Abstract
Purpose
Cardiac dysfunction and hyperdynamic systemic circulation may be present in patients with cirrhosis. The purpose of this study was to identify relations between plasma levels of N-terminal-proBNP (NT-proBNP), reflecting early ventricular dysfunction, and the severity of liver disease and cardiac dysfunction in cirrhotic patients.
Materials and Methods
Sixty-three cirrhotic patients and 15 controls (group 1) were enrolled in this study. Plasma levels of NT-proBNP were determined in echocardiographically examined patients, which were allocated to 1 of 3 groups according to Child-Pugh classification or into 2 groups, i.e., a compensated group without ascites (group 2) and decompensated group with ascites (group 3).
Results
Plasma NT-proBNP levels were significantly higher in cirrhotic patients (groups 2 and 3) than in age-matched controls (155.9 and 198.3 vs. 40.3 pg/mL, respectively, p < 0.05). NT-proBNP levels were significantly increased in Child class C patients than in classes B and A (250.0 vs. 168.6 and 119.6 pg/mL, respectively, p < 0.05). Left atrial dimension, wall thickness of left ventricle, and EF or E/E' were significantly increased, and EDT was prolonged in cirrhotic patients than in controls. Increased LVMI and decreased E/A ratio were noted in the group of patients with ascites as compared with the other groups.
Conclusion
Plasma NT-proBNP levels were high in cirrhotic patients and are likely to be related to the severity of disease. Advanced cirrhosis is associated with advanced cardiac dysfunction, and NT-proBNP levels has predictive value for concomitant cardiac dysfunction and cirrhosis progression.
Keywords: N-terminal-proBNP, cirrhosis, cardiac dysfunction
INTRODUCTION
Systemic circulation in patients with cirrhosis is hyperdynamic and is characterized by an increased heart rate and cardiac output and reduced systemic vascular resistance with normal or decreased arterial blood pressure.1-3 In cirrhotic patients, cardiac output is increased at rest, and it has been assumed that systolic function is normal or even supranormal.4,5 However, many cirrhotic patients present with dyspnea, fluid retention, and limited exercise capacity.6,7 As a solution to these clinical manifestions, the newly introduced clinical entity, formerly known as latent alcoholic cardiomyopathy, cirrhotic cardiomyopathy, reflects a condition with defective myocardial contractility under physical and pharmacological strain.8,9 Although this cardiac dysfunction due to hepatic failure has not yet been finally classified and the mechanisms are not fully understood, early detection of this condition is crucial. Several studies have shown increased plasma levels of brain natriuretic peptide (BNP) and NT-proBNP in some patients with cirrhosis, and these findings may suggest cardiac dysfunction.10-12 NT-proBNP has recently suggested been to be a even better indicator of early cardiac dysfunction than BNP because of its stability and longer biological half-life.13-15 However, until now very few studies have assessed the levels of plasma NT-proBNP in cirrhotic patients, therefore, the exact role of NT-proBNP in the noninvasive diagnosis of cardiac dysfunction in cirrhotic patients remains to be fully clarified. Consequently, this study was undertaken to examine relationships between plasma levels of NT-proBNP and severities of liver disease and cardiac dysfunction, further investigate cardiac structures and functions by echocardiography and assess their correlations with disease severity and cardiac dysfunction in cirrhotic patients.
