Abstract
Purpose
Vascular endothelial growth factor (VEGF) levels in malignant ascites have high diagnostic value for their discrimination from asictes of non-malignant origin. However, there have been no reports on the comparison of VEGF levels between malignant ascites of chemonaive and chemotreated patients.
Materials and Methods
VEGF levels were measured in 44 ascites patients (cirrhosis ascites, 10; chemonaive patients, 21; chemotreated patients, 13) and compared to the level of carcinoembryonic antigen (CEA) and carbohydrate antigen 19 - 9 (CA 19 - 9). The diagnostic parameters of sensitivity, specificity, and correlation among 3 markers were evaluated.
Results
VEGF levels in malignant ascites of chemonaive and chemotreated patients were significantly higher than those in cirrhotic ascites (p< 0.05). VEGF levels in ascites of chemonaive patients were significantly higher than those in chemotreated patients (p< 0.05). A cutoff value of 10.4 pg/mL was calculated using receiver operating characteristic curves (ROCs) for VEGF in chemotreated and chemonaive patients, which gave sensitivities of 75.0% and 53.8% and specificities of 69.6% and 47.1%, respectively. Positive correlations were observed between VEGF and CEA (r = 0.353, p< 0.05) as well as between VEGF and CA19 - 9 (r = 0.367, p< 0.05) in ascites.
Conclusion
VEGF levels could be a useful tumor marker for malignant ascites, but its value should carefully be interpreted because of lesser reliability in chemotreated ones.
Keywords: Vascular endothelial growth factor, chemotherapy, malignant ascites, carbohydrate antigen 19-9, carcinoembryonic antigen
INTRODUCTION
The differential diagnosis of ascites is important for detection of underlying disease and treatment in clinical practice. Lymphatic obstruction and other factors, including vascular permeability factors and metalloproteinase, have been considered as major pathophysiological mechanism of ascites formation.1 However, the precise mechanism of its development is unknown. The detection of malignant cells in asctic fluid has been the gold standard for the diagnosis of malignant ascites, but it has a sensitivity of only 40 - 60%.2 Other parameters, such as lactic acid dehydrogenase,3 C-reactive protein,4 fibronectin,5 and cholesterol,6 have also been studied, nevertheless, none of them proved to be a fully satisfactory discriminant between malignant and non-malignant ascites.
VEGF is a unique angiogenic dimeric glycoprotein with a molecular mass of 34 to 42 kDa, and it has potent endothelial cell mitotic activity and vascular permeability activity.7 VEGF is involved in the formation of ascites, but its value in the diagnosis of malignant ascites has not been fully elucidated.8,9 It has been reported that VEGF levels are significantly high in malignant ascites and serum from patients with ovarian or gastrointestinal carcinoma, especially with metastatic disease.8,10-12 On the other hand, several chemotherapeutic agents appear to inhibit VEGF- induced angiogenesis in several animal studies.13-16 The purpose of this study was to evaluate differences in VEGF levels in malignant ascites between no chemotreated (chemonaive) patients and chemoreceived patients due to various malignancies.
CEA and CA19 - 9 are the most widely used tumor markers for various cancers, especially gastrointestinal cancers.17-19 Tumor markers such as CEA and CA19 - 9 could also be useful tools in the differential diagnosis of benign and malignant effusions.20 It has been demonstrated that CEA and CA19 - 9 showed significant sensitivity difference between patients with malignant-related ascites and those without malignant-related ascites.21,22 Therefore, we measured CEA and CA19 - 9 to assess the utility of discriminating malignant from nonmalignant ascites. Furthermore, we compared VEGF in ascites of chemonaive and chemotreated patients with conventional tumor markers such as CEA and CA19 - 9.
