Abstract
Purpose
In this randomized, double-blind study, we investigated the analgesic efficacy and side effects of continuous constant-dose infusions of remifentanil after total abdominal hysterectomy and compared it to fentanyl.
Materials and Methods
Fifty-six adult female patients scheduled for elective total abdominal hysterectomy were enrolled in this study. Patients were randomly assigned to two groups according to fentanyl (group F, n = 28) or remifentanil (group R, n = 28) for postoperative analgesia. Patients in group F were given fentanyl intravenously with an infusion rate of fentanyl 0.5 µg/kg/hr; group R was given remifentanil with an infusion rate of remifentanil 0.05 µg/kg/min for 2 days. Pain intensity at rest, occurrence of postoperative nausea and vomiting (PONV), dizziness, pruritus, and respiratory depression were assessed 1 hr after arrival at the post-anesthesia care unit, at 6; 12; 24; and 48 hr postoperation and 6 hr post-infusion of the study drug. Pain was evaluated by using visual analogue scale (VAS; 0 - 10). The time that patients first requested analgesics was recorded as well as additional analgesics and antiemetics.
Results
There were no significant differences in VAS, time to first postoperative analgesics, and additional analgesics between the 2 groups. The incidences and severities of PONV and opioid related side effects were not different between the groups; however, there were 3 episodes (10.7%) of serious respiratory depression in group R.
Conclusion
Continuous infusion technique of remifentanil did not reveal any benefits compared to fentanyl. Furthermore, it is not safe for postoperative analgesia in the general ward.
Keywords: Postoperative nausea and vomiting, postoperative pain control, remifentanil, respiratory depression
INTRODUCTION
Postoperative pain is one of the most important problems that confront surgical patients because it affects the cardiovascular, respiratory, and endocrine systems. Proper perioperative analgesia management may reduce the occurrence of serious postoperative complications.1
Continuous infusion of analgesics has been a method used to manage postoperative pain. This technique minimizes the fluctuation of analgesic medication in the blood, which provides more efficient pain management than the intermittent injection of analgesics.
Remifentanil, µ agonist opioid, has a very fast onset and an ultra-short duration of action because of rapid hydrolysis of the methyl ester linkage by nonspecific blood and tissue esterases.2-4 The time required for a 50% reduction in blood concentration after the discontinuation of an infusion that attains a steady state (context-sensitive half-time) is about 3 minutes, and it does not increase with the duration of infusion. These unique pharmacokinetic properties of remifentanil should confer ease of titration to changing intraoperative conditions and make it an ideal agent for postoperative analgesia. A previous study observed that remifentanil with an infusion rate of 0.05 - 0.15 µg/kg/min provides good postoperative analgesia for major surgical procedures,5 and a recent study showed that remifentanil reduces the incidence of side effects, especially postoperative nausea and vomiting (PONV), compared to fentanyl.6
However, intravenous administration of remifentanil has the potential to induce opioid-induced side effects, such as respiratory depression, and there are few studies that investigate the efficacy and side effects of remifentanil for postoperative pain control after abdominal surgery.
This randomized, double-blinded study was conducted for 2 purposes; to compare the analgesic efficacy of continuous constant-dose infusions of remifentanil after total abdominal hysterectomy (TAH) compared to fentanyl and assess the side effects of opioids infusions, especially in PONV.
MATERIALS AND METHODS
The Consolidated Standards of Reporting Trials (CONSORT) recommendations for reporting randomized, controlled clinical trials were followed. After obtaining approval of the Institutional Review Board from Yonsei University Medical Center and written informed consent from patients, 56 ASA physical status I adult female patients scheduled for elective TAH were enrolled in this study. Patients under 18 or over 65 years of age; with neurological, psychiatric, endocrinologic, renal or hepatic disorders; allergy to opioids; or taking analgesic, antiemetic or psychoactive drugs were excluded. The principles of pain control device and visual analogue scale (VAS) for pain assessment were explained to patients on the day of the pre-anesthetic visit.