MATERIALS AND METHODS
Study population
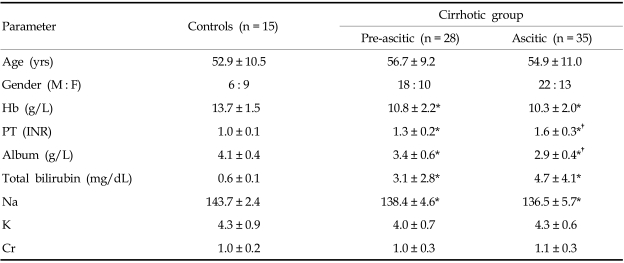
From March 2004 to October 2005, 63 cirrhotic patients were enrolled in this study. They consisted of 40 men and 23 women with a mean age of 55.8 ± 10.1 years (range, 40 - 71 years). Diagnoses were based on established clinical, biochemical, and ultrasonography criteria. The cause of cirrhosis was post-hepatic cirrhosis due to hepatitis B virus infection in 32 patients, hepatitis C virus infection in 6, alcoholic in 19, autoimmune in 2, and cryptogenic in 4. According to Child-Pugh classification, 16 patients were in class A, 29 in class B, and 18 in class C. The age-matched control group (group 1) comprised of 15 healthy adults with a mean age of 52.9 ± 10.5 years (range, 41 - 67 years). We divided the cirrhotic patients into a compensated group without ascites (group 2, pre-ascitic, n = 28) and a decompensated group with ascites (group 3, ascitic, n = 35). Patients in group 3 had received diuretic therapy along with a less strict dietary salt restriction (440 mmol sodium per day) but additional cardiovascular medication, including beta blockers, was not prescribed for any of these patients. Seventeen patients had a hepatic encephalopathy history. Exclusion criteria for study subjects were presence of cardiac, pulmonary, and renal diseases; hypertension; and other major organic diseases. All patients and controls received a normal cardiac physical examination, chest X-ray, and electrocardiography (ECG). The baseline clinical and biochemical characteristics of the patients and controls are summarized in Table 1.
Table 1.
Baseline Clinical and Biochemical Characteristics of the Cirrhotic Patients and Controls
M, male; F, female; Hb, hemoglobin; PT, prothrombin time; Cr, creatinine.
*p < 0.05, compared with control. †p < 0.05, compared with preascitic group.
Data are the number of the patients or mean ± SD.
Study protocol
Blood was drawn from a forearm vein after at least 10 minutes of resting supine, collected in standard sampling tubes for NT-proBNP analysis, and in appropriate tubes for other laboratory determinations. A complete blood count, prothrombin time [PT, as reflected by the international normalized ratio (INR)], and liver and renal function tests were performed using standard laboratory automated techniques. Plasma NT-proBNP levels were determined using a commercially available immunoassay based on the sandwich technique (Elecsys proBNP, Roche Diagnostics); its lower and upper limits of detection were 5 pg and 35,000 pg/mL, respectively. This is a rapid assay with a test time of approximately 18 minutes for a single sample, and test results were calculated automatically. All patients and controls were studied by M-mode, 2-dimensional, and Doppler echocardiography via trans-thoracic approach. Cardiac dimensions and left ventricular hypertrophy were evaluated by measuring echocardiographic parameters, including interventricular septum thickness in diastole (IVSd), left ventricular posterior wall thickness in diastole (LVPWd), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular mass index (LVMI), and left atrial diameter (LAD). Systolic pump function of the left ventricle (LV) was evaluated by ejection fraction (EF). Diastolic function of the left ventricle was evaluated by the peak filling velocity of E (E) and A waves (A) of Doppler mitral valve inflow velocities, E/A ratio, E wave deceleration time (EDT), mitral valve annular velocities (E') by Doppler tissue imaging, and E'/E. In addition, LV-Tei index was used as a parameter of combined systolic and diastolic myocardial performance of the LV.
Statistical analysis
All statistical evaluations were performed using SPSS statistical package (version 12.0, SPSS, Chicago, IL, USA). Results are expressed as means ± SD for raw values. Kruskal-Wallis test was used for assessment of statistical differences in baseline characteristics, biochemical or echocardiographic parameters, and plasma NT-pro BNP levels between groups or Child classes. The p < 0.05 were considered statistically significant.