MATERIALS AND METHODS
Subjects
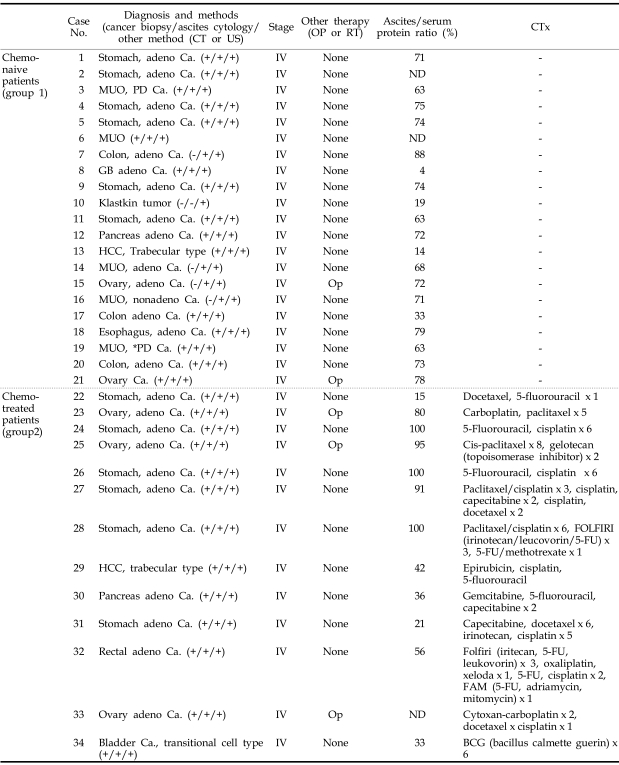
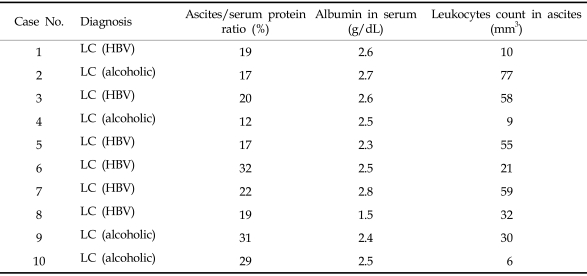
Forty-four patients (aged 10 - 90 yr; 26 males and 18 females), who presented with clinically detectable ascites, were studied at Uijeongbu St. Mary's Hospital of Catholic University of Korea from March 2005 to April 2006. Patients with malignant ascites who had not undergone systemic chemotherapy (n = 21) were defined as group 1, patients with malignant ascites who had undergone chemotherapy as group 2 (n = 13, Table 1), and patients with cirrhotic ascites as group 3 (n = 10, Table 2), which were transudate ascites of viral origin (6 cases) and alcoholic origin (4 cases). The diagnosis of malignant tumors in all patients was established by primary cancer biopsy (n = 29) and cytology of ascites (n = 4). Klastkin tumor (group 1, case 10) was diagnosed by CT scan (Table 1). Liver carcinomas of 2 patients were not accompanied by liver cirrhosis. Ascites samples were collected from the supernatants of all subjects after centrifugation (900 × g) and stored at -80℃ until use. The study protocol was approved by the ethics committee of Uijeongbu St. Mary's Hospital. Informed consent was obtained from all patients.
Table 1.
Clinical and Pathological Characteristics of Malignant Ascites Patients
PD Ca., poorly differentiated carcinoma; Adeno Ca., adenocarcinoma; CT, computed tomography; US, ultrasonography; MUO, metastasis of unknown origin; OP, operation; RT, radiation therapy; ND, not done; CTx, chemotherapy; P, progression; PR, partial response; FU, fluorouracil.
Table 2.
Clinical and Pathological Characteristics in Cirrhotic Patients (Group 3)
LC, liver cirrhosis; HBV, hepatitis B virus.
Methods
The ascitic fluid of each patient was analyzed for VEGF, CEA, CA19 - 9, protein concentration, and leukocytes counts. In cirrhotic ascites (group 3), leukocyte counts were performed to exclude bacterial peritonitis as a complication of cirrhosis. The samples were analyzed in a laboratory of the Department of Laboratory Medicine, Uijeongbu St. Mary's Hospital. The concentrations of VEGF in the ascites were measured using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's guidelines. The samples were analyzed in duplicate; human recombinant VEGF 165 was serially diluted and used as the standard. VEGF concentrations were measured according to a standard curve. Levels of CEA and CA19 - 9 concentrations were measured in all patients by radioimmune assay.23 Previously published cutoff values for ascitic CEA24 and CA19 - 925 were used in this study (normal value; CEA < 5 ng/mL, CA19 - 9 < 37 U/mL). The nature of the ascites (transudate or exudate) was classified by the use of Light's criteria to give a diagnostic sensitivity of 98% and specificity of 98% for an exudate.26 Ascites showing an ascites/serum protein ratio greater than 50% were regarded as exudate. The results of chemotherapy in group 2 were evaluated as follows: complete response, partial response, progressive disease, and stable disease. The response criteria were defined according to WHO guidelines.27,28
Statistical analysis
Differences between the groups were examined using Mann-Whitney test. Differences between patients with adenocarcinoma and those with nonadenocarcinoma in group 1 and group 2 were examined using Mann-Whitney test. The data are presented as means±standard deviations. The relationships between VEGF and the conventional parameters, CEA and CA19-9, were analyzed using Spearman's correlation analysis. Data were analyzed using SPSS software (version 10.0, Chicago, IL, USA). Differences were considered significant at p values less than 0.05.
