Abstract
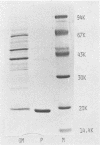
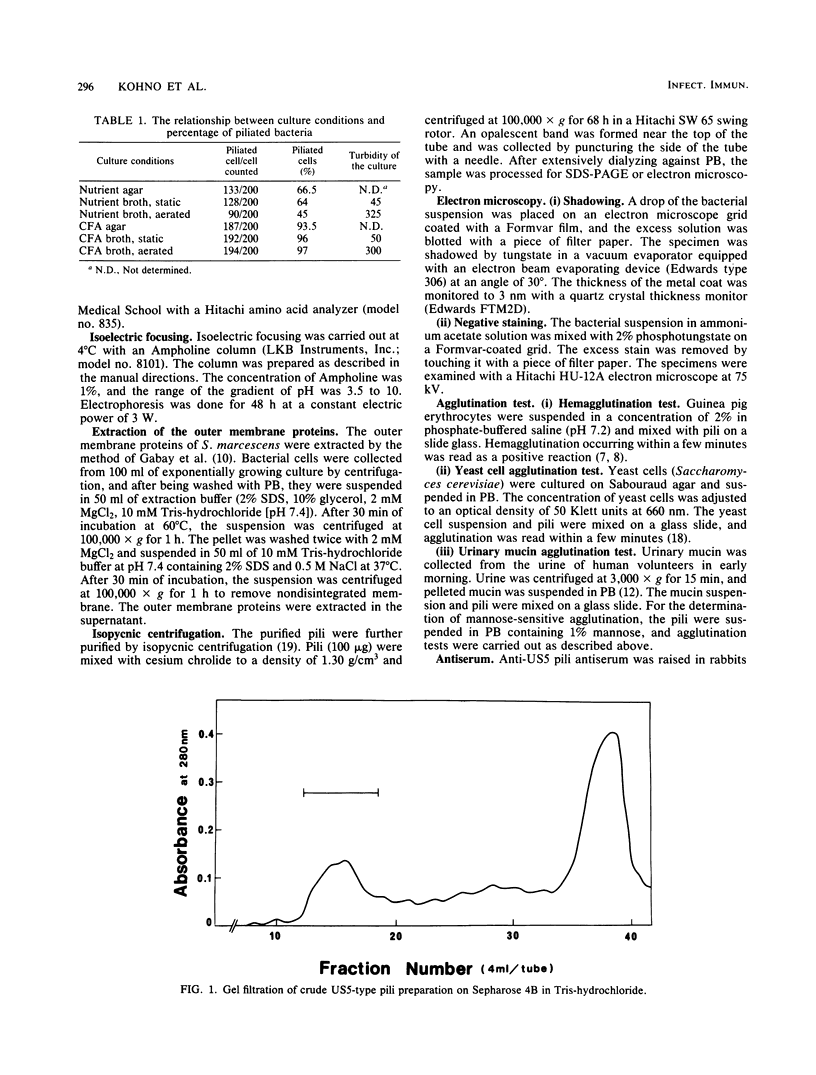
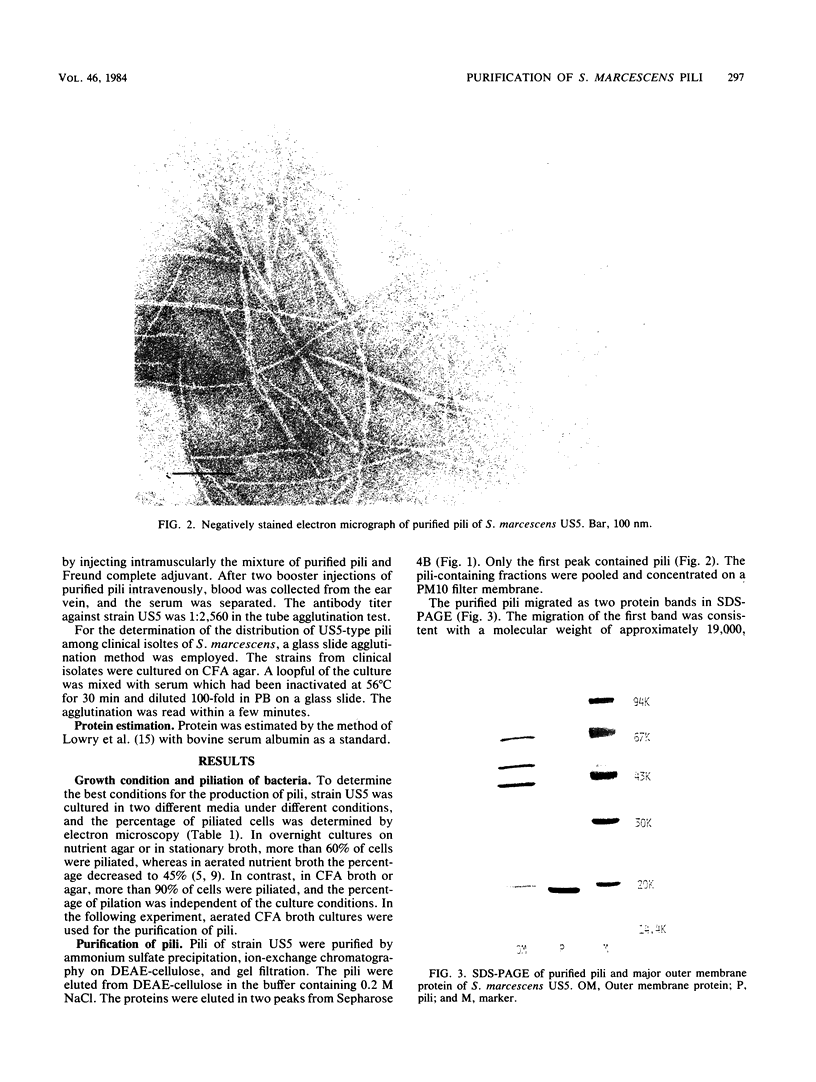
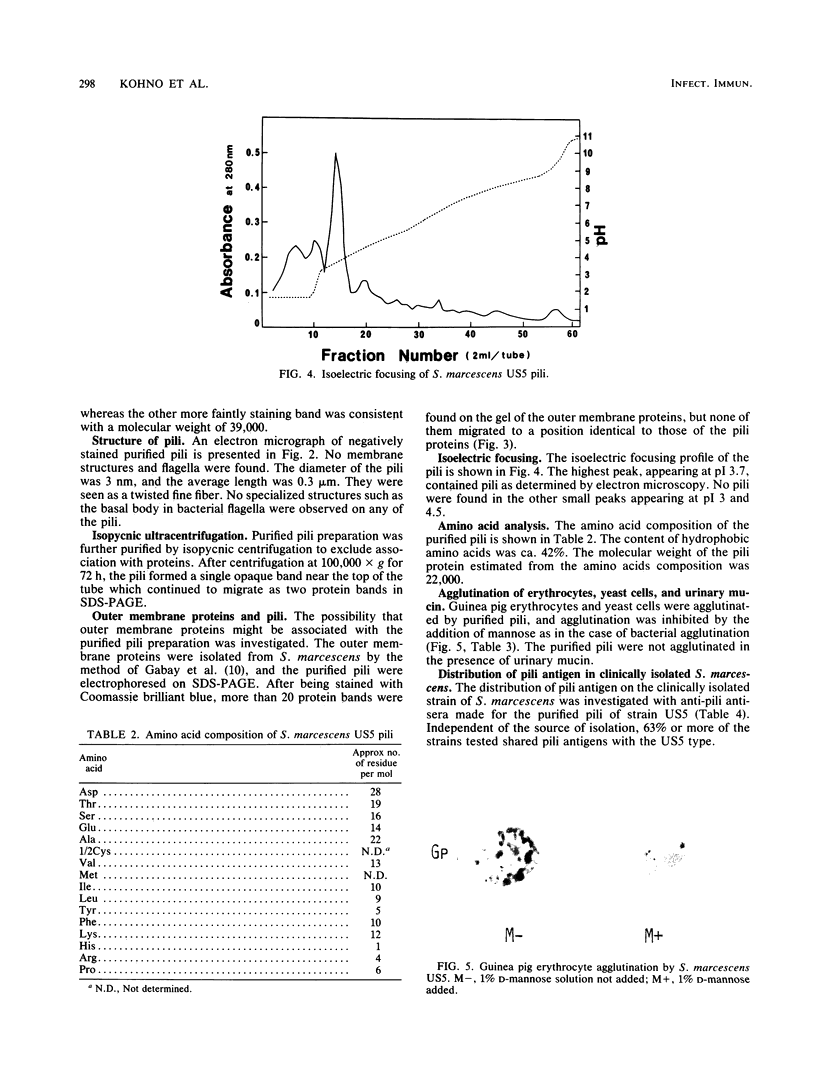
The pili of Serratia marcescens US5 isolated from a patient with urinary tract infection were purified and characterized. During the aeration culture, the pili were detached from the bacteria and were precipitated by the addition of ammonium sulfate. The purification of the pili was carried out by ion-exchange chromatography and gel filtration on Sepharose 4B. In electron microscopy, the purified pilus showed a filament of 3 nm in diameter and 0.3 micron in average length. The molecular weight of the protein subunit of the purified pili was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Two protein bands appeared. One has a molecular size of 19,000 daltons, and the other has a molecular size of 39,000 daltons. The isoelectric point was 3.7. The content of hydrophobic amino acids in purified pili subunits was 42% of the total amino acid content. Further purification of pili by isopycnic centrifugation failed to remove the large protein band. No identical protein