Abstract
We describe a case of septic shock due to Vibrio alginolyticus presenting with fever and bilateral leg pain. Despite intensive management with antibiotics and inotropic agents, the patient died from septic shock 1 day after hospitalization. V. alginolyticus was isolated from both leg wounds and a blood culture. To the best of our knowledge, this is the first reported case of V. alginolyticus bacteremia in Korea.
Keywords: Vibrio alginolyticus, bacteremia, septic shock, Korea
INTRODUCTION
Vibrios have been recognized as human pathogens, and they can be acquired through either ingestion of contaminated seafood or contact of traumatized skin with seawater or brackish water. In addition to V. cholerae, three additional major groups of vibrios have been associated with human disease. These include the halophilic V. parahemolyticus, V. vulnificus, and other halophilic vibrios, including V. alginolyticus, V. fluvialis, V. hollisae, V. damsela, V. furnissii, V. metschnikovii, and V. cincinnatiensis. Among them, the epidemiologic and clinical features of V. vulnificus, which is a common cause of invasive human diseases, are well delineated.1
V. alginolyticus has been etiologically associated with superficial wound infections mainly in the skin and ear, which have a benign course, in striking contrast with the other pathogenic vibrios such as V. vulnificus. V. alginolyticus occasionally causes life-threatening infections in immunocompromised individuals.
Here, we describe a septic shock case caused by V. alginolyticus in a cirrhotic patient after eating raw fish. To the best of our knowledge, this is the first reported case of V. alginolyticus bacteremia in Korea.
CASE REPORT
A 59-year-old man was admitted to Kyung Hee University Medical with fever, shaking chills, and bilateral leg pain. He felt general malaise and high fever about two days before admission, and he had eaten a bowl of rice capped with raw fish one week before admission. Except for hepatitis B, his medical and family history were unremarkable. He had never been evaluated for Hepatitis B. He did not consume alcohol nor did he smoke.
On arrival, his temperature was 36.9℃, heart rate was 108 beats/min, respiratory rate was 20 breaths/min, and blood pressure was 60/30 mmHg. Examination of the patient's legs revealed erythema on both posterior calves. Severe pain was present on the erythematous lesions, but no obvious external wound was present. The rest of the physical examination was unremarkable except for bilateral crackles with inspiration over the lower chest. The laboratory data on admission were as follows: peripheral white blood cell count 1,240/mm3, with 82% segmented neutrophils, hemoglobin 12.5 mg/dL, platelet cell count 38,000/mm3, C-reactive protein 2.2 mg/dL, prothrombin time 2.33 INR, serum albumin 2.0 g/dL, total bilirubin 2.81 mg/dL, aspartate aminotransferase 82 IU/L, alanine aminotransferase 28 IU/L, blood urea nitrogen 5.6 mg/dL, creatinine 2.0 mg/dL, creatinine kinase 992 U/L, myoglobin 2,678 ng/mL, lactic dehydrogenase 599 IU/L, arterial pH 7.30, PCO2 29.8 mmHg, PO2 98.3 mmHg, and bicarbonate 14.4 mmol/L on O2 2 L/min via nasal prong. The chest radiography taken on admission revealed pulmonary congestion on both lung fields, and coarse liver parenchyma was observed on abdominal ultrasonography.
V. vulnificus had been suspected in this cirrhotic patient who developed a septicemic illness associated with necrotizing cutaneous lesions after ingesting seafood. Empirical antibiotic therapy with vancomycin, ciprofloxacin, and doxycycline was started to treat V. vulnificus and community acquired soft tissue infections. Massive fluid and intravenous bicarbonate was administered to treat septic shock. The erythema had gradually enlarged in the first 20 hours after admission, and tense hemorrhagic bullae were also present on the erythema (Fig. 1). As time progressed, he complained of severe pain in both legs, and his leg showed marked edema with shallow pulses and physical findings of tender flesh. Both legs showed signs consistent with necrotizing fasciitis. Surgical exploration was not done because of the uncorrected septic shock and refusal by the patient and his family members. Twenty-four hours after hospitalization, the patient died from uncontrolled septic shock.
Fig. 1.
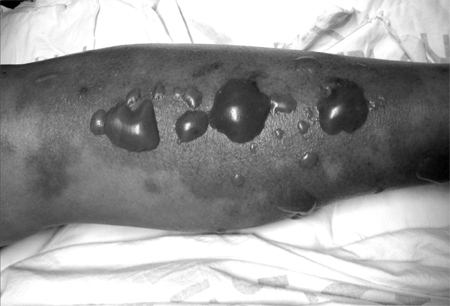
Various sized, scattered, hemorrhagic tense bullae on ill-defined purpuric erythematous patches formed on both lower legs 20 hours after admission.