RESULTS
VEGF levels in the malignant ascites of either chemonaive patients (349.13 ± 490.76 pg/mL) and chemotreated patients (51.68 ± 89.26 pg/mL) were significantly higher than those of patients with cirrhotic ascites (0.26 ± 0.82 pg/mL) (p< 0.05). VEGF levels in malignant ascites of the untreated group were also significantly higher than in the chemotreated malignant group (p< 0.05). When patients were evaluated according to pathological type, VEGF levels in ascites did not differ between those with adenocarcinoma (n = 17 in group 1, n = 11 in group 2) and those with nonadenocarcinomas (n = 4 in group 1, n = 2 in group 2; p > 0.05).
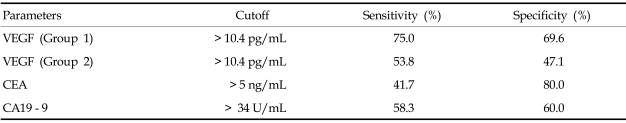
A cutoff value of 10.4 pg/mL was selected using ROC curves for VEGF in malignant ascites of patients in group 1 and group 2, which gave sensitivities of 75.0% and 53.8%, respectively, and specificities of 69.6% and 47.1%, respectively Figs. 1A and B. When cutoff values for CEA and CA19 - 9 were applied, the results obtained were classified into true positive, false positive, true negative and false negative. Sensitivity and specificity were then calculated for each parameter, and the results are shown in Table 3. Significant correlations were observed between VEGF levels and ascitic CA19 - 9 in malignant ascites (r = 0.367, p< 0.05, Fig. 2A). There was also a significant correlation between VEGF levels and CEA concentrations in malignant ascites (r = 0.353, p< 0.05, Fig. 2B).
Fig. 1.
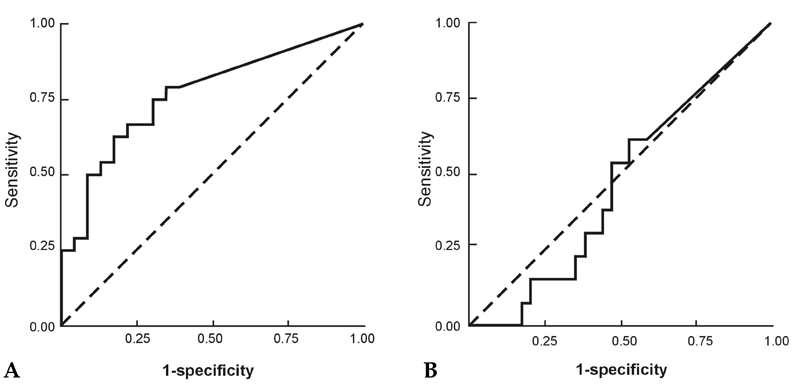
(A) Malignant ascites of chemonaive patients. The areas under the ROC curve were 0.766 (p = 0.002). (B) Malignant ascites of chemotreated patients. The areas under the ROC curve were 0.462 (p = 0.686). ROC, receiver operating characteristic.
Table 3.
Diagnostic Value of Individual Parameters in Differentiating Malignant from Nonmalignant Ascites
VEGF, vascular endothelial growth factor; Group 1, malignant ascites of chemonaive patients; Group 2, malignant ascites of chemotreated patients; CEA, carcinoembryonic antigen; CA19 - 9, carbohydrate antigen 19 - 9.
