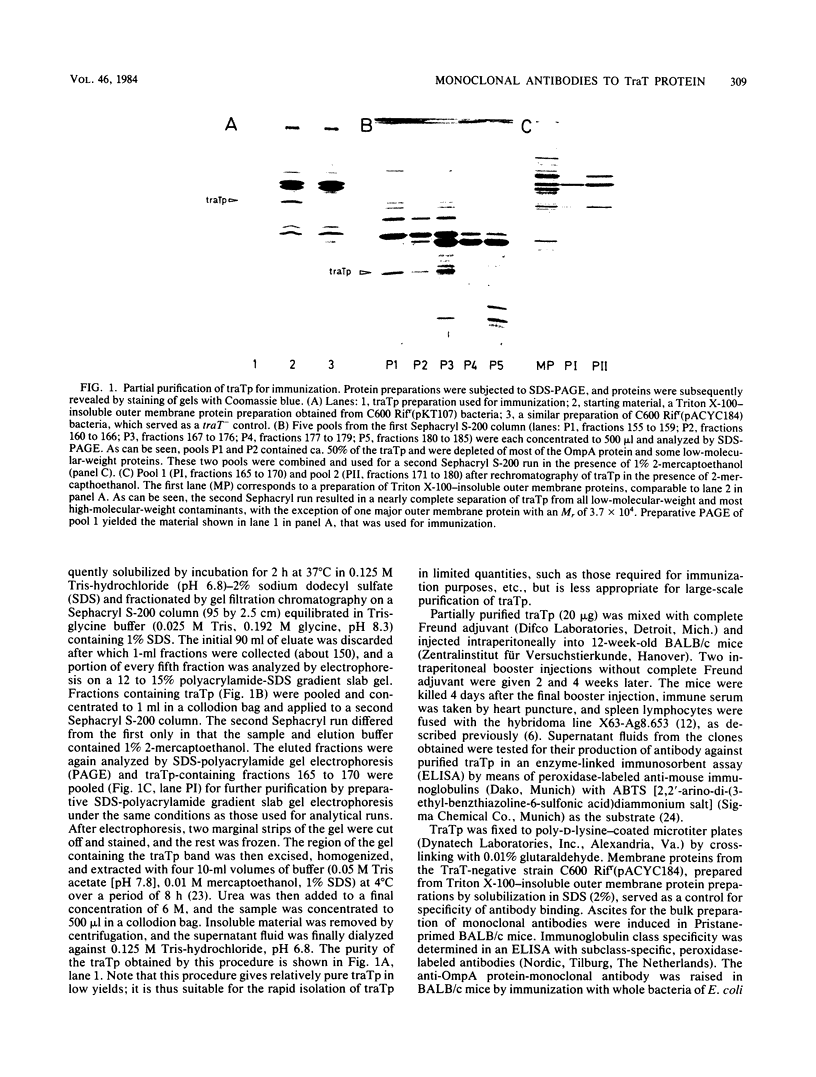
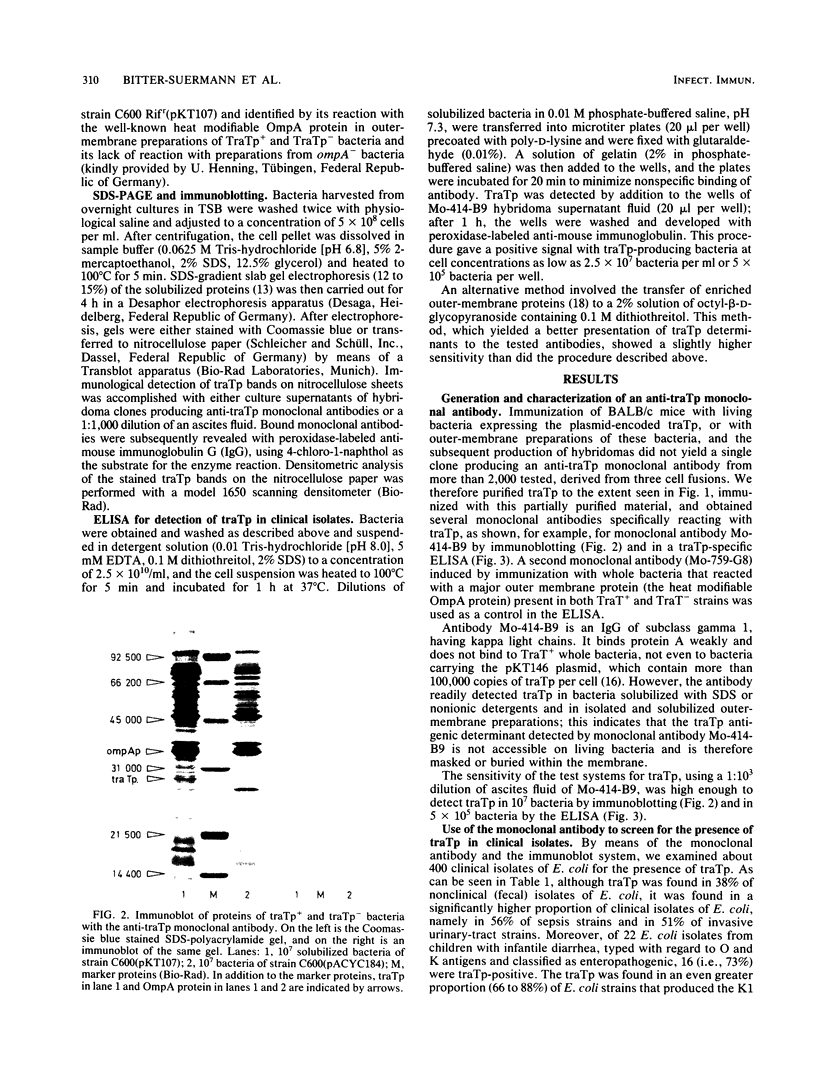
Abstract
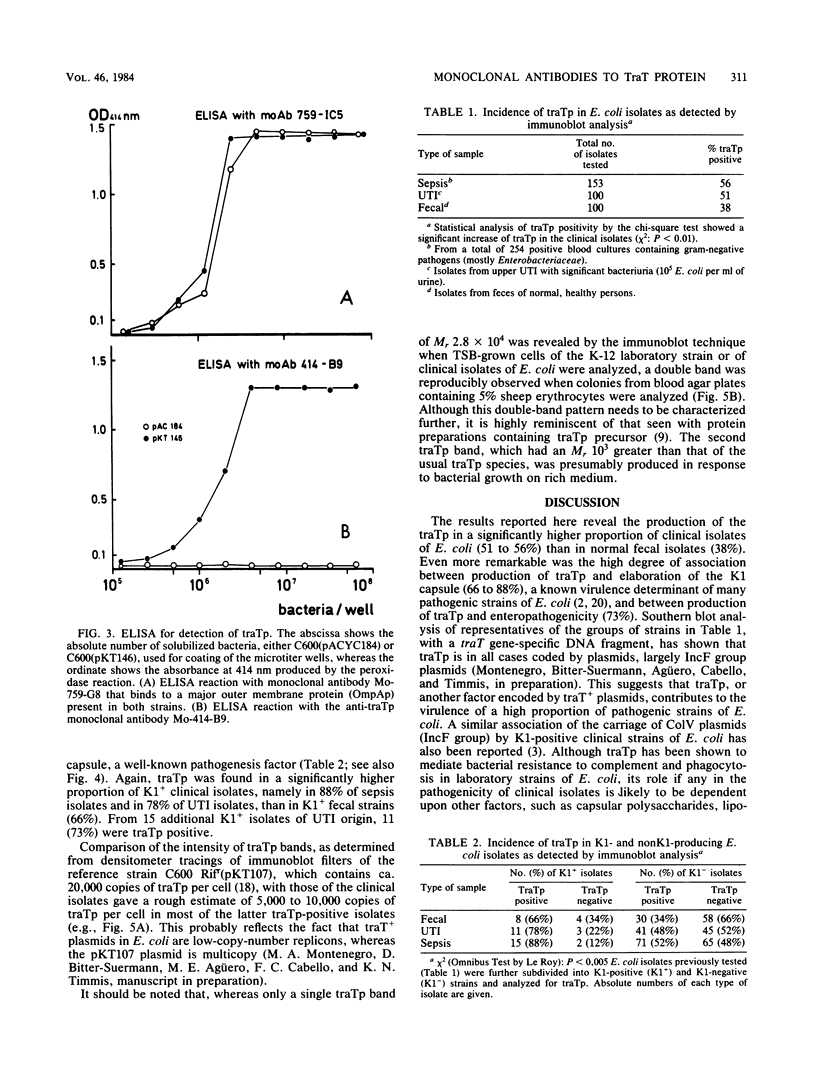
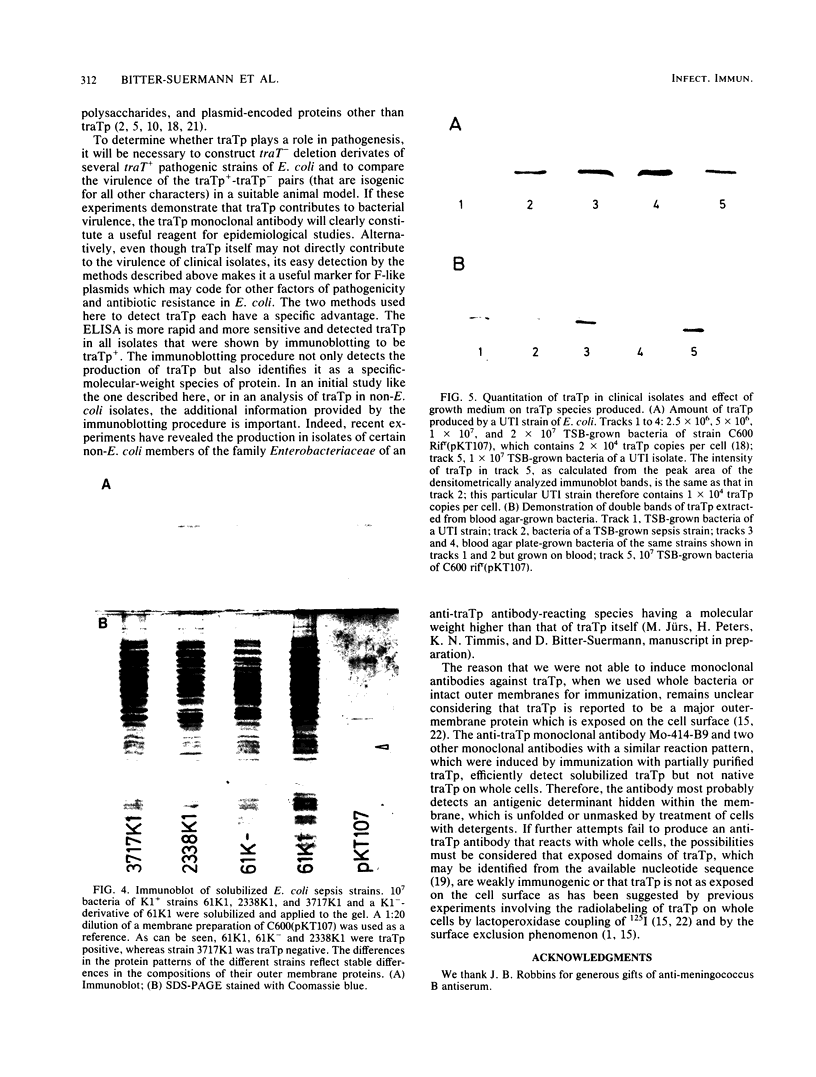
The TraT protein specified by IncF group plasmids mediates surface exclusion and bacterial resistance to the lethal activities of serum. In this study, an anti-TraT protein monoclonal antibody was generated which failed to react with TraT+ bacteria but which efficiently detected solubilized TraT protein in Western blots and in an enzyme-linked immunosorbent assay. Use of this antibody to screen clinical and nonclinical isolates of Escherichia coli for the production of TraT protein revealed its presence in a modest proportion (38%) of normal fecal strains, a significantly higher proportion of clinical strains (51 to 73%), and an even higher proportion (78 to 88%) of clinical strains concomitantly producing the K1 capsule, an important virulence factor of E. coli.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Kennedy N., Skurray R. Cell--cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agüero M. E., Cabello F. C. Relative contribution of ColV plasmid and K1 antigen to the pathogenicity of Escherichia coli. Infect Immun. 