RESULTS
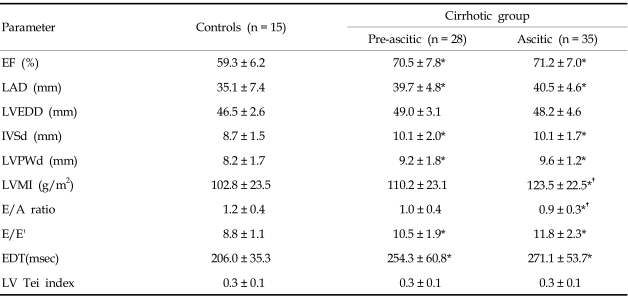
Echocardiographic parameters according to the decompensation component of cirrhosis are given in Table 2. Left atria were significantly enlarged in cirrhotic patients (groups 2 and 3) compared with the group (group 1) of normal controls (39.7 ± 4.8 mm in group 2 and 40.5 ± 4.6 mm in group 3 vs. 35.1 ± 7.4 mm in group 1) and wall thickness of LV was increased in cirrhotic patients compared with controls (10.1 ± 2.0 mm or 9.2 ± 1.8 mm in group 2 and 10.1 ± 1.7 mm or 9.6 ± 1.2 mm in group 3 vs. 8.7 ± 1.5 mm or 8.2 ± 1.7 mm in group 1, IVSd or LVPWd in each group, p < 0.05). EF or E/E' was also increased and EDT was prolonged in patients with cirrhosis vs controls (70.5 ± 7.8 % or 10.5 ± 1.9 or 254.3 ± 60.8 msec in group 2 and 71.2 ± 7.0 % or 11.8 ± 2.3 or 271.1 ± 53.7 msec in group 3 vs. 59.3 ± 6.2 % or 8.8 ± 1.1 or 206.0 ± 35.3 msec in group 1, respectively, p < 0.05). On the other hand, no statistically significant differences were observed for LVEDD and LV-Tei index between groups 1, 2, and 3. Increased LVMI and decreased E/A ratio were noted in the group of patients with ascites compared with the other groups (123.5 ± 22.5 g/m2 or 0.9 ± 0.3 in group 3 vs. 110.2 ± 23.1 g/m2 or 1.0 ± 0.4 in group 2 and 102.8 ± 23.5 g/m2 or 1.2 ± 0.4 in group 1, respectively, p < 0.05). Plasma NT-proBNP levels were significantly higher in cirrhotic patients than in normal controls (155.9 ± 69.8 pg/mL in group 2 and 198.3 ± 146.7 pg/mL in group 3 vs. 40.3 ± 13.6 pg/mL in group 1, respectively, p < 0.05), but no significant NT-proBNP level difference was observed between groups 2 and 3 (p > 0.05; Fig. 1). When patients were grouped according to Child-Pugh classification, plasma NT-proBNP levels were significantly higher in Child class C than in classes B and A, and in controls (250.0 ± 176.6 pg/ mL in Child class C vs. 168.6 ± 71.3 pg/mL in Child class B, 119.6 ± 67.3 pg/mL in Child class A, and 40.3 ± 13.6 pg/mL in controls, respectively, p < 0.05; Fig. 2), and no significant NT-proBNP level difference was observed between Child classes B and A (p > 0.05).
Table 2.
Echocardiographic Parameters in Cirrhotic Patients and Controls
EF, ejection fraction of left ventricle; LAD, left atrium diameter; LVEDD, left ventricular end-diastolic diameter; IVSd, interventricular septum thickness in diastole; LVPWd, left ventricular posterior wall thickness in diastole; LVMI, left ventricular mass index; E/A ratio, ratio of velocity of E wave to velocity of A wave of Doppler mitral valve inflow; EDT, deceleration time of E wave; E', mitral valve annular velocities.
*p < 0.05, compared with control. †p < 0.05, compared with preascitic group.
Fig. 1.