Fig. 2.
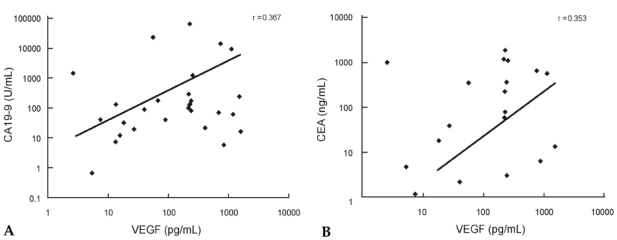
(A) Correlation between ascitic VEGF concentrations and CA19 - 9 concentrations in malignant ascites. (B) Correlation between ascitic VEGF concentrations and CEA concentrations in malignant ascites. VEGF, vascular endothelial growth factor.
Forty-one samples (chemonaive 19/21, chemotreated 12/13, and cirrhotic ascites 10/10) out of 44 patients were evaluated for exudative or transudative ascites (Table 1). The fraction of exudative ascites was 78.9% (15/19) in group 1, 58.3% (7/12) in group 2, and 0% in group 3, according to Light's criteria. In 3 patients, ascites measurements were not done. When applying Light's criteria to all patients, 71.0% and 100% of sensitivity and specificity, respectively, were found.
DISCUSSION
Malignant ascites are frequently found during the progression of cancer and incur significant morbidity. It has been suggested that VEGF is involved in the formation of ascites,9 but its role as a marker for malignant ascites has not yet been fully established. A similar observation earlier described that VEGF plays a role in the formation of malignant ascites by increasing vascular permeability.7,8 This finding suggests that VEGF might be a useful marker of malignant ascites, distinguishing it from benign ascites. In the current study, VEGF concentrations in the malignant ascites of the chemonaive group or chemotreated group were significantly higher than those of patients with cirrhotic ascites as a control. On the other hand, VEGF had better sensitivity than that of CA19 - 9 or CEA for discriminating between malignant and nonmalignant ascites. Furthermore VEGF in ascites alone is an excellent tumor marker only in chemonaive patients compared to 2 conventional tumor markers.
Several chemotherapeutic agents inhibit VEGF-induced angiogenesis. There has been no report of VEGF secretion in malignant ascites, particularly in chemotreated patients. In this study, we demonstrated that VEGF levels in chemotreated group decreased more than those in the untreated group. This result reaffirms that chemotherapeutic agents exert antiangiogenic activity. From a clinical perspective, our study demonstrated that the clinical significance of VEGF levels in ascites of chemotreated patients should be interpreted with care due to low sensitivity and specificity.
In our study, a cutoff value of 10.4 pg/mL was selected from the ROC curves for VEGF in groups 1 and 2, and this gave sensitivities of 75.0% and 53.8% and specificities of 69.6% and 47.1%, respectively. Our results were lower than the previously reported values for sensitivity (91.3%) and specificity (90.9%) for VEGF levels in ascites.8 We do not know the reasons for this, nevertheless, these results might be due to the proportion of metastatic carcinoma of unknown origin (MUO). Furthermore, the standard deviations of VEGF measurements in this study were very large, possibly due to significant case differences, including various kinds of malignancy, and 3 patients in group 1 had extremely high levels of ascitic VEGF concentrations (1000 pg/mL or more). Two patients in group 2 had ascitic VEGF concentrations of 200 pg/mL or more resulting in a large standard deviation.
In this study, there were no significant differences in VEGF levels between patients with adenocarcinomas and those with nonadenocarcinomas. In both chemonaive and chemotreated patients, the presence of different cell types could not explain their different VEGF levels. These results are in concordance with earlier studies that VEGF values did not differ significantly according to histologic subtype.12
In this study, significant correlations were found between VEGF levels and both CEA and CA19 - 9 concentrations. Until now, there has been no study on the correlations between VEGF levels and both CEA and CA19 - 9 concentrations. However, similar results were reported about the role of serum VEGF in gastrointestinal cancer; serum VEGF levels correlated well with those of CEA and CA19 - 9, according to the progression of cancer.29 In our analysis, sensitivity for evaluation of exudative ascites was lower than expected because of applying only protein ratio: Light's criteria consist of 3 criteria (protein ratio, LDH ratio, and LDH activity). But Loewenstein et al. reported that a CEA level (> 10 ng/mL) in ascites suggests malignancy, even if the fluid is transudative.30 In our study, 4 cases of malignant ascites were correctly classified as transudative malignancy by measurement of CEA. Thererfore, it is necessary in the future to elucidate the correlation between several recent tumor markers as well as conventional markers. The present data again confirmed the possible role of VEGF as a new marker for malignant ascites, however, its value has to be carefully interpreted in patients with past history of chemotherapy.