bands to pili proteins were detected in the electrophoresis pattern of the outer membrane proteins extracted from S. marcescens US5 in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. These two proteins might be a dimer of a small molecule. A survey of clinically isolated strains of S. marcescens revealed that more than 60% of the strains had this type of pili. These results suggest that these pili are widely distributed among strains of S. marcescens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adegbola R. A., Old D. C. New fimbrial hemagglutinin in Serratia species. Infect Immun. 1982 Oct;38(1):306–315. doi: 10.1128/iai.38.1.306-315.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amako K., Ohshima H., Yasunaka K., Kono K. Pili mediated agglutination of Serratia marcescens in human urine. Microbiol Immunol. 1981;25(10):981–992. doi: 10.1111/j.1348-0421.1981.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Speth V., Jann K. Participation of pili and cell wall adhesion in the yeast agglutination activity of Escherichia coli. Infect Immun. 1981 Dec;34(3):980–986. doi: 10.1128/iai.34.3.980-986.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E. Screening of uropathogenic Escherichia coli for expression of mannose-selective adhesins: importance of culture conditions. J Clin Microbiol. 1981 Jun;13(6):1088–1095. doi: 10.1128/jcm.13.6.1088-1095.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J., Yasunaka K. Interaction of the lamB protein with the peptidoglycan layer in Escherichia coli K12. Eur J Biochem. 1980 Feb;104(1):13–18. doi: 10.1111/j.1432-1033.1980.tb04393.x. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E. K99 surface antigen of Escherichia coli: purification and partial characterization. Infect Immun. 1977 Jan;15(1):272–279. doi: 10.1128/iai.15.1.272-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K. [The mechanism of the interaction of Serratia marcescens pili and human urinary mucin]. Nihon Saikingaku Zasshi. 1984 Mar;39(2):85–102. [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Altmann G., Eshdat Y. Screening of bacterial isolates for mannose-specific lectin activity by agglutination of yeasts. J Clin Microbiol. 1980 Apr;11(4):328–331. doi: 10.1128/jcm.11.4.328-331.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]