V. alginolyticus was isolated from the fluid culture of the bullae and three sets of blood cultures obtained on admission. After 48 hours of incubating the culture-positive samples, straight or curved, Gram-negative, oxidase-positive colonies were seen on blood agar, MacConkey agar, and thiglycollate medium. The colonies were smooth, convex, creamy in consistency, and gray-white with full margins. The bacteria were initially identified as V. alginolyticus by the API 20NE system (version 7.0 La Balme-Les Grotte, France) code number 6466644 at a 92.3% confidence level, but were unidentified by the Vitek system (Biomeriux, Hazelwood, MO, USA). After 24 hours of incubation on thiosulfate-citrate-bile salts-sucrose (TCBS) agar, yellow colonies were seen. Further identification was confirmed with the following biochemical tests. The colonies showed a positive reaction to indol production, methyl red, Voges-Proskauer, lysine decarboxylase, and ornithine decarboxylase. Negative findings included: growth on Simmons' citrate, H2S on TSI, gas production, urea hydrolysis, and arginine dihydrolysis. Sensitivity of the bacteria was determined by a Kirby-Bauer disk diffusion method, and the results were interpreted by the CLSI recommendations. The organism was susceptible to a variety of antibiotics including ampicillin, amoxillin-clavulanate, cephalothin, cefuroxime, gentamicin, ciprofloxacin, and trimethoprim-sulfamethoxazole.
DISCUSSION
V. alginolyticus is a curved, gram-negative, oxidase-positive bacillus organism. The organism derived its name from its ability to utilize alginate. This species is the most salt-tolerant among the pathogenic Vibrio species and can even grow in extremely high salt concentrations (as high as 10%).1 This organism lives mainly in the coastal waters of temperate and tropical regions.2-4 Its virulence is related to its ability to produce hemolysis, hemagglutination, and protease.5 It forms large, yellow (sucrose fermenting) colonies on TCBS medium but it also forms pronounced swarming on non-selective solid media. The differential reactions on TCBS agar are helpful in making a presumptive identification of V. cholerae, V. alginolyticus, V. parahemolyticus, and V. vulnificus. After 18 to 24 hours of incubation on TCBS agar, V. alginolyticus which can also ferment sucrose, will produce yellow colonies; V. parahemolyticus and V. vulnificus, which do not utilize sucrose, produce blue-green colonies.
V. alginolyticus is considered relatively nonpathogenic in humans, unlike the notoriously virulent V. vulnificus. The reason for V. alginolyticus's lack of virulence remains unclear. V. alginolyticus has been etiologically associated with cellulitis and acute otitis media or externa.3,6-10 As a whole, these infections have responded well to appropriate antibiotics. Seven Korean cases of V. alginolyticus infection have previously been reported. Four cases were related with otitis media11,12 or myringitis,13 two with soft tissue infection,14,15 and the last one was related with gastroenteritis.16
It has been rarely reported that V. alginolyticus causes deep-seated or invasive infections.7 Only 10 cases of invasive V. alginolyticus infection, as defined as bloodstream infection or deep-seated or necrotizing soft tissue infection, have been reported in the English literature since 1976. The route for these infections is direct contact with contaminated sea-water or ingestion of raw seafood, which is the same as that of other vibrios or superficial soft tissue infections. Predisposing underlying conditions are immunocompromised states like cirrhosis, burns, cancer, post-splenectomy, and steroid therapy.7,9 However necrotizing fasciitis has also been reported to develop following a soft tissue injury from a coral reef to the leg of an immune-competent individual.17 Compared with V. vulnificus sepsis, V. alginolyticus sepsis is similar in underlying disease and its disease course, but is different with respect to its lower pathogenicity. The relative mortality of V. alginolyticus sepsis is also lower than that of V. vulnificus.
Vibrio species are usually resistant to penicillins and vancomycin, but are susceptible to tetracycline, chloramphenicol, aminoglycosides, and other β-lactam antibiotics, in particular, V. paraheamolyticus and V. alginolyticus, which may have β-lactamase activity.8 In vitro, V. alginolyticus isolates are usually sensitive to most antibiotics including trimethoprim-sulfamethoxazole, tetracycline, chloramphenicol, gentamicin, quinolones, and the third generation cephalosporins.10
Soft tissue infections caused by vibrios generally respond well to the appropriate antibiotics and surgical drainage. Early administration of antibiotics is critical because the cellulitis can spread very rapidly. Bactremic infections in compromised hosts respond less well to therapy. The reported mortality rate was lowest for bacteremic patients begun on antibiotics within 24 hours of the onset of illness but was still unacceptably high at 33%.1 In this case, we could not perform surgical exploration due to the refusal of the patient and his family members. If he had undergone surgical intervention, he might have lived longer.