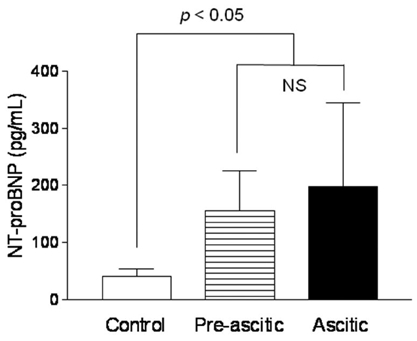
Plasma NT-proBNP levels in controls and cirrhotic patients who were divided into a compensated group without ascites (pre-ascitic) and a decompensated group with ascites (ascitic). NS, no significant differences.
Fig. 2.
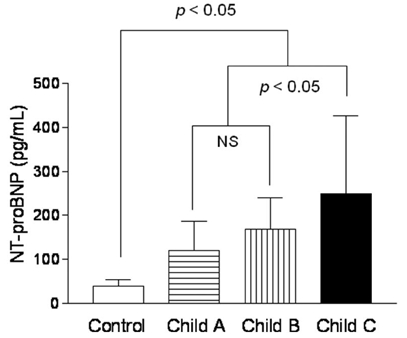
Plasma NT-proBNP levels in controls and cirrhotic patients grouped as class A, B, and C according to child-pugh classification. NS, no significant differences.
DISCUSSION
Cirrhosis is associated with many cardiovascular changes or abnormalities, including hyperdynamic circulation, portal hypertension, hepatopulmonary syndrome, and hepatorenal syndrome. The main change of cardiovascular function in cirrhotic patients, i.e., a hyperdynamic circulation, as manifested by increased cardiac output and decreased arterial pressure, has been recognized for a long time.1-3 Although it tends to be assumed that cardiac contractile function is normal or even supranormal due to the presence of increased cardiac output at rest, many cirrhotic patients present with clinical manifestations that are suggestive of early cardiac dysfunction or overt heart failure.2,5-7 Thus, to consider cardiovascular changes from a functional point of view, the heart in cirrhosis is both hyperdynamic and dysfunctional. For many years, it has been known that the performance of LV is usually reduced by stimuli such as drugs or exercise in alcoholic heart muscle disease or alcoholic cardiomyopathy, which was presented by some studies of cardiac function in patients with alcoholic cirrhosis.16,17 Depressed contractile response to physiologic or pathophysiologic stimuli has recently been referred to as cirrhotic cardiomyopathy, a clinical entity which is clinically and pathophysiologically different from alcoholic heart muscle disease.4,8 On the other hand, cirrhotic cardiomyopathy includes compound changes in increased cardiac output related to normal or increased systolic performance without stimuli at rest, but depressed contractile response to various strain, decreased beta-adrenergic receptor function, postreceptor dysfunction, defective excitation contraction coupling, and conductance abnormalities in some patients.9,19 Therefore, in patients with all forms of cirrhosis, clinically significant cardiac dysfunction and latent heart failure associated with impaired cardiac contractility may be outcome when preload or afterload increased. Since heart failure is characterized by complicated cardiorenal, hemodynamic, and neurohormonal alterations, information on numerous variables is required. For instance, clinical status, exercise capacity, hemodynamic variables, echocardiographic parameters, several markers of neurohumoral activation, and biochemical markers of end-organ dysfunction might be considered to assess and monitor patients with heart failure.20,21 In general, structural or functional changes in the cirrhotic heart are easily evaluated by M-mode, 2-dimensional, and Doppler echocardiographic studies via the trans-thoracic approach.18,22-25 Main structural changes in the cirrhotic heart are observed in the left heart and include dilatation of the left atrium and hypertrophy or dilatation of the left ventricle. In cirrhotic patients, systolic function is normal or even supranormal at rest without a stimulus but cardiac contractile function in response to strains attenuates these functions compared with healthy controls. Diastolic dysfunction in the cirrhotic heart appears to be more prevalent than systolic dysfunction, therefore, echocardiography may reveal abnormal diastolic function under circumstances without strain or even at rest.22-25 In the present study, left atrial enlargement, increased wall thickness and EF of the left ventricle, and E/E' of mitral annulus velocity were noted in cirrhotic patients as compared with controls. In addition, increased LVMI and decreased E/A ratio were noted in the group of ascitic patients as compared with pre-ascitic patients or healthy controls. On the other hand, no statistically significant difference was observed between these three groups in terms of LVEDD or LV-Tei index. These findings are similar to the structural and functional changes reported by previous studies. It is expected that E/E' measurements make up for the weak points of Doppler transmitral inflow measurement, and that E/E' may be used as a reliable marker of diastolic dysfunction in cirrhotic patients. Additionally, more