Footnotes
This work was supported by the grant from Catholic University in 2006.
References
- 1.Aslam N, Marino CR. Malignant ascites: new concepts in pathophysiology, diagnosis, and management. Arch Intern Med. 2001;161:2733–2737. doi: 10.1001/archinte.161.22.2733. [DOI] [PubMed] [Google Scholar]
- 2.Colli A, Buccino G, Cocciolo M, Parravicini R, Mariani F, Scaltrini G. Diagnostic accuracy of fibronectin in the differential diagnosis of ascites. Cancer. 1986;58:2489–2493. doi: 10.1002/1097-0142(19861201)58:11<2489::aid-cncr2820581123>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Sevinc A, Sari R, Fadillioglu E. The utility of lactate dehydrogenase isoenzyme pattern in the diagnostic evaluation of malignant and nonmalignant ascites. J Natl Med Assoc. 2005;97:79–84. [PMC free article] [PubMed] [Google Scholar]
- 4.Yildirim B, Sari R, Isci N. Patients with spontaneous bacterial peritonitis, and mlignant and cirrhotic ascites. J Natl Med Assoc. 2005;97:276–280. [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui RA, Kochhar R, Singh V, Rajwanshi A, Goenka MK, Metha SK. Evaluation of fibronectin as a marker of malignant ascites. J Gastroenterol Hepatol. 1992;7:161–164. doi: 10.1111/j.1440-1746.1992.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 6.Rana SV, Babu SG, Kocchar R. Usefulness of ascitic fluid cholesterol as a marker for malignant ascites. Med Sci Monit. 2005;11:CR136–CR142. [PubMed] [Google Scholar]
- 7.Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999;6:373–378. doi: 10.1007/s10434-999-0373-0. [DOI] [PubMed] [Google Scholar]
- 8.Dong WG, Sun XM, Yu BP, Luo HS, Yu JP. Role of VEGF and CD44v6 in differentiating benign from malignant ascites. World J Gastroenterol. 2003;9:2596–2600. doi: 10.3748/wjg.v9.i11.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramov Y, Anteby SO, Fasouliotis SJ, Barak V. Markedly elevated levels of vascular endothelial growth factor, fibroblast growth factor, and interleukin 6 in Meigs syndrome. Am J Obstet Gynecol. 2001;184:354–355. doi: 10.1067/mob.2001.110028. [DOI] [PubMed] [Google Scholar]
- 10.Sun XM, Dong WG, Yu BP, Luo HS, Yu JP. Clinical significance of detecting VEGF, CD44v6, MMP-2 and MMP-9 in malignant ascites. Ai Zheng. 2004;23:85–89. [PubMed] [Google Scholar]
- 11.Nascimento I, Schaer R, Lemaire D, Freire S, Paule B, Carvalho S, et al. Vascular endothelial growth factor (VEGF) levels as a tool to discriminate between malignant and nonmalignant ascites. APMIS. 2004;112:585–587. doi: 10.1111/j.1600-0463.2004.apm1120904.x. [DOI] [PubMed] [Google Scholar]
- 12.Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178–187. [PubMed] [Google Scholar]
- 13.Kuwahara K, Sasaki T, Kobayashi K, Noma B, Serikawa M, Iiboshi T, et al. Gemcitabine suppresses malignant ascites of human pancreatic cancer: correlation with VEGF expression in ascites. Oncol Rep. 2004;11:73–80. [PubMed] [Google Scholar]
- 14.Manenti L, Riccardi E, Marchini S, Naumova E, Floriani I, Garofalo A, et al. Circulating plasma vascular endothelial growth factor in mice bearing human ovarian carcinoma xenograft correlates with tumor progression and response to therapy. Mol Cancer Ther. 2005;4:715–725. doi: 10.1158/1535-7163.MCT-04-0305. [DOI] [PubMed] [Google Scholar]
- 15.Keyes K, Cox K, Treadway P, Mann L, Shih C, Faul MM, et al. An in vitro tumor model: analysis of angiogenic factor expression after chemotherapy. Cancer Res. 2002;62:5597–5602. [PubMed] [Google Scholar]