Premedication was not prescribed. All patients were monitored by electrocardiography, noninvasive blood pressure, and pulse oximetry when they arrived at the operating theater. The anesthetic techniques were standardized. Anesthesia was induced with 1.5 mg/kg of propofol and 1 µg/kg of remifentanil. For neuromuscular blockade, 0.6 mg/kg of rocuronium was given by IV. After tracheal intubation, the lungs were ventilated with 50% air in oxygen, and anesthesia was maintained with 1 - 2% sevoflurane and 0.1 µg/kg/min of remifentanil. No other sedative, analgesic, or antiemetic drug was administered.
Patients were randomly allocated to 2 groups to receive either fentanyl (group F, n = 28) or remifentanil (group R, n = 28) for their postoperative analgesia. Randomization was achieved by using sequentially numbered, opaque-sealed envelopes containing computer-generated random allocations in a ratio of 1 : 1 in balanced blocks of 8. Patients in group F were given fentanyl with an infusion rate of 0.5 µg/kg/hr IV and group R patients were given remifentanil with an infusion rate of 0.05 µg/kg/min IV for 2 days, which is minimum amount of known efficacy of remifentanil in major surgical procedures.5 The study solution was prepared by an anesthesiologist who was not involved in the trial. Approximately 30 min before the anticipated end of surgery, an infusion pump (Infusor SV2, Boxter Health care Co., Deerfield, IL, USA) was attached to a continuously infusing intravenous catheter. At the end of surgery, neuromuscular block was reversed with 0.03 mg/kg of neostigmine and 0.004 mg/kg of glycopyrrolate by IV. After adequate spontaneous respiration, the trachea was extubated.
The anesthesiologist, who did not know the study protocol, assessed patients in the post-anesthesia care unit (PACU) and subsequently in the general ward. Each patient was assessed 1 hr after arrival at PACU, at 6; 12; 24; and 48 hr post-operation and 6 hr post-infusion of the study drug by an investigator blinded to the patient group. Respiratory depression was assessed in postoperative periods. A respiratory rate ≤ 10 was defined as respiratory depression. Pain intensity at rest, occurrences and severities of PONV, dizziness, and pruritus were also assessed (0 = none, 1 = mild, 2 = moderate and required treatment, 3 = severe and refractory to the treatment). Pain was evaluated by using VAS at resting state with 10 meaning maximum pain and 0 meaning no pain. Rescue analgesia was provided with 30 mg of ketorolac by IV when pain intensity on VAS was ≥ 5, the daily maximum dose of which was 90 mg. The time when patients first requested analgesics and additional analgesics was recorded.
An additional analysis of the severity of PONV was performed,7 which was categorized into 4 degrees using the following standardized scoring algorithm that has been used in similar trials:8,9
"No PONV": Absence of any emetic episodes and nausea.
"Mild PONV": 1) Patients had only mild nausea. 2) One emetic episode or short lasting nausea of any severity (< 10 min) occurred but was triggered by an exogenous stimulus, such as drinking, eating, or postoperative movement. After the stimulus, nausea diminished and patients felt well again throughout the entire study period. No antiemetics drug was necessary.
"Moderate PONV": 1) Patients had 1 - 2 emetic episodes or moderate to severe nausea without exogenous stimulus. 2) Patients required antiemetics therapy once.
"Severe PONV": Patients had more than 2 emetic episodes or were nauseated more than two times (moderate or severe). The administration of at least more than 1 antiemetic was necessary.
Four mg of ondansetron was administered by IV when patients requested. The additional doses of antiemetics were recorded.
Power calculation indicated that 30 patients per group would be required to detect a difference of 50% in case of PONV, which was 40% in the previous study5 with a power of 90%.
All data were expressed as mean ± standard deviation or number (n). Comparisons of demographic data between the groups were made using Student t-test. Comparisons of VAS, side effects of opioids, additional analgesics, and use of antiemetics were made using Mann-Whitney U test. A p value < 0.05 was considered statistically significant. SPSSTM version 12.5 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
RESULTS
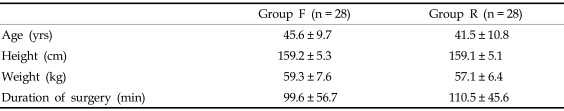
Fifty-six patients were enrolled in this study. Demographic characteristics of patients and the duration of surgery were similar between the 2 groups (Table 1). Four patients in group R left the study because of 1 case of severe PONV and 3 cases of serious respiratory depression.