prevalent left ventricular hypertrophy and relaxation abnormality in decompensated patients with more advanced cirrhosis suggest that advanced cirrhosis is associated with more advanced cardiac dysfunction. Meanwhile, the natriuretic peptides have recently been highlighted as major markers for the diagnosis, severity, and prognosis of heart failure, whereas other conventional cardiac function tests are time consuming and often do not correlate well with symptomatic changes in patients' conditions. B-type natriuretic peptide (BNP) is a neurohormone synthesized along with atrial natriuretic peptide (ANP) in cardiac ventricles. BNP is released as preproBNP and then enzymatically cleaved to NT-proBNP and BNP, depending on ventricular myocyte stretching and volume overload.15 In cases of heart failure, ventricular BNP production is markedly elevated, and circulating BNP concentrations are consistently elevated in untreated heart failure.26 Accordingly, blood measurements of BNP and NT-proBNP have been found to be of diagnostic value in congestive heart failure (CHF) related to CHF severity. Moreover, they could be used for adjusting treatment and have been shown to be powerful prognostic markers in both chronic and acute heart failure, independent of standard echocardiographic parameters.15,27-30 However, NT-proBNP is influenced by age and the age-related normal decline in glomerular filtration rate, therefore 2 cutoff points must be used; i.e., NT-proBNP > 125 pg/mL in patients younger than 75 years and > 450 pg/mL in patients older than 75 years.15 Despite a close relationship between age and renal function, NT-proBNP has recently been suggested to be an even better marker of early cardiac dysfunction or heart failure than BNP because it is more stable and less sensitive to rapid fluctuations caused by short-term secretion stimuli due to its appreciably longer biological half-life.13-15 A study by Henriksen et al.12 showed that circulating proBNP concentrations are significantly increased in patients with advanced cirrhosis and that they are closely related to BNP concentrations, however, no signs of reduced hepatic degradation of proBNP or of BNP are present in patients with cirrhosis, suggesting that elevated levels of proBNP and BNP are related to markers of cirrhotic severity and indicate the presence of cardiac dysfunction in advanced cirrhosis. In the present study, plasma NT-proBNP levels were found to be significantly higher in cirrhotic patients than in controls, and also higher in Child class C patients than in classes B and A or controls. Importantly, a significant correlation was observed between NT-proBNP and Child class, suggesting that plasma NT-proBNP levels are likely to be related to the severity of cirrhosis. Ascites is 1 of the parameters of Child-Pugh classification that indicates liver disease severity. Thus, progressive increases in the ascites ratios in cirrhotic patients from Child classes A, B, and C is expected, however, no significant plasma NT-proBNP level differences were observed between pre-ascitic and ascitic patients in the present study. This result represents 1 of the limitations of this study, which may be attributable to diuretic therapy before blood sampling in cirrhotic patients with ascites. De Lemos et al.30 have advocated that the net effect of beta-blockers and diuretics is often to reduce BNP concentration. Therefore, further experimental and clinical research is needed to assess the significance of nonspecific and specific therapies influencing preload and afterload, including drug therapy with diuretics, beta-blockers, and angiotensin inhibitors. Another limitation of this study is the relatively small study population and short follow-up period. The inclusion of a larger population and long-term follow-up may be necessary. In conclusion, the present study shows that plasma NT-proBNP levels, LAD and LVMI, E/A ratio, EDT, and E/E' may be reliable indicators of the extent of cardiac abnormalities in cirrhotic patients. After all, increased levels of NT-proBNP have significant clinical implications in patients with all forms of cirrhosis because it was found to be related to disease severity. Thus, it may be suggested that cardiac dysfunction is related to an increased plasma NT-proBNP levels, which in turn is more prominent in severe disease, and that NT-proBNP has predictive value in cases of concomitant cardiac dysfunction and cirrhotic progression.