- 16.Lissoni P, Fugamalli E, Malugani F, Ardizzoia A, Secondino S, Tancini G, et al. Chemotherapy and angiogenesis in advanced cancer: vascular endothelial growth factor (VEGF) decline as predictor of disease control during taxol therapy in metastatic breast cancer. Int J Biol Markers. 2000;15:308–311. doi: 10.1177/172460080001500405. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes LC, Kim SB, Saad SS, Matos D. Value of carcinoembryonic antigen and cytokeratins for the detection of recurrent disease following curative resection of colorectal cancer. World J Gastroenterol. 2006;12:3891–3894. doi: 10.3748/wjg.v12.i24.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe D, Lee J, Schade R, Chaudhary A. Patient with markedly elevated CA 19-9 not associated with malignancy. South Med J. 2006;99:306–308. doi: 10.1097/01.smj.0000202695.97123.2b. [DOI] [PubMed] [Google Scholar]
- 19.Nowak J, Jakubowska D, Wiczkowski A, Sprzaczkowska K, Stechly T, Zmudzińs W, et al. Carbohydrate antigens CA 19-9, CA 242, CA 50 in liver diseases. Wiad Lek. 1998;51:484–491. [PubMed] [Google Scholar]
- 20.Trapé J, Molina R, Sant F. Clinical evaluation of the simultaneous determination of tumor markers in fluid and serum and their ratio in the differential diagnosis of serous effusions. Tumour Biol. 2004;25:276–281. doi: 10.1159/000081392. [DOI] [PubMed] [Google Scholar]
- 21.Chen SJ, Wang SS, Lu CW, Chao Y, Lee FY, Lee SD, et al. Clinical value of tumour markers and serum-ascites albumin gradient in the diagnosis of malignancy-related ascites. J Gastroenterol Hepatol. 1994;9:396–400. doi: 10.1111/j.1440-1746.1994.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 22.Cascinu S, Del Ferro E, Barbanti I, Ligi M, Fedeli A, Catalano G. Tumor markers in the diagnosis of malignant serous effusions. Am J Clin Oncol. 1997;20:247–250. doi: 10.1097/00000421-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Miles LE, Hales CM. Labelled antibodies and immunological assay systems. Nature. 1968;219:186–189. doi: 10.1038/219186a0. [DOI] [PubMed] [Google Scholar]
- 24.Denstman F, Rosen L, Khubchandani IT, Sheets JA, Stasik JJ, Reither RD. Comparing predictive decision rules in postoperative CEA monitoring. Cancer. 1986;58:2089–2095. doi: 10.1002/1097-0142(19861101)58:9<2089::aid-cncr2820580921>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 25.Del Villano BC, Brennan S, Brock P, Bucher C, Liu V, McClure M, et al. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19-9. Clin Chem. 1983;29:549–552. [PubMed] [Google Scholar]
- 26.Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–513. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 27.WHO Handbook for Reporting Results of Cancer Treatment. Geneva: World Health Organization; 1979. (WHO Offset Publication No. 48). [Google Scholar]
- 28.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Hyodo I, Doi T, Endo H, Hosokawa Y, Nishikawa Y, Tanimizu M, et al. Clinical significance of plasma vascular endothelial growth factor in gastrointestinal cancer. Eur J Cancer. 1998;34:2041–2045. doi: 10.1016/s0959-8049(98)00282-2. [DOI] [PubMed] [Google Scholar]
- 30.Loewenstein MS, Rittgers RA, Feinerman AE, Kupchik HZ, Marcel BR, Koff RS, et al. Carcinoembryonic antigen assay of ascites and detection of malignancy. Ann Intern Med. 1978;88:635–638. doi: 10.7326/0003-4819-88-5-635. [DOI] [PubMed] [Google Scholar]