1983 Apr;40(1):359–368. doi: 10.1128/iai.40.1.359-368.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agüero M. E., Harrison H., Cabello F. C. Increased frequency of ColV plasmids and mannose-resistant hemagglutinating activity in an Escherichia coli K1 population. J Clin Microbiol. 1983 Dec;18(6):1413–1416. doi: 10.1128/jcm.18.6.1413-1416.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Binns M. M., Mayden J., Levine R. P. Further characterization of complement resistance conferred on Escherichia coli by the plasmid genes traT of R100 and iss of ColV,I-K94. Infect Immun. 1982 Feb;35(2):654–659. doi: 10.1128/iai.35.2.654-659.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R., Deubel U., Hadding U., Bitter-Suermann D. Identification of functionally relevant determinants on the complement component C3 with monoclonal antibodies. J Immunol. 1982 Nov;129(5):2042–2050. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazza D., Levy S. B. Biochemical and immunological characterization of an R plasmid-encoded protein with properties resembling those of major cellular outer membrane proteins. J Bacteriol. 1980 Oct;144(1):149–158. doi: 10.1128/jb.144.1.149-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazza D., Levy S. B. Biosynthesis of a plasmid-encoded outer membrane surface exclusion protein involves processing from a precursor polypeptide. J Biol Chem. 1980 Oct 10;255(19):8955–8958. [PubMed] [Google Scholar]
- Gross R. J., Cheasty T., Rowe B. Isolation of bacteriophages specific for the K1 polysaccharide antigen of Escherichia coli. J Clin Microbiol. 1977 Dec;6(6):548–550. doi: 10.1128/jcm.6.6.548-550.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Beutin L., Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol. 1980 Apr;142(1):285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Timmis J. K., Moll A., Timmis K. N. Mutants that overproduce TraTp, a plasmid-specified major outer membrane protein of Escherichia coli. Mol Gen Genet. 1982;187(3):426–431. doi: 10.1007/BF00332623. [DOI] [PubMed] [Google Scholar]
- Moll A., Manning P. A., Timmis K. N. Plasmid-determined resistance to serum bactericidal activity: a major outer membrane protein, the traT gene product, is responsible for plasmid-specified serum resistance in Escherichia coli. Infect Immun. 1980 May;28(2):359–367. doi: 10.1128/iai.28.2.359-367.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Winters C., Levine R. P. Nucleotide sequence analysis of the complement resistance gene from plasmid R100. J Bacteriol. 1982 Aug;151(2):819–827. doi: 10.1128/jb.151.2.819-827.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Robinson M. K. Determinants that increase the serum resistance of Escherichia coli. Infect Immun. 1980 Jul;29(1):278–280. doi: 10.1128/iai.29.1.278-280.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]
- Zimmer B., Hartung H. P., Scharfenberger G., Bitter-Suermann D., Hadding U. Quantitative studies of the secretion of complement component C3 by resident, elicited and activated macrophages. Comparison with C2, C4 and lysosomal enzyme release. Eur J Immunol. 1982 May;12(5):426–430. doi: 10.1002/eji.1830120513. [DOI] [PubMed] [Google Scholar]