Table 1.
Patient Demographic Data and Intraoperative Characteristics
Group F, fentanyl 0.5 µg/kg/hr IV; Group R, remifentanil 0.05 µg/kg/min IV for postoperative pain control.
There were no significant differences between the groups.
Values are mean ± SD.
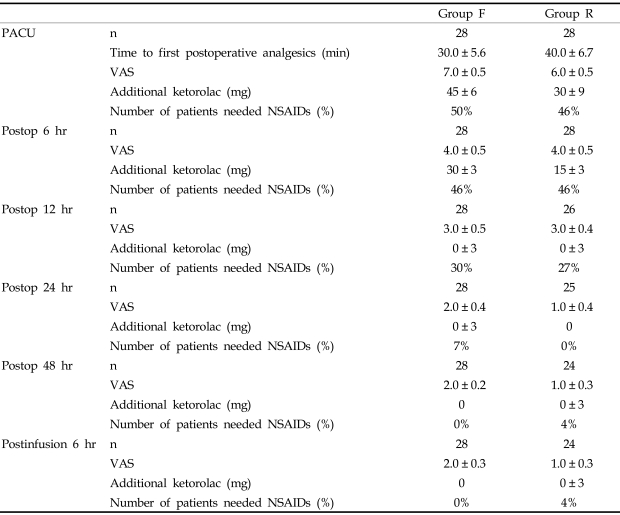
There were no significant differences in VAS, time to first postoperative analgesics, and additional analgesics between the groups (Table 2).
Table 2.
Postoperative VAS and Additional Analgesics
Group F, fentanyl 0.5 µg/kg/hr IV; Group R, remifentanil 0.05 µg/kg/min IV for postoperative pain control.
PACU, postoperative anesthesia care unit; Postop, postoperative; Postinfusion 6 hr, 6 hr after the end of infusion of the study drug; n, number of analyzed patients; Time to the first analgesics was defined from arrival at PACU; Additional ketorolac, the dosage of ketorolac required during the time intervals.
Values are median ± SE or number of patients.
There were no significant differences between the groups.
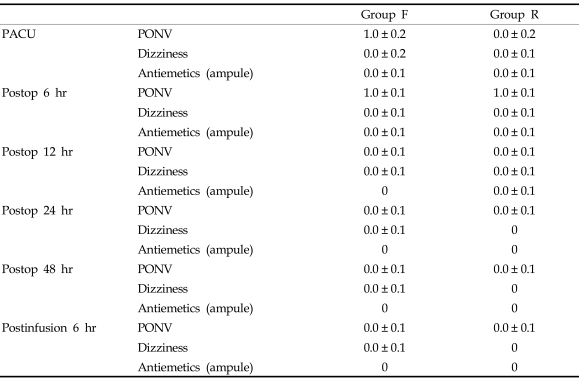
Although 1 patient experienced severe PONV and infusion of the study solution was stopped, the incidence and severity of PONV were not different between the groups (Table 3). Furthermore, there were no statistical differences in the antiemetics used between the groups. The incidence and severity of dizziness were not different between the groups (Table 3). No patients in either group complained of pruritus. However, there were 3 episodes (10.7%) of serious respiratory depression in group R, but none in group F, therefore, the current study inevitably came to an end.
Table 3.
Side Effects of Opioids and Use of Antiemetics
Group F, fentanyl 0.5 µg/kg/hr IV; Group R, remifentanil 0.05 µg/kg/min IV for postoperative pain control.
The severity of side effects related to opioid was rated as: 0 = none, 1 = mild, 2 = moderate, 3 = severe and refractory to the treatment.
PACU, postoperative anesthesia care unit; Postop, postoperative; Postinfusion 6 hr, 6 hr after the end of infusion of the study drug; Antiemetics; the number of required antiemetics during the time intervals.
Values are median ± SE.