ACKNOWLEDGMENTS
We'd like to express sincere thanks to our colleagues, Dr. Soochan Bae (Cardiovascular Division, Beth Israel Deaconess Medical Center, Harvard University) for help with preparing manuscripts and Dr. Sora Choi (Department of Occupational & Environmental Medicine, Chosun University College of Medicine) for help with statistics.
Footnotes
This study was supported by a research fund from Chosun University in 2005.
References
- 1.Llach J, Ginès P, Arroyo V, Rimola A, Tito L, Badalamenti S, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94:482–487. doi: 10.1016/0016-5085(88)90441-6. [DOI] [PubMed] [Google Scholar]
- 2.Møller S, Henriksen JH. Circulatory abnormalities in cirrhosis with focus on neurohumoral aspects. Semin Nephrol. 1997;17:505–519. [PubMed] [Google Scholar]
- 3.Naschitz JE, Slobodin G, Lewis RJ, Zuckerman E, Yeshurun D. Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J. 2000;140:111–120. doi: 10.1067/mhj.2000.107177. [DOI] [PubMed] [Google Scholar]
- 4.Lee SS. Cardiac abnormalities in liver cirrhosis. West J Med. 1989;151:530–535. [PMC free article] [PubMed] [Google Scholar]
- 5.Baik SK, Lee SS. Cirrhotic cardiomyopathy: causes and consequences. J Gastroenterol Hepatol Suppl. 2004;7:S185–S190. [Google Scholar]
- 6.Epstein SK, Ciubotaru RL, Zilberberg MD, Kaplan LM, Jacoby C, Freeman R, et al. Analysis of impaired exercise capacity in patients with cirrhosis. Dig Dis Sci. 1998;43:1701–1707. doi: 10.1023/a:1018867232562. [DOI] [PubMed] [Google Scholar]
- 7.Fallon MB, Abrams GA. Pulmonary dysfunction in chronic liver disease. Hepatology. 2000;32:859–865. doi: 10.1053/jhep.2000.7519. [DOI] [PubMed] [Google Scholar]
- 8.Ma Z, Lee SS. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology. 1996;24:451–459. doi: 10.1002/hep.510240226. [DOI] [PubMed] [Google Scholar]
- 9.Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9–15. doi: 10.1136/heart.87.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Villa G, Romanelli RG, Casini Raggi V, Tosti Guerra C, De Feo ML, Marra F, et al. Plasma levels of brain natriuretic peptide in patients with cirrhosis. Hepatology. 1992;16:156–161. doi: 10.1002/hep.1840160126. [DOI] [PubMed] [Google Scholar]
- 11.Wong F, Siu S, Liu P, Blendis LM. Brain natriuretic peptide: is it a predictor of cardiomyopathy in cirrhosis? Clin Sci (Lond) 2001;101:621–628. [PubMed] [Google Scholar]
- 12.Henriksen JH, Gøtze JP, Fuglsang S, Christensen E, Bendtsen F, Møller S. Increased circulating pro-brain natriuretic peptide (proBNP) and brain natriuretic peptide (BNP) in patients with cirrhosis: relation to cardiovascular dysfunction and severity of disease. Gut. 2003;52:1511–1517. doi: 10.1136/gut.52.10.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campell DJ, Mitchelhill KI, Schlicht SM, Booth RJ. Plasma amino-terminal pro-brain natriuretic peptide: a novel approach to the diagnosis of cardiac dysfunction. J Card Fail. 2000;6:130–139. [PubMed] [Google Scholar]
- 14.Gøtze JP, Kastrup J. Plasma pro-brain natriuretic peptides are strong biochemical markers in clinical cardiology. Scand J Clin Lab Invest Suppl. 2001;234:47–51. [PubMed] [Google Scholar]
- 15.McCullough PA, Omland T, Maisel AS. B-type natriuretic peptides: A diagnostic breakthrough for clinicians. Rev Cardiovasc Med. 2003;4:72–80. [PubMed] [Google Scholar]