In summary, we present a case of septic shock due to V. alginolyticus in a patient in whom the clinical presentation did not suggest the presence of this organism. He was treated with the appropriate antibiotics, but his late visit to the hospital and failure to achieve surgical debridement may have caused his death. Immunocompromised hosts should be careful about eating raw fish, especially during the warm seasons. At present, thorough cooking of seafood is the only effective means of prevention. Early administration of antibiotics and surgical intervention, if needed, is critical for controlling these invasive vibrios infections.
References
- 1.Neill MA, Carpenter CJ. Other pathogenic vibrios. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett's: principles and practice of infectious diseases. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2005. pp. 2544–2548. [Google Scholar]
- 2.Chakraborty S, Nair GB, Shinoda S. Pathogenic vibrios in the natural aquatic environment. Rev Environ Health. 1997;12:63–80. doi: 10.1515/reveh.1997.12.2.63. [DOI] [PubMed] [Google Scholar]
- 3.Patterson TF, Bell SR, Bia FJ. Vibrio alginolyticus cellulitis following coral injury. Yale J Biol Med. 1988;61:507–512. [PMC free article] [PubMed] [Google Scholar]
- 4.Maugeri TL, Caccamo D, Gugliandolo C. Potentially pathogenic vibrios in brackish waters and mussels. J Appl Microbiol. 2000;89:261–266. doi: 10.1046/j.1365-2672.2000.01096.x. [DOI] [PubMed] [Google Scholar]
- 5.Zanetti S, Deriu A, Volterra L, Falci MP, Molicotti P, Fadda G, et al. Virulence factors in Vibrio alginolyticus strains isolated from aquatic environments. Ann Ig. 2000;12:487–491. [PubMed] [Google Scholar]
- 6.Lessner AM, Webb RM, Rabin B. Vibrio alginolyticus conjunctivitis. First reported case. Arch Ophthalmol. 1985;103:229–230. doi: 10.1001/archopht.1985.01050020081026. [DOI] [PubMed] [Google Scholar]
- 7.Ho PL, Tang WM, Lo KS, Yuen KY. Necrotizing fasciitis due to Vibrio alginolyticus following an injury inflicted by a stingray. Scand J Infect Dis. 1998;30:192–193. doi: 10.1080/003655498750003636. [DOI] [PubMed] [Google Scholar]
- 8.Joseph SW, DeBell RM, Brown WP. In vitro response to chloramphenicol, tetracycline, ampicillin, gentamicin, and beta-lactamase production by halophilic Vibrios from human and environmental sources. Antimicrob Agents Chemother. 1978;13:244–248. doi: 10.1128/aac.13.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard RJ, Pessa ME, Brennaman BH, Ramphal R. Necrotizing soft-tissue infections caused by marine vibrios. Surgery. 1985;98:126–130. [PubMed] [Google Scholar]
- 10.Mukherji A, Schroeder S, Deyling C, Procop GW. An unusual source of Vibrio alginolyticus-associated otitis: prolonged colonization or freshwater exposure? Arch Otolaryngol Head Neck Surg. 2000;126:790–791. doi: 10.1001/archotol.126.6.790. [DOI] [PubMed] [Google Scholar]
- 11.Doh YJ, Kim MH, Kim ES. A case of Vibrio alginolyticus isolated from otorrhea of chronic otitis media. Korean J Infect Dis. 1997;29:153–157. [Google Scholar]
- 12.Nam R, Kang MS, Han JY, Lee EY, Kim SH. Two cases of Vibrio alginolyticus isolated from chronic otitis media. Korean J Clin Pathol. 1989;9:453–458. [Google Scholar]
- 13.Lee HJ, Choi JW. A case of Vibrio alginolyticus myringitis. Korean J Clin Pathol. 1995;15:101–105. [Google Scholar]
- 14.Cho SR, Lee KW, Chong YS, Kwon OH, Jahng JS. Isolation of Vibrio alginolyticus from an infected wound of a foot. Korean J Clin Pathol. 1995;15:281–285. [Google Scholar]
- 15.Kang MS, Yoon HS, Chong YS, Lee SY, Hahn SB. Isolation of Vibrio alginolyticus from a infected wound of a hand. J Clin Pathol Qual Control. 1986;8:175–180. [Google Scholar]
- 16.Kim JS, Park SY, Kil YC, Lee HJ, Suh JT. A case of simultaneous isolation of Vibrio parahemolyticus and Vibrio alginolyticus. Korean J Clin Microbiol. 2000;3:147–152. [Google Scholar]
- 17.Gomez JM, Fajardo R, Patiño JF, Arias CA. Necrotizing fasciitis due to Vibrio alginolyticus in an immunocompetent patient. J Clin Microbiol. 2003;41:3427–3429. doi: 10.1128/JCM.41.7.3427-3429.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