There were no significant differences between the groups.
The first patient was a 27 years old woman with a body weight of 48 kg. On the first postoperative day (POD 1), an antibiotic cefotiam diluted by 4 mL of distilled water was injected into the patient (skin test was negative). The patient was found cyanotic and experienced loss of consciousness (LOC). Cardiopulmonary resuscitation (CPR) started immediately and the patient regained consciousness after about 10 min. There was no abnormality found on the ECG, chest X-ray, complete blood count, serum electrolytes, arterial blood gas analysis, and cardiac enzyme levels. The patient was discharged on POD 7 without problems. The second patient was a 43 years old woman with a body weight of 61 kg. On POD 2, a new fluid bag was connected to the IV tubing, and several drops of fluid ran into the tubing while changing the fluid bag. The patient became apneic, CPR was initiated, and the patient was intubated. Soon after, she began breathing spontaneously and was extubated. On neurologic examination and brain MRI, no abnormalities were found. The patient was discharged on POD 8. The third patient was a 46 years old woman with a body weight of 52 kg. She received an IV dose of cimetidine in the same intravenous line followed by 2 mL of flush solution. She immediately became cyanotic and apneic. We initiated CPR and she awoke shortly afterward. Subsequent examination revealed normal results, and the patient recovered without incident. In patients 2 and 3, we suspected malfunction of the infusor. After collecting Infusor SV2, we ran it for 24 hr to look for any malfunctions, but we did not find any abnormalities.
DISCUSSION
In the present study, the continuous infusion of remifentanil provided the same quality of analgesia after TAH as fentanyl in these clinical settings. There were no differences in VAS, incidences and severities of PONV, dizziness, additional analgesics, and antiemetics between the 2 groups. However, there were 3 episodes of serious respiratory depression in group R.
An ultra-short acting opioid such as remifentanil is preferred to a long-acting opioid such as morphine because remifentanil does not have any significant adverse effects on the cardiovascular system and does not accumulate in the body compared to other opioids.4 In addition, intraoperative remifentanil also produces less PONV than intraoperative fentanyl.6 These features of remifentanil could make it an ideal agent for postoperative analgesia. In this study, we employed a continuous background infusion of remifentanil since the pharmacokinetic profile of remifentanil makes it suitable for the continuous infusion.
There are some articles that advocate favorable results of remifentanil infusion for postoperative pain,5,10-14 especially in critical patients,10,11,14 but the authors stopped the current study when 3 patients from the group R had serious respiratory depression.
The amount of remifentanil that we chose for this study was the minimum amount of known efficacy of remifentanil in major surgical procedures and was comparable to previous studies as well as clinical experience.5,10-14 Although there have been no studies so far about equipotent doses of fentanyl and remifentanil for postoperative pain control, fentanyl in this study was infused at the recommended basal infusion rate15 and remifentanil was also infused at the basal infusion rate known to be effective for postoperative pain control.5,11,12,14 Patient-controlled analgesia technique is more effective in cases of IV pain control, but our study used only basal infusion for fear that bolus doses of remifentanil might cause respiratory depression.5,16-19
It might have been appropriate to measure drug concentration during the study period in an effort to compare whether our infusions were indeed equipotent. However, our aim was to compare these agents in a study that was clinically relevant and not as a direct comparison of their respective pharmacokinetic properties. Since there were no differences in the amount of additional analgesics given and the severity of pain under basal infusion of fentanyl and remifentanil in this study, it is assumed that the basal infusion rates used in this study are comparable.
