CJ, Cole JH, Chauvel PJ. The effect of botulinum toxin type A on the functional ability of the child with spastic hemiplegia a randomized controlled trial. Eur J Neurol. 2001;8 Suppl 5:50–58. doi: 10.1046/j.1468-1331.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- 30.Mehrholz J, Wagner K, Meissner D, Grundmann K, Zange C, Koch R, et al. Reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in adult patients with severe brain injury: a comparison study. Clin Rehabil. 2005;19:751–759. doi: 10.1191/0269215505cr889oa. [DOI] [PubMed] [Google Scholar]
- 31.Sätilä H, Iisalo T, Pietikäinen T, Seppänen RL, Salo M, Koivikko M, et al. Botulinum toxin treatment of spastic equinus in cerebral palsy: a randomized trial comparing two injection sites. Am J Phys Med Rehabil. 2005;84:355–365. doi: 10.1097/01.phm.0000160006.51859.ae. quiz 66-7, 392. [DOI] [PubMed] [Google Scholar]