- 16.Gould L, Zahir M, Shariff M, DiLieto M. Cardiac hemodynamics in alcoholic patients with chronic liver disease and a presystolic gallop. J Clin Invest. 1969;48:860–868. doi: 10.1172/JCI106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limas CJ, Guiha NH, Lekagul O, Cohn JN. Impaired left ventricular function in alcoholic cirrhosis with ascites. Ineffectiveness of ouabain. Circulation. 1974;49:754–760. doi: 10.1161/01.cir.49.4.755. [DOI] [PubMed] [Google Scholar]
- 18.Hu W, Sun X, He J, Ma Y, Wang L. The study by echocardiography in 44 patients with cirrhotic cardiomyopathy. J Gastroenterol Hepatol Suppl. 2004;5:A653. [Google Scholar]
- 19.Bernardi M, Calandra S, Colantoni A, Trevisani F, Raimondo ML, Sica G, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27:28–34. doi: 10.1002/hep.510270106. [DOI] [PubMed] [Google Scholar]
- 20.Mann DL. Mechanisms and models of heart failure: a combinational approach. Circulation. 1999;100:999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 21.Mair J. Monitoring of patients with heart failure. Scand J Clin Lab Invest Suppl. 2005;240:99–106. doi: 10.1080/00365510500236234. [DOI] [PubMed] [Google Scholar]
- 22.Wong F, Liu P, Lilly L, Bomzon A, Blendis L. Role of cardiac structural and functional abnormalities in the pathogenesis of hyperdynamic circulation and renal sodium retention in cirrhosis. Clin Sci (Lond) 1999;97:259–267. [PubMed] [Google Scholar]
- 23.Finucci G, Desideri A, Sacerdoti D, Bolognesi M, Merkel C, Angeli P, et al. Left ventricular diastolic function in liver cirrhosis. Scand J Gastroenterol. 1996;31:279–284. doi: 10.3109/00365529609004879. [DOI] [PubMed] [Google Scholar]
- 24.Gaskari SA, Honar H, Lee SS. Therapy insight: cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol. 2006;3:329–337. doi: 10.1038/ncpgasthep0498. [DOI] [PubMed] [Google Scholar]
- 25.Alexander J, Mishra P, Desai N, Ambadekar S, Gala B, Sawant P. Cirrhotic cardiomyopathy: Indian scenario. J Gastroenterol Hepatol. 2007;22:395–399. doi: 10.1111/j.1440-1746.2006.04507.x. [DOI] [PubMed] [Google Scholar]
- 26.Mair J, Hammerer-Lercher A, Puschendorf B. The impact of cardiac natriuretic peptide determination on the diagnosis and management of heart failure. Clin Chem Lab Med. 2001;39:571–588. doi: 10.1515/CCLM.2001.093. [DOI] [PubMed] [Google Scholar]
- 27.Packer M. Should B-type natriuretic peptide be measured routinely to guide the diagnosis and management of chronic heart failure? Circulation. 2003;108:2950–2953. doi: 10.1161/01.CIR.0000109205.35813.8E. [DOI] [PubMed] [Google Scholar]
- 28.Yu CM, Sanderson JE. Plasma brain natriuretic peptide - an independent predictor of cardiovascular mortality in acute heart failure. Eur J Heart Fail. 1999;1:59–65. doi: 10.1016/S1388-9842(98)00010-5. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, et al. NT-proBNP in severe chronic heart failure: rationale, design, and preliminary results of the COPERNICUS NT-proBNP substudy. Eur J Heart Fail. 2004;6:343–350. doi: 10.1016/j.ejheart.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 30.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–322. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]