Bowdle et al. evaluated the use of an infusion of remifentanil to provide postoperative analgesia during PACU stay.5 One hundred fifty-seven patients from 7 medical centers were enrolled, and apnea occurred in 11 patients (7%). Of the 11 apneic patients, 9 had received boluses of remifentanil prior to the onset of apnea. They reported 1 case of respiratory depression with a possible explanation: The remifentanil infusion was piggybacked into a standard IV infusion line. The "dead space" of the main IV tubing between the port where the remifentanil was inserted into the main IV tubing and the patient's vein could have varied from approximately 1 to 5 mL. Thus, changes in the rate of flow in the main IV tubing could have a substantial impact on the moment-to-moment delivery of remifentanil. If the flow in the main IV tubing were suddenly increased substantially, the remifentanil contained in the dead space of the IV tubing would suddenly be flushed into the patient. Such a bolus could easily produce respiratory depression, including apnea.5 In our cases, these events occurred right after side shooting of a medication or fluid flush of the main IV tubing. A small amount of remifentanil accumulated in the IV tubing can cause serious respiratory depression during postoperative pain control even in young and ASA I patients, especially in a general ward where continuous vital monitoring is not a common practice.
In the current study, an IV infusor was connected via a 3-way stopcock that was attached to a 70-cm-long, 5 mL volume IV extension line. The exact amount of remifentanil delivered to the patient while mishaps with IV tubing cannot be measured. Nevertheless, we can estimate the amount of remifentanil introduced into the IV main tubing. During the post-op period, the main fluid is usually infused at a rate of 120 mL/hr. In patient 1, 4 mL of diluted antibiotics was injected; therefore, about 4.6 µg of remifentanil could be injected. In patient 3, when 2 mL of fluid was flushed to the main IV tubing, about 2.5 µg of remifentanil could be injected. However, if the main fluid was injected at a slower rate, more study solution could have built up in the IV tubing.
Egan et al. demonstrated that bolus injection of remifentanil would be potentially safe and effective in clinical situation.20 However, some younger subjects also experienced respiratory depression at a relatively low dose of remifentanil and episodes of apnea occurred in 4 subjects. In addition, after 25 µg of remifentanil injections, respiratory depression developed in both younger and older subjects.
The primary side effect of concern in association with remifentanil in spontaneously ventilating patients is respiratory depression and apnea. Rapid onset opioids such as remifentanil are especially troublesome in this regard because the carbon dioxide ventilation-response curve (i.e. the relationship between minute volume and PaCO2) is altered before the patient's PaCO2 rises sufficiently to sustain ventilatory drive.17,18
Even though there are reports of successful remifentanil injection via bolus or IV PCA (patient-controlled analgesia), it can cause significant complications. While using remifentanil infusion for postoperative pain control, an extremely small dose of remifentanil can cause serious respiratory depression. Careful monitoring of respiratory function and skills in the recognition and treatment of inadequate respiration would be obligatory in a clinical setting when using remifentanil.
The present results show that a background infusion technique using remifentanil provides equal pain relief as measured by VAS and cannot reduce the incidence of PONV compared to fentanyl administration. However, serious respiratory depression was observed during continuous infusion of remifentanil but not fentanyl. Our study indicates that IV continuous infusion technique using remifentanil for postoperative analgesia would cause serious respiratory depression in spontaneously ventilating patients.
References
- 1.Gust R, Pecher S, Gust A, Hoffmann V, Böhrer H, Martin E. Effect of patient-controlled analgesia on pulmonary complications after coronary artery bypass grafting. Crit Care Med. 1999;27:2218–2223. doi: 10.1097/00003246-199910000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Feldman PL, James MK, Brackeen MF, Bilotta JM, Schuster SV, Lahey AP, et al. Design, synthesis, and pharmacological evaluation of ultrashort- to long-acting opioid analgesics. J Med Chem. 1991;34:2202–2208. doi: 10.1021/jm00111a041. [DOI] [PubMed] [Google Scholar]
- 3.Michelsen LG, Salmenperä M, Hug CC, Jr, Szlam F, VanderMeer D. Anesthetic potency of remifentanil in dogs. Anesthesiology. 1996;84:865–872. doi: 10.1097/00000542-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B) Anesth Analg. 1993;77:1031–1040. doi: 10.1213/00000539-199311000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Bowdle TA, Camporesi EM, Maysick L, Hogue CW, Jr, Miguel RV, Pitts M, et al. A multicenter evaluation of remifentanil for early postoperative analgesia. Anesth Analg. 1996;83:1292–1297. doi: 10.1097/00000539-199612000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Rama-Maceiras P, Ferreira TA, Molíns N, Sanduende Y, Bautista AP, Rey T. Less postoperative nausea and vomiting after propofol + remifentanil versus propofol + fentanyl anaesthesia during plastic surgery. Acta Anaesthesiol Scand. 2005;49:305–311. doi: 10.1111/j.1399-6576.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 7.Korttila K. The study of postoperative nausea and vomiting. Br J Anaesth. 1992;69(7 Suppl 1):20S–23S. doi: 10.1093/bja/69.supplement_1.20s. [DOI] [PubMed] [Google Scholar]
- 8.Eberhart LH, Seeling W, Bopp TI, Morin AM, Georgieff M. Dimenhydrinate for prevention of post- operative nausea and vomiting in female in-patients. Eur J Anaesthesiol. 1999;16:284–289. doi: 10.1046/j.1365-2346.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 9.Eberhart LH, Seeling W, Ulrich B, Morin AM, Georgieff M. Dimenhydrinate and metoclopramide alone or in combination for prophylaxis of PONV. Can J Anaesth. 2000;47:780–785. doi: 10.1007/BF03019481. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan K, Elliot SC, Berridge JC, Mallick A. Remifentanil patient-controlled analgesia following cardiac surgery. Acta Anaesthesiol Scand. 2005;49:876–879. doi: 10.1111/j.1399-6576.2005.00729.x. [DOI] [PubMed] [Google Scholar]
- 11.Yalcin Cok O, Ozkose Z, Atabekoglu S, Yardim S. Intravenous patient-controlled analgesia using remifentanil in a child with Axenfeld-Rieger syndrome. Paediatr Anaesth. 2005;15:162–166. doi: 10.1111/j.1460-9592.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 12.Calderón E, Pernia A, De Antonio P, Calderón-Pla E, Torres LM. A comparison of two constant-dose continuous infusions of remifentanil for severe postoperative pain. Anesth Analg. 2001;92:715–719. doi: 10.1097/00000539-200103000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Kucukemre F, Kunt N, Kaygusuz K, Kiliccioglu F, Gurelik B, Cetin A. Remifentanil compared with morphine for postoperative patient-controlled analgesia after major abdominal surgery: a randomized controlled trial. Eur J Anaesthesiol. 2005;22:378–385. doi: 10.1017/s0265021505000657. [DOI] [PubMed] [Google Scholar]
- 14.Gurbet A, Goren S, Sahin S, Uckunkaya N, Korfali G. Comparison of analgesic effects of morphine, fentanyl, and remifentanil with intravenous patient-controlled analgesia after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:755–758. doi: 10.1053/j.jvca.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Sinatra RS. Acute pain management and acute pain services. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anesthesia and management of pain. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. pp. 793–835. [Google Scholar]
- 16.Schüttler J, Albrecht S, Breivik H, Osnes S, Prys-Roberts C, Holder K, et al. A comparison of remifentanil and alfentanil in patients undergoing major abdominal surgery. Anaesthesia. 1997;52:307–317. doi: 10.1111/j.1365-2044.1997.24-az0051.x. [DOI] [PubMed] [Google Scholar]
- 17.Bouillon T, Bruhn J, Radu-Radulescu L, Andresen C, Cohane C, Shafer SL. A model of the ventilatory depressant potency of remifentanil in the non-steady state. Anesthesiology. 2003;99:779–787. doi: 10.1097/00000542-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Gross JB. When you breathe IN you inspire, when you DON'T breathe, you... expire: new insights regarding opioid-induced ventilatory depression. Anesthesiology. 2003;99:767–770. doi: 10.1097/00000542-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Mallick A, Elliot S. Remifentanil is too potent to be given by bolus. Br J Anaesth. 2004;93:305–306. doi: 10.1093/bja/aeh589. [DOI] [PubMed] [Google Scholar]
- 20.Egan TD, Kern SE, Muir KT, White J. Remifentanil by bolus injection: a safety, pharmacokinetic, pharmacodynamic, and age effect investigation in human volunteers. Br J Anaesth. 2004;92:335–343. doi: 10.1093/bja/aeh075. [DOI] [PubMed] [